Abstract
AIM
To determine the relationship between chronic kidney disease (CKD) awareness (CKD-A), self-management behaviors (CKD-SMB) knowledge, performance of CKD-SMBs, health literacy (HL) and kidney function.
METHODS
Participants were eligible patients attending an outpatient nephrology clinic. Participants were administered: Newest Vital Sign to measure HL, CKD self-management knowledge tool (CKD-SMKT) to assess knowledge, past performance of CKD-SMB, CKD-A. Estimated GFR (eGFR) was determined using the MDRD-4 equation. Duration of clinic participation and CKD cause were extracted from medical charts.
RESULTS
One-hundred-fifty patients participated in the study. eGFRs ranged from 17-152 mL/min per 1.73 m2. Majority (83%) of respondents had stage 3 or 4 CKD, low HL (63%), and were CKD aware (88%). Approximately 40% (10/25) of patients in stages 1 and 2 and 6.4% (8/125) in stages 3 and 4 were unaware of their CKD. CKD-A differed with stage (P < 0.001) but not by HL level, duration of clinic participation, or CKD cause. Majority of respondents (≥ 90%) correctly answered one or more CKD-SMKT items. Knowledge of one behavior, “controlling blood pressure” differed significantly by CKD-A. CKD-A was associated with past performance of two CKD-SMBs, “controlling blood pressure” (P = 0.02), and “keeping healthy body weight” (P = 0.01). Adjusted multivariate analyses between CKD-A and: (1) HL; and (2) CKD-SMB knowledge were non-significant. However, there was a significant relationship between CKD-A and kidney function after controlling for demographics, HL, and CKD-SMB (P < 0.05).
CONCLUSION
CKD-A is not associated with HL, or better CKD-SMBs. CKD-A is significantly associated with kidney function and substantially lower eGFR, suggesting the need for focused patient education in CKD stages 1.
Keywords: Chronic kidney disease awareness, Health literacy, Kidney function, Self-management behaviors, Self-management behavior performance, Epidermal growth factor receptor, Chronic kidney disease knowledge
Core tip: Chronic kidney disease (CKD) awareness has not been examined in a specialty clinic environment. This study examined the associations between CKD awareness, health literacy, CKD self-management behaviors, past performance of self-management behaviors, and kidney function. We found that majority of the participants in our study were aware of having CKD. CKD awareness increased as CKD worsened; however, nearly 40% of patients in CKD stages 1 and 2 and about 6% of patients in CKD stages 3 and 4 were unaware of having CKD. CKD awareness was not related to health literacy, self-management behavior knowledge, or past performance of behaviors.
INTRODUCTION
Chronic kidney disease (CKD) is marked by slow and progressive decline in kidney function, leading to end stage renal disease (ESRD), a condition associated with high morbidity, mortality, and economic burden[1]. The prevalence of CKD in the United States is approximately 15%[2] and rising[1]. Despite this, CKD awareness (CKD-A) is low among primary care patients, ranging from 6% to 9%[3-6], with greater awareness in more advanced stages of CKD[7,8]. Improving CKD-A is the goal of public health initiatives such as Kidney Early Evaluation Program[8] and National Kidney Disease Education Program[9].
Awareness of CKD is an essential step for patient adherence to multiple CKD-specific health behaviors to slow further renal function deterioration. Behavioral management of CKD is complex and includes managing blood pressure (BP), weight, cholesterol, blood glucose levels in those with diabetes, fluid intake, dietary modifications, medication adherence, and engaging in physical activity[10]. Optimal performance of these health behaviors requires patients to be knowledgeable and health literate about these behaviors. Knowledge and health literacy (HL) are closely related, though distinct concepts, as health knowledge, while essential for adequate HL[11], does not ensure that an individual is health literate. Low HL is a significant problem impacting 23%-28% of patients with CKD[12,13]. Low HL has been linked to poor kidney function[14,15] and lower kidney disease knowledge[16]. A review by Mackey et al[17] examining the relationship between HL and development of self-management skills, concluded that low HL presents a significant issue in development of self-management skills.
Previous research examining relationships between CKD-A and clinical risk reduction targets, clinical markers, and specific health behaviors[3,6,18] found no relationship between CKD-A and most clinical markers or targets and with two out of three health behaviors. However, since these studies were based on larger databases designed for other purposes, they may not capture the entire range of patient-reported CKD-specific self-management behaviors. Previous work has not examined whether CKD-A is associated with HL, and importantly, with actual performance of behaviors, a vital issue in CKD self-management. While previous work has shown that there is greater awareness at higher CKD stages[7,8], these have not been examined in the context of patients participating in a specialty nephrology clinic.
The purpose of this study was to examine the relationship between CKD-A and: (1) Knowledge of current CKD self-management behaviors (CKD-SMB); (2) performance of CKD-SMB in previous three months as a measure of engagement in behavior; (3) HL; and (4) kidney function (eGFR) in patients attending a specialty nephrology clinic.
MATERIALS AND METHODS
The study was conducted at an outpatient specialty nephrology clinic at the University of New Mexico Health Sciences Center (UNM HSC) between April and August 2012. Inclusion criteria included: (1) At least 21 years of age; (2) English-speaking; (3) having CKD stages 1-4; and (4) one prior visit at the outpatient nephrology clinic. Exclusion criteria included acute kidney injury, and cognitive impairment. Patients were excluded if they had acute kidney injury and if their medical charts showed signs of poor cognitive functioning. Cognitive impairment was further assessed using the six item screener, a psychometrically valid and reliable tool to identify patients with cognitive impairment[19]. Patients with a score of less than 4 were excluded. Visual acuity was measured using the pocket vision screener (Rosenbaum, Graham-Field Surgical Co Inc., New York, NY, United States)[20]. Those with visual acuity worse than 20/100 were also excluded. Patients were given a $20 merchandise gift card as compensation for participating, irrespective of whether they met vision or cognitive screening tests. Individuals who consented to participate were evaluated for cognitive impairment and visual acuity. Detailed description of the study methodology is provided elsewhere[14].
