Abstract
Renal capsular invasion (RCI) and lymphovascular invasion (LVI) are potential prognostic factors of significance in renal cell carcinoma (RCC). We evaluated the independent prognostic implications of RCI and LVI in localized clear cell RCC based on a large multi-institutional cohort. 6, 849 patients who had undergone radical or partial nephrectomy for RCC were included. Associations between recurrence and RCI or LVI were analyzed by constructing statistical models that combined Cox proportional hazard regression and propensity score matching. To analyze RCI, 2, 733 patients including 603 patients with RCI were enrolled. To analyze LVI, 3, 586 patients including 121 patients with LVI were enrolled. Recurrence was observed in 75 (12.4%) patients with RCI and 134 (6.3%) patients without RCI. In all statistical models, RCI was significantly associated with an increased risk of recurrence. Recurrence was observed 29 (24.0%) patients with LVI and 207 (6.0%) patients without LVI. LVI was significantly associated with an increased risk of recurrence only in non-adjusted univariate models, but not in multivariate adjusted analysis or propensity score matching models. In conclusion, these findings suggest that RCI could be a significant risk factor for localized clear cell RCC recurrence. In contrast to RCI, LVI cannot be an independent prognostic variable.
Introduction
The prognosis of patients with renal cell carcinoma (RCC) is currently assessed by the TNM staging system after surgical treatment such as radical or partial nephrectomy. Currently, the TNM staging system in RCC has used tumor size as the single deciding factor for classifying T1-2 RCC. However, despite appropriate surgical treatment in localized RCC, some patients experience unexpected disease progression or recurrence1. Therefore, it is insufficient to predict prognosis based only on tumor size because all localized RCCs do not demonstrate the same biological behavior and postsurgical clinical course. Although tumor grade currently provides valuable prognostic information, additional reliable factors are needed to predict prognosis more accurately.
Renal capsular invasion (RCI) and lymphovascular invasion (LVI) are two potential prognostic factors of significance. There is conflicting information regarding the prognostic implications of RCI. Data presented by Klatte et al.2, May et al.3, and Cho et al.4 suggested that RCI is a poor prognostic factor, but Süer E et al.5 and Rouach et al.6 reported that RCI is not an independent prognostic factor for disease-specific survival.
Penetration and migration of tumors into the blood or lymphatic vessels could be an essential step in entering the circulation7. Although macroscopic invasion into the vessels has been recognized as a prognostic factor for quite a while8, the International Society of Urological Pathology recommended that microvascular invasion should not be used as a prognostic factor in routine evaluations based on the accumulated evidence9. However, LVI has been investigated as an independent predictor of poor prognosis in several solid tumors10–12 and predisposing patients to disease progression. Studies addressing the prognostic implications of LVI provided conflicting suggestions13–21 and did not reach solid conclusions.
Large cohort studies on LVI and RCI that exclude and control for potential bias are currently not available. Therefore, there is still some controversy regarding the prognostic significance of RCI and LVI in RCC. The main aim of the current study was to present the independent prognostic implications of RCI and LVI in localized clear cell RCC based on a large multi-institutional cohort study.
Methods
Study populations
The data for this study was derived from the KORCC (Korean Renal Cell Carcinoma) database, a nationwide multicenter database from 8 academic centers in Korea. In total, 6, 849 patients who had undergone surgical treatments for RCC between 1999 and 2011 were included in the KORCC database22. Clinical data were reviewed and collected in the form of a standardized electronic case report that included the following: (1) preoperative data, including age, gender, body mass index (BMI), previous medical history, Eastern Cooperative Oncology Group (ECOG) performance status, American Society of Anesthesiologists (ASA) status classification system, and symptoms at diagnosis; (2) preoperative laboratory findings; (3) surgical data, including the operative method, operative time, and estimated blood loss; (4) pathologic data, including TNM stage, histologic subtype, Fuhrman’s nuclear grade, mass size, sarcomatoid differentiation, renal capsule invasion and lymphovascular invasion; Pathology specimens were assessed by experienced pathologists at each institution without centralized review. RCI was defined as the presence of tumor cells within the fibrous renal capsule without perirenal fat tissue infiltration, and LVI was defined as the presence of tumor cells inside small blood vessels or lymphatic channels within the tumor (excluding the renal vein and its muscle-containing segmental branches); (5) postoperative follow-up data, including disease recurrence, death, and cause of death. Pathological staging was performed based on the 7th edition of the American Joint Committee on Cancer classification system23, and histological differentiation was classified following the AJCC and Heidelberg recommendations24. Information on deaths and their causes (‘cancer-related death’ or ‘non-cancer-related death’) was updated by reviewing medical records and the Korean National Statistical Office database. After approval by the Institutional Review Boards of the Catholic University of Korea, Seoul St. Mary’s Hospital, we reviewed data from 3, 576 patients with localized clear cell RCC treated with curative surgery who were eligible for analysis.