Eligible patients who consented were administered the following instruments by trained interviewers: (1) Newest Vital Sign (NVS) a validated HL instrument[21]; (2) CKD-SMKT, a 10 item CKD self-management knowledge tool measuring patients’ knowledge about common CKD SMBs[22]. After responding to the knowledge aspect for each CKD-SMKT behavior item, respondents indicated whether they had performed the respective behavior in the previous three months using a dichotomous (Yes/No) response. This instrument has been content validated[20] and reported in a previous study[14]; and (3) one question about CKD awareness, “Have you ever been told that you have weak or failing kidneys (excluding kidney stones, bladder infections, or incontinence (i.e., no bladder control)”. This question is similar to previous studies that have assessed CKD awareness[5,6,7,18,23]. Self-reported demographic data were also collected. We also extracted data on medically reported cause of CKD as well as patients’ first visit date to the specialty clinic from medical records to determine the length of time attending specialty clinic. Kidney function was estimated using the Modification of Diet in Renal Disease (MDRD) equation (MDRD-4) traceable to IDMS, which uses serum creatinine concentration, age, gender, and race. Patients received a $20 merchandise gift card for study participation. Approval to conduct the study was obtained from the institutional review boards of both Southern Illinois University Edwardsville and University of New Mexico Health Sciences Center.
Statistical analysis
Sample size calculations were based on the effect size for kidney function, with a power of 0.9, alpha of 0.05[14]. Descriptive statistics were used for quantitative variables; frequencies and percentages were used for categorical variables. Duration of time attending the specialty clinic was calculated by determining the number of months from the first visit date recorded in the specialty clinic and the date on which the survey was conducted. CKD-A was a dichotomous variable. HL derived from NVS scores, was combined into two categories: (1) limited HL (high likelihood and possible likelihood of limited HL); and (2) adequate HL. Knowledge of CKD-SMB was calculated as percent of correct CKD-SMB responses on the 10-item CKD-SMKT. eGFR was log transformed to address deviations from normality.
Chi-square contingency coefficient and Fisher’s exact test were used for the following analyses: (1) demographics and CKD-A; (2) CKD-A and performance of each activity over the previous three months. Demographic categories were collapsed to reduce the number of cells with expected frequencies < 5. Median test was used to examine whether duration of participation in specialty clinic affected CKD awareness.
Hierarchical regression was performed to determine whether CKD-A was associated with knowledge of CKD-SMB (measured as percent knowledge) after controlling for demographics and HL. Logistic regression was used to examine the relationship between CKD-A and HL after controlling for demographics and knowledge of CKD-SMB. The relationship between CKD-A and kidney function after controlling for key demographics, and length of time attending clinic was examined using both logistic and hierarchical regression as appropriate. As age, gender, and race are used to calculate eGFR, they were removed from multivariate analyses that involved eGFR, similar to a previously published study[14]. Analyses were conducted using SPSS Inc. Released 2009 PASW statistical version 18.0 (SPSS Inc. Chicago, IL, United States). Results were considered statistically significant at P < 0.05[14]. The statistical review of the study was performed by Dr. Junvie Pailden, from SIUE Department of Mathematics and Statistics.
RESULTS
Patients
Of the 181 patients approached, 150 met the eligibility criteria and participated in the study (83% participation rate)[14]. Non-participants included 15 patients who declined to participate, and 16 patients who agreed to participate but failed to meet screening criteria. Among those who failed screening criteria, majority (n = 15) of the patients were excluded because they had a score of 4 or less on the six item screener (SIS), and one was excluded because they had recent acute kidney injury. Table 1 shows the demographics of the sample.
Table 1.
Sample demographics
| Characteristic | Total n (%), n = 150 | CKD aware n (%), n = 132 (88%) | CKD unaware, n = 18 (12%) | P valuea |
| Gender | 0.087 | |||
| Male | 70 (47) | 65 (49) | 5 (27) | |
| Female | 80 (53) | 67 (51) | 13 (72) | |
| Race | ||||
| White | 60 (40) | 54 (41) | 6 (33) | 0.625 |
| Native American | 9 (6) | 8 (6) | 1 (6) | |
| Black | 6 (4) | 6 (4) | 0 (0) | |
| Hispanic/Latin American | 62 (41) | 54 (41) | 8 (44) | |
| Otherb | 13 (9) | 10 (8) | 3 (17) | |
| Age (yr, 21-90) | ||||
| 21-40 | 15 (10) | 10 (8) | 5 (28) | 0.027 |
| 41-60 | 67 (45) | 61 (46) | 6 (33) | |
| ≥ 61 | 68 (45) | 61 (46) | 7 (39) | |
| Education | ||||
| Some high school | 22 (15) | 21 (16) | 1 (6) | 0.258 |
| High school graduate or GEDd | 38 (25) | 31 (61) | 7 (39) | |
| Some college and above | 90 (60) | 80 (61) | 10 (56) | |
| Health Insurance | ||||
| Medicare | 35 (23) | 33 (25) | 2 (11) | 0.437 |
| Medicaid | 12 (8) | 11 (8) | 1 (6) | |
| Medicare and Medicaid | 18 (12) | 17 (13) | 1 (6) | |
| Private and Other | 30 (20) | 25 (19) | 5 (28) | |
| Otherc | 55 (37) | 46 (35) | 9 (50) | |
| Annual household income | ||||
| < $15000 | 69 (46) | 64 (48) | 5 (28) | 0.176 |
| $15000-30000 | 42 (28) | 34 (26) | 8 (44) | |
| > $30000 | 39 (26) | 34 (26) | 5 (28) | |
| CKD stage (KDOQI guidelines) | ||||
| Stage 1 | 8 (5) | 4 (3) | 4 (22) | < 0.001 |
| Stage 2 | 17 (11) | 11 (8) | 6 (33) | |
| Stage 3 | 75 (50) | 69 (52) | 6 (33) | |
| Stage 4 | 50 (33) | 48 (36) | 2 (11) | |
| CKD cause | ||||
| Hypertension | 30 (20) | 28 (21) | 2 (11) | 0.088 |
| Diabetes | 19 (13) | 18 (14) | 1 (6) | |
| Diabetes and hypertension | 28 (19) | 25 (19) | 3 (17) | |
| Glomerulonephritis | 26 (17) | 18 (14) | 8 (44) | |
| Cystic disease | 4 (3) | 4 (3) | 0 (0) | |
| Urologic disease | 8 (5) | 8 (6) | 0 (0) | |
| Other | 30 (20) | 27 (21) | 3 (17) | |
| Unknown | 5 (3) | 4 (3) | 1 (6) | |
| Health literacy level | ||||
| Low | 95 (63) | 84 (64) | 11 (61) | 0.513 |
| Adequate | 55 (37) | 48 (36) | 7 (39) |
χ2 contingency coefficient comparing demographics and CKD awareness; bold numerals, P < 0.05 ;
Race: Other: Asian 6 (4%) and other 3 (2%);
Health insurance “Other”: Uninsured 4 (3%), private 6 (4%), Indian Health Service 1 (0.7%), and other 51 (34%). CKD: Chronic kidney disease; GED: General Education Development; KDOQI: Kidney Disease Outcomes Quality Initiative.
Participant demographics: Respondents were female (53%), white (40%) or Hispanic/Latin American (41%), over the age of 40 (90%), high school graduates or above (85%), with private or government insurance (63%), and earning up to $30000 annually (74%). The majority of respondents had Stage 3 or 4 CKD (83%) and limited HL (63%), and were aware that they had CKD (88%). CKD-A differed by age (P = 0.027) with 67% (10/15) of patients in the 21-40 age group compared to 90% (61/68) of patients over 60 years old being aware of having CKD. CKD-A was higher with higher CKD stage (P < 0.001). Fifty percent (n = 4) of those in CKD stage 1 were aware compared to 65% (n = 11) in stage 2, 92% (n = 69) in stage 3, and 96% (n = 48) in stage 4. CKD-A did not differ by HL level.
Association between CKD-A And CKD-SMB knowledge and performance
Table 2 shows the correct responses to each item in the CKD-SMB scale along with the comparison of each item by CKD-A. The majority of respondents (≥ 90 percent) correctly answered one or more items in the CKD-SMB scale. Only one item, “control my blood pressure,” differed between those who were aware and those who were unaware (P = 0.02) with fewer CKD unaware correctly identifying controlling blood pressure being important to help their kidneys. Knowledge (determined as percent of CKD-SMB items answered correctly) did not differ by CKD-A (t = 1.98, df = 146, P = 0.162).
Table 2.
Correct responses to current chronic kidney disease self-management behaviors and comparison with chronic kidney disease awareness (n = 150)
| CKD-self-management knowledge items | Correct answer; % (n) | Total aware, n = 132 Percent aware giving correct answer % (n) | Total unaware, n = 18 Percent unaware giving correct answer % (n) | Significance with CKD awarenessa |
| To help my kidneys, I need to: | ||||
| Control my blood pressure | True; 93.3 (140) | 96 (126) | 78 (14) | 0.020 |
| Take my blood pressure medicine(s) | True; 90 (135) | 91 (120) | 83.3 (15) | 0.390 |
| Have my urine (“pee”) tested | True; 93.3 (140) | 93 (123) | 94.4 (17) | 0.657 |
| Eat more salt | False; 95.3 (143) | 96 (127) | 89 (16) | 0.199 |
| Get my blood checked | True; 90.6 (136) | 92 (122) | 78 (14) | 0.068 |
| Keep a healthy body weight | True; 96.0 (144) | 97 (128) | 89 (16) | 0.153 |
| Not take some types of over-the-counter medicines (motrin, aleve, ibuprofen, naproxen) | True; 93.3 (140) | 92 (122) | 100 (18) | 0.61 |
| With diabetes | (Total n = 63) | (n = 57) | (n = 5) | |
| Keep track of my blood sugar | True; 95.2 (60) | 97 (55) | 100 (5) | 1 |
| Eat less sugar | True; 95.2 (60) | 97 (55) | 100 (5) | 1 |
| Take my diabetes medicine(s) | True; 96.8 (61) | 93 (52) | 100 (5) | 1 |
Fishers’ exact test used as more than 20% of cells had expected frequency < 5, P < 0.05.
Table 3 describes whether each CKD-SMB performed in the past 3 mo differed by CKD-A. A significantly greater percent of respondents who were aware reported that they “controlled their BP” (P = 0.02) and kept a healthy body weight (P = 0.013) in the past three months compared to those who were unaware. Specifically, those who were aware were 5.9 times more likely to perform the activity of controlling blood pressure and four times more likely of “keeping a healthy body weight” over the past 3 mo.
Table 3.
Performance of chronic kidney disease self-management behaviors in previous three months compared with chronic kidney disease awareness (n = 150)
| Items | Percent who performed the activity in the past 3 mo, % (n) | Total aware (n = 132) Percent aware that performed the activity in past 3 mo, % (n) | Total unaware (n = 18) Percent not aware that performed the activity in the past 3 mo, % (n) | Odds ratio (95%CI)b | P valuea |
| Control my blood pressure | 93 (139) | 95.4 (125) | 77.8 (14) | 5.9 (1.5-23.7) | 0.02 |
| Take my blood pressure medicine(s) | 88 (130) | 88.5 (115) | 83% (15) | 1.5 (0.4-5.9) | 0.46 |
| Have my urine (“pee”) tested | 92 (138) | 93.1 (122) | 89 (16) | 1.7 (0.3-8.5) | 0.62 |
| Eat more salt | 9.4 (14) | 9.2 (12) | 11.1 (2) | 0.8 (0.2-3.9) | 0.67 |
| Get my blood checked | 98 (145) | 98.5 (129) | 94.1 (16) | 4.0 (.3-47.0) | 0.31 |
| Keep a healthy body weight | 68 (101) | 72 (94) | 39 (7) | 4.0 (1.4-11.1) | 0.013 |
| Not take some types of over-the-counter medicines (motrin, Aleve, ibuprofen, naproxen) | 89 (133) | 90 (118) | 83.3 (15) | 1.8 (0.5-7.1) | 0.41 |
| Items included for those with diabetes | (n = 63) | (n = 58) | (n = 5) | ||
| Keep track of my blood sugar | 90 (55) | 89 (50) | 100 (5) | --- | 1 |
| Eat less sugar | 93.5 (58) | 93 (53) | 100 (5) | 1 | |
| Take my diabetes medicine(s) | 95 (57) | 94.5 (52) | 100 (5) | 1 |
P < 0.05;
Reference category: “Not performed activity” for odds ratio.
eGFR ranged from 17 to 152 mL/min per 1.73 m2. Approximately 17% of the eGFRs were 60 or above. Figure 1 shows the bivariate analysis of mean eGFR by awareness (t = -3.42, df = 18.82, P = 0.003) demonstrating increased CKD-A with more advanced CKD. We also examined CKD awareness by CKD stage (results not reported) and found similar results.
Figure 1.
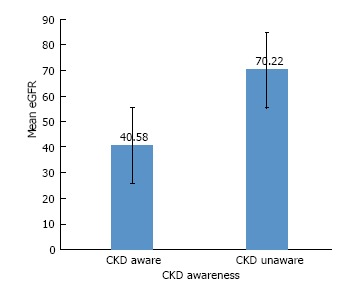
Comparison of mean eGFRs between those who are aware of their chronic kidney disease vs those who are unaware (P = 0.003)a. aFigure shows mean eGFRs and standard errors for the chronic kidney disease (CKD) aware and CKD unaware group.
Multivariate analyses
Multivariate analysis examining the relationship between CKD-A and HL after controlling for demographics and CKD-SMB knowledge was non-significant (P > 0.05). Additionally, multivariate comparison of CKD-A and CKD-SMB knowledge after controlling for demographics and HL was not significant (P > 0.05). As the literature does not offer guidance on the directionality of the relationship between CKD-A and kidney function, we performed multivariate analyses to examine the relationship between CKD-A and kidney function after controlling for demographics, and length of time attending clinic considering CKD-A as an independent as well as dependent variable. For both analyses, the overall model was significant. Hierarchical regression analyses with CKD awareness as independent variable and log eGFR as the dependent variable showed that CKD awareness predicted 29.4% of the variance in log eGFR. The relationship between CKD-A and log eGFR was negative suggesting a lower eGFR with awareness. Logistic regression results with awareness as a dependent variable, suggested the estimated odds ratio to be 0.926 (95%CI: 0.891-0.926, P < 0.05) suggesting a lower odds of awareness at higher eGFR.
CKD-unaware patients
We examined CKD unaware patients (n = 18) further to identify reasons for their unawareness despite participation in specialty clinic. As reported in Table 1, majority of CKD unaware patients were female (72%) and Hispanic/Latin American (44%). Proportional comparison to the demographic characteristics of the overall sample showed that a greater percent of the CKD-unaware patients were in the 21-40 age group (33%), were high school graduates or GED (18.4%), had private or other insurance (33%), incomes between $15000-$30000 (19%), had CKD stages 1 and 2 (40%), and had adequate health literacy (12.7%). The primary medically reported cause for CKD unaware patients in the initial stages of CKD (stages 1 and 2) was glomerulonephritis. However, 4 out of 8 patients in stages 3 to 4 with diabetes and hypertension also indicated that they were unaware of having CKD. There were no patients with cystic or urologic disease who indicated that they were unaware of having CKD. Fourteen out of 18 CKD unaware patients were clinic patients for more than 6 mo.
Duration of participation in specialty clinic and medically reported cause of CKD were examined to further understand potential reasons for unawareness among patients visiting the specialty clinic. Duration of clinic participation ranged from 2 to 118 mo (media n = 22.5 mo). However, duration of participation in the clinic was not significantly different between those who were aware and those who were unaware (chi sq = 0.253, P = 0.802). Similar results were obtained for duration of clinic participation across different health literacy groups (chi sq = 0.258, P = 0.735). We also examined CKD awareness by comparing it with medically reported cause of CKD. No significant relationships were present (chi sq = 12.39, df = 7, P = 0.088).
DISCUSSION
This study examined CKD-A in patients with CKD stages 1-4 in a nephrology specialty clinic and as expected, found that awareness of having CKD was high among patients in this outpatient nephrology specialty clinic. However, we found that 10/25 (40%) of patients in stages 1 and 2, and 8/125 (6.4%) in stages 3 and 4 were unaware of their CKD. We did not find any relationships between CKD-A and HL, and with most CKD-SMBs. No relationship between CKD-A and knowledge of CKD-SMBs and performance of the same in the previous three months was found either. However, similar to other studies, we found CKD-A was significantly higher with worse renal function[12,24,25], and this relationship remained significant even after controlling for demographics and length of time attending clinic. This study extends findings from prior studies that focused only on knowledge of CKD-specific behaviors to an assessment of patient-reported engagement in behaviors. While previous studies examined the relationship of CKD awareness with clinical targets and markers as outcome measures[3,6,18], our study examined patient-reported knowledge and performance of self-management behaviors as outcomes, which are more patient-centered outcomes. Nevertheless, our study corroborates prior research that showed non-significant relationships between CKD-A and outcomes.
While our study determined that there is a relationship between CKD awareness and kidney function (eGFR), the direction of this relationship is unclear. CKD-A can impact kidney function and it is possible that kidney function decline can promote awareness. Given the potential for a bi-directional relationship, we conducted analyses using CKD awareness as both a dependent as well as independent variable. While we can envision awareness to impact kidney function via self-management behaviors[8] our study did not find such a relationship. It is possible that the cross-sectional nature of our study or small sample size limited our findings. Future longitudinal studies are needed to elucidate the pathway by which CKD awareness impacts kidney function.
The vast majority (88%) of patients in our study were aware that they had CKD, although the 40% unawareness in stages 1 and 2 in a specialty nephrology clinic setting is cause for concern and suggests an urgent need to improve awareness and education of CKD in the earlier stages of disease. This was expected as our study was conducted in a specialty nephrology clinic and the majority of our sample had stage 3 or 4 CKD. Even patients in stages 1 and 2 were mostly aware (60% aware), possibly due to having a primary renal disease (e.g., glomerulonephritis) requiring nephrology management. This contrasts with low awareness rates reported in other studies, which were conducted in primary care settings[3-7].
Our study used the NHANES question to assess awareness. This question has been previously used to gauge CKD-A and has comparable sensitivity and specificity to other single item awareness questions, including a modified version to enhance sensitivity and specificity[9]. Irrespective of the question used for gauging CKD-A, no association was found with clinical markers or specific CKD-SMBs[3,18]. For example, one study examined the relationship of seven clinical markers of renal dysfunction to awareness using the NHANES CKD-A question and determined that a high percentage of individuals with 4 to 5 clinical markers were unaware of their CKD[6]. Another study examined three health behaviors, three clinical risk reduction targets, and their association with CKD-A using a different question[3]. Only one clinical marker, albuminuria, and one health behavior, tobacco avoidance, was related to CKD-A. However, their study did not examine all CKD-specific behaviors or HL. Tuot et al[18], examined the relationship between CKD-A and specific clinical measures reporting no relationship between the two, and called for assessment of the relationship between awareness and behaviors related to CKD-A.
We found no relationship between CKD-A and HL, which suggests that people who are aware may be no more health literate than those who are unaware. Poorer HL has been linked to several important CKD outcomes such as lower eGFR, poor knowledge, poor referral for transplantation, and increased mortality in ESRD[12]. While CKD-A may be the essential first step for patient engagement and adherence[9,26], our results highlight the need for targeted HL interventions in order to realize the positive impact on patient outcomes, rather than assuming that awareness of having CKD would intrinsically motivate patients to become health literate.
Our finding of a lack of relationship between CKD-A and most of the recommended CKD-SMB knowledge items is noteworthy. Evidence from this study are counter to the commonly perceived notion that awareness of CKD is essential for patients to have greater knowledge of CKD-SMBs or to engage in CKD SMBs[8,26]. In contrast to previous studies, the patients in our study were seen in a nephrology clinic and a majority were aware of having CKD, were knowledgeable about CKD-specific behaviors, were in the advanced stages of CKD (Stages 3 and 4) and reported performing many of the CKD SMBs in the previous three months. Yet, being aware of having CKD was not significantly associated with self-reported performance of majority of the CKD-SMBs, though the behaviors were performed at a relatively high (70%) rate regardless of awareness. One explanation for the lack of a significant association between most behaviors and CKD-A may be that patients perceived these as general or cardiovascular-related behaviors (i.e., hypertension) rather than CKD-specific behaviors[3]. This may explain the lack of relationships between CKD-A and performance of behaviors in the previous 3 mo. The finding of a relationship between CKD-A and performance of two specific behaviors, namely, “blood pressure control” and “maintaining healthy body weight” supports patients’ thinking of these as cardiovascular or general health behaviors.
Previous studies examining CKD-A and BP control (measured clinically) have reported conflicting results[18,27]. Our study asked patients about their maintenance of BP control as a useful behavior to protect their kidneys and found that a significantly greater percent of CKD aware patients performed the activity. This may be associated with a greater percent of CKD aware patients self-reporting having hypertension and consequently recognizing that behavior as a cardiovascular-related behavior. Although providers at the nephrology clinic may have equally emphasized all key CKD-specific health behaviors, the frequent comorbidity of hypertension along with public health education about BP control may result in higher patient knowledge of the importance of optimal BP control. Greater educational efforts by providers to highlight the importance of other CKD-specific behaviors are needed if CKD-A is to translate into actual behavior performance. Nevertheless, from a clinical practitioner perspective, it is encouraging to note the high numbers of patients in both aware and unaware categories that performed most behaviors.
Other explanations of lack of relationship between CKD-A and CKD-SMBs may relate to the relative lack of symptoms in CKD, especially in early stages[3], or that CKD SMBs may have been less familiar to patients who developed CKD caused by non-diabetes or non-hypertension causes (e.g., glomerulonephritis). In our study, 18 (72%) of the 25 patients in CKD stages 1 and 2 had non-diabetes or non-hypertension CKD causes. Consistent with prior studies[7,8], our study found that mean eGFR of those who were aware was significantly lower (worse CKD) than those who were unaware, suggesting that awareness increases with higher CKD stage or worse eGFR. The relationship with eGFR remained significant even after controlling for demographics and length of time attending clinic. Given that renal function trajectory is variable among individuals, affected by numerous factors[28], and sometimes slow[29], the relationship with kidney function offers promising opportunity for education about CKD-SMBs, especially if targeted in earlier CKD stages. Such tailored behavior education can improve adherence and slow the rate of eGFR loss[30]. Clinicians may keep this in mind when counseling patients, and use different educational strategies for those with higher eGFRs. Also, telehealth technology such as text messaging and interactive voice response (IVR) systems have been recently suggested as ways to improve patient awareness and knowledge of CKD particularly in the earlier stages when minimal symptoms are experienced[31].
We were surprised to find 8 out of 18 patients in stages 3 and 4 CKD, the majority of whom were seen in this specialty nephrology clinic for more than 6 mo, were unaware of having CKD. We do not have data about the number of visits that they made to the clinic over the duration of time that they participated in the clinic. Providers should not assume that the patients that they are seeing are aware of their disease and should proactively discuss and educate patents about their disease condition without assuming that their participation in the specialty clinic, or their performance of CKD-specific behaviors would imply awareness.
This study has several limitations, including that this was a relatively small cross-sectional study conducted at a single center. We were unable to include non-English speakers due to the nature of the study instruments. While we used validated measures such as NVS, we were unable to evaluate critical or interactive HL[14]. Also, construct validity of the CKD-SMB tool has not been established, although content validity of the instrument has been previously published[22]. Further, the item in CKD SMKT that referred to taking BP medication, isn’t relevant to those not taking BP medication. Nevertheless, as only 12% reported that they did not perform the activity, it implies that patients may have interpreted the item as “taking medication” rather than taking BP medication. Additionally, we were unable to confirm behaviors through chart review. Finally, we estimated eGFR using MDRD-4 equation, rather than the (more recent) CKD-EPI equation, which is more accurate at higher GFR[32]. However, in a study examining CKD-A and healthy behaviors, sensitivity analyses performed using the MDRD equation showed that their study results remained unchanged irrespective of the type of equation used to assess eGFR[3]. It is unlikely that the GFR estimating equation would have significantly influenced our results.
In conclusion, CKD-A was not associated with health literacy, did not translate into better CKD-SMB knowledge, or result in improved performance of CKD-SMBs even in worse stages of CKD. However, CKD-A did have a significant association with kidney function, especially with lower eGFR, which is consistent with existing literature. Future work should focus on targeted efforts to enhance education and training of CKD-SMBs differentiating it from behaviors for other related conditions such as hypertension, and beginning in the early stages of CKD. It is important to determine the effect of awareness on renal function and vice versa, as worse renal function may improve one’s awareness, and, conversely, improved awareness may impact the trajectory of renal disease. Longitudinal research is essential to evaluate the impact of CKD–SMB education on renal function trajectory.
ARTICLE HIGHLIGHTS
Research background
Chronic kidney disease (CKD) awareness is low in the primary care patient population with greater awareness in the advanced stages of CKD. Awareness of CKD is the first step for patient adherence to the numerous CKD-specific health behaviors necessary for optimal management of CKD. In order for patients to perform these behaviors appropriately, it is necessary that they be knowledgeable and health literate about these behaviors. Previous studies on CKD awareness have been based on larger databases and have focused on clinical markers and only few health behaviors. Additionally, previous work has not studied whether CKD awareness is associated with health literacy, or with actual performance of self-management behaviors, particularly in a specialty nephrology clinical practice setting.
Research motivation
While it may be assumed that being aware of having CKD will motivate patients to improve their health outcomes particularly in a specialty nephrology clinic setting, it has never been previously studied within this setting. Additionally, CKD awareness and kidney function relationships have not been previously examined, nor has actual performance of CKD specific behaviors been previously examined. This study examines the relationships between CKD awareness, health literacy, kidney function, CKD self-management behavior knowledge and its performance in a specialty practice setting. Examining these relationships will offer useful information to clinicians regarding how to design the best interventions for different stages of CKD, taking into account factors such as CKD awareness, health literacy, and self-management behavior performance.
Research objectives
The purpose of this study was to examine the relationship between CKD-A and: (1) Knowledge of current CKD self-management behaviors (CKD-SMB); (2) performance of CKD-SMB in previous three months as a measure of engagement in behavior c) HL, and d) kidney function (eGFR) in patients attending a specialty nephrology clinic. Learning more about these relationships will allow clinicians to use more targeted interventions with their patients.
Research methods
The authors used surveys to measure health literacy, self-management behavior knowledge, and performance, and CKD awareness. At the same time, the authors extracted information from medical records to determine CKD cause, serum creatinine levels, and length of time attending specialty clinic. Serum creatinine levels were converted to estimate glomerular filtration rates (EGFR) values using the MDRD-4 equation. The uniqueness of our study is that the authors used actual prospective data collection rather than retrospective data which most current studies in the literature have used.
Research results
This study examined CKD-A in patients with CKD stages 1-4 in a nephrology specialty clinic and as expected, found that awareness of having CKD was high among patients in this outpatient nephrology specialty clinic. However, the authors found that 10/25 (40%) of patients in stages 1 and 2, and 8/125 (6.4%) in stages 3 and 4 were unaware of their CKD. The authors did not find any relationships between CKD-A and HL, and with most CKD-SMBs. No relationship between CKD-A and knowledge of CKD-SMBs and performance of the same in the previous three months was found either. However, similar to other studies, the authors found CKD-A was significantly higher with worse renal function, and this relationship remained significant even after controlling for demographics and length of time attending clinic. Future efforts should include longitudinal studies that focus primarily on CKD-specific behaviors and beginning in the early stages of CKD. Also, since the authors saw that awareness was associated with kidney function, future work should determine the effect of awareness on renal function trajectory.
Research conclusions
Some new findings from our study include: (1) although awareness of CKD was high, it did not translate into better CKD self-management behavior knowledge or actual performance of behaviors even in worse stages of CKD; (2) the authors also found that awareness was significantly associated with kidney function but not with health literacy; (3) The authors found that large percent of patients reported being knowledgeable about CKD self-management behaviors, but there was no relationship between knowledge and performance of those behaviors. Based on this study, the authors theorize that among the several factors that affect kidney function, CKD awareness is a significant factor. However, the pathway by which awareness affects kidney function is unknown. It is possible that awareness arises only after kidney function decline, implying that raising awareness in the earlier stages of the disease may minimize the rate of kidney function decline. As health literacy and self-management behaviors are not associated with awareness, it suggests that just being aware of having CKD does not guarantee that individuals will perform self-management behaviors, or become health literate. The mechanisms to improve kidney function through awareness pathway need to be further assessed by longitudinal studies. CKD-A is not associated with HL, nor does it translate into better CKD-SMBs. CKD-A is significantly associated with kidney function, with awareness occurring with substantially lower eGFR. While the current literature indicates that CKD awareness is low, our study found a high level of CKD awareness. One reason for a high level of awareness may be that it was conducted in a specialty practice setting. Also, current literature does not address awareness and self-management behaviors or kidney function, both which our study addresses. The new hypothesis that this study proposes include: a) CKD awareness arises after kidney function decline. This study proposes examination of the relationship between CKD awareness and kidney function using longitudinal studies. Clinical practitioners should enhance and focus patient education of CKD in the earlier stages of CKD when awareness may be low. Also, practitioners should differentiate between CKD-specific behaviors and general health behaviors, so that patients clearly understand the CKD-specific behaviors needed to prevent kidney function decline.
Research perspectives
A larger sample size would help confirm the study results. Also, conducting this study in multiple practice settings will help improve generalizability. Future research should be longitudinal in nature and examine the relationship between CKD awareness and renal function trajectory. Future research should be longitudinal with a larger sample size and multiple specialty clinics.
Footnotes
Supported by SIUE STEP.
Institutional review board statement: This study was reviewed and approved by the Southern Illinois University Edwardsville Institutional Review Board and University of New Mexico Institutional Review Board.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: None of the authors have any conflicts of interest.
Data sharing statement: Dataset available from the corresponding author at rdevraj@siue.edu. Consent was not obtained for data sharing, but the presented data are anonymized and risk of identification is low.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: August 18, 2017
First decision: October 9, 2017
Article in press: December 1, 2017
P- Reviewer: Robles NR, Taheri S S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
Contributor Information
Radhika Devraj, School of Pharmacy, Southern Illinois University Edwardsville, Edwardsville, IL 62026, United States. rdevraj@siue.edu.
Matthew E Borrego, College of Pharmacy, University of New Mexico, Albuquerque, NM 87131, United States.
A Mary Vilay, College of Pharmacy, University of New Mexico, Albuquerque, NM 87131, United States.
Junvie Pailden, College of Arts and Sciences, Southern Illinois University Edwardsville, Edwardsville, IL 62026, United States.
Bruce Horowitz, Division of Nephrology and Hypertension, University of Utah, Salt Lake City, UT 84112, United States.
References
- 1.Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, Williams DE, Zhuo X. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65:403–411. doi: 10.1053/j.ajkd.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data system: CKD in the General population. [accessed 2016 Nov 18] Available from: https://www.usrds.org/2016/view/v1_01.aspx.
- 3.Tuot DS, Plantinga LC, Judd SE, Muntner P, Hsu CY, Warnock DG, Gutiérrez OM, Safford M, Powe NR, McClellan WM; REGARDS Investigators. Healthy behaviors, risk factor control and awareness of chronic kidney disease. Am J Nephrol. 2013;37:135–143. doi: 10.1159/000346712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhave JC, Troyanov S, Mongeau F, Fradette L, Bouchard J, Awadalla P, Madore F. Prevalence, awareness, and management of CKD and cardiovascular risk factors in publicly funded health care. Clin J Am Soc Nephrol. 2014;9:713–719. doi: 10.2215/CJN.06550613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirazian S, Diep R, Jacobson AM, Grant CD, Mattana J, Calixte R. Awareness of Chronic Kidney Disease and Depressive Symptoms: National Health and Nutrition Examination Surveys 2005-2010. Am J Nephrol. 2016;44:1–10. doi: 10.1159/000446929. [DOI] [PubMed] [Google Scholar]
- 6.Tuot DS, Plantinga LC, Hsu CY, Jordan R, Burrows NR, Hedgeman E, Yee J, Saran R, Powe NR; Centers for Disease Control Chronic Kidney Disease Surveillance Team. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER 3rd, Saran R, Messer KL, Levey AS, Powe NR. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168:2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17:225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuot DS, Zhu Y, Velasquez A, Espinoza J, Mendez CD, Banerjee T, Hsu CY, Powe NR. Variation in Patients’ Awareness of CKD according to How They Are Asked. Clin J Am Soc Nephrol. 2016;11:1566–1573. doi: 10.2215/CJN.00490116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devraj R, Gordon EJ. Health literacy and kidney disease: toward a new line of research. Am J Kidney Dis. 2009;53:884–889. doi: 10.1053/j.ajkd.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Baker DW. The meaning and the measure of health literacy. J Gen Intern Med. 2006;21:878–883. doi: 10.1111/j.1525-1497.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser SD, Roderick PJ, Casey M, Taal MW, Yuen HM, Nutbeam D. Prevalence and associations of limited health literacy in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2013;28:129–137. doi: 10.1093/ndt/gfs371. [DOI] [PubMed] [Google Scholar]
- 13.Jain D, Green JA. Health literacy in kidney disease: Review of the literature and implications for clinical practice. World J Nephrol. 2016;5:147–151. doi: 10.5527/wjn.v5.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devraj R, Borrego M, Vilay AM, Gordon EJ, Pailden J, Horowitz B. Relationship between Health Literacy and Kidney Function. Nephrology (Carlton) 2015;20:360–367. doi: 10.1111/nep.12425. [DOI] [PubMed] [Google Scholar]
- 15.Ricardo AC, Yang W, Lora CM, Gordon EJ, Diamantidis CJ, Ford V, Kusek JW, Lopez A, Lustigova E, Nessel L, et al. Limited health literacy is associated with low glomerular filtration in the Chronic Renal Insufficiency Cohort (CRIC) study. Clin Nephrol. 2014;81:30–37. doi: 10.5414/CN108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright JA, Wallston KA, Elasy TA, Ikizler TA, Cavanaugh KL. Development and results of a kidney disease knowledge survey given to patients with CKD. Am J Kidney Dis. 2011;57:387–395. doi: 10.1053/j.ajkd.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackey LM, Doody C, Werner EL, Fullen B. Self-Management Skills in Chronic Disease Management: What Role Does Health Literacy Have? Med Decis Making. 2016;36:741–759. doi: 10.1177/0272989X16638330. [DOI] [PubMed] [Google Scholar]
- 18.Tuot DS, Plantinga LC, Hsu CY, Powe NR. Is awareness of chronic kidney disease associated with evidence-based guideline-concordant outcomes? Am J Nephrol. 2012;35:191–197. doi: 10.1159/000335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Horton JC, Jones MR. Warning on inaccurate Rosenbaum cards for testing near vision. Surv Ophthalmol. 1997;42:169–174. doi: 10.1016/s0039-6257(97)00055-6. [DOI] [PubMed] [Google Scholar]
- 21.Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devraj R, Wallace LS. Application of the content expert process to develop a clinically useful low-literacy Chronic Kidney Disease Self-Management Knowledge Tool (CKD-SMKT) Res Social Adm Pharm. 2013;9:633–639. doi: 10.1016/j.sapharm.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 24.Flessner MF, Wyatt SB, Akylbekova EL, Coady S, Fulop T, Lee F, Taylor HA, Crook E. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2009;53:238–247. doi: 10.1053/j.ajkd.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders MR, Kim SD, Patel N, Meltzer DO, Chin MH. Hospitalized patients frequently unaware of their chronic kidney disease. J Hosp Med. 2015;10:619–622. doi: 10.1002/jhm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narva AS, Norton JM, Boulware LE. Educating Patients about CKD: The Path to Self-Management and Patient-Centered Care. Clin J Am Soc Nephrol. 2016;11:694–703. doi: 10.2215/CJN.07680715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright-Nunes JA, Luther JM, Ikizler TA, Cavanaugh KL: Patient knowledge of blood pressure target is associated with improved blood pressure control in chronic kidney disease. Patient Education and Counseling. 2012;88:184–188. doi: 10.1016/j.pec.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosansky SJ. Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol. 2012;36:1–10. doi: 10.1159/000339327. [DOI] [PubMed] [Google Scholar]
- 29.Bayliss EA, Bhardwaja B, Ross C, Beck A, Lanese DM. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol. 2011;6:704–710. doi: 10.2215/CJN.06610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Vargas PA, Tong A, Howell M, Craig JC. Educational Interventions for Patients With CKD: A Systematic Review. Am J Kidney Dis. 2016;68:353–370. doi: 10.1053/j.ajkd.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Tuot DS, Boulware LE. Telehealth Applications to Enhance CKD Knowledge and Awareness Among Patients and Providers. Adv Chronic Kidney Dis. 2017;24:39–45. doi: 10.1053/j.ackd.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurella Tamura M, Anand S, Li S, Chen SC, Whaley-Connell AT, Stevens LA, Norris KC. Comparison of CKD awareness in a screening population using the Modification of Diet in Renal Disease (MDRD) study and CKD Epidemiology Collaboration (CKD-EPI) equations. Am J Kidney Dis. 2011;57:S17–S23. doi: 10.1053/j.ajkd.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