Statistical Analysis
Differences in clinicopathologic features including age, gender, body mass index (BMI), comorbidities (diabetes, hypertension and chronic renal disease), ECOG performance status, tumor size and Fuhrman grade were evaluated by descriptive statistics. Continuous and categorical variables were described using the independent t-test and χ2 test, respectively. The primary oncologic outcome measured in this study is recurrence. Recurrence-free survival rates were calculated using the cumulative incidence method and analyzed using the Kaplan-Meier method. Associations between recurrence and RCI or LVI were analyzed by constructing statistical models that combined Cox proportional hazard regression and propensity score matching.
To account for inherent differences among patients, such as baseline characteristics or uneven pathologic distributions between the two groups, the estimated propensity score was obtained from the fit of a logistic regression model to adjust for age, sex, BMI, tumor size, Fuhrman grade, sarcomatoid differentiation, ECOG performance status, and smoking status (c-statistic of 0.862 for RCI and 0.862 for LVI). 1:5 matching was performed using the greedy matching method, and the balance of the patients according to RCI or LVI was evaluated using the standardized difference and significance testing (independent t-test and χ² test or Fisher’s exact test) after propensity score matching. All statistical analyses were performed using the software package SAS version 9.3 (SAS Institute, Inc, Cary, NC, USA). A value of p < 0.05 was considered statistically significant.
Results
Comparison of clinical characteristics
To evaluate the prognostic significance of RCI, 2, 733 patients including 603 patients with RCI (incidence of RCI was 22.1%) were enrolled. To analyze LVI, 3, 586 patients including 121 patients with LVI (incidence of LVI was 3.4%) were enrolled. RCI and LVI were significantly associated with several other adverse clinicopathologic features, such as Fuhrman grade, and histology type (Tables 1 and 2).
Table 1.
Baseline description of renal cell carcinoma patients with or without capsular invasion according to pre- and post-propensity matching.
| Pre-propensity cohort | Post-propensity cohort | |||||
|---|---|---|---|---|---|---|
| RCI (−) | RCI (+) | P value | RCI (−) | RCI (+) | P value | |
| Age (years) | 55.5 ± 12.6 | 57.2 ± 12.4 | 0.0032 | 55 ± 13 | 56.6 ± 12.6 | 0.0795 |
| BMI | 24.6 ± 3.3 | 24.5 ± 3.1 | 0.3677 | 24.7 ± 3.3 | 24.9 ± 3 | 0.3214 |
| Sex | 0.9549 | 0.0584 | ||||
| Male | 1532 (71.92) | 433 (71.81) | 838 (73.19) | 169 (73.8) | ||
| Female | 598 (28.08) | 170 (28.19) | 307 (26.81) | 60 (26.2) | ||
| DM | 0.1511 | 0.0584 | ||||
| No | 1668 (83.86) | 438 (81.26) | 961 (84.15) | 181 (79.04) | ||
| Yes | 321 (16.14) | 101 (18.74) | 181 (15.85) | 48 (20.96) | ||
| HTN | 0.1616 | 0.476 | ||||
| No | 1218 (62.02) | 314 (58.69) | 716 (63.2) | 139 (60.7) | ||
| Yes | 746 (37.98) | 221 (41.31) | 417 (36.8) | 90 (39.3) | ||
| CKD | 0.3148 | 0.1759 | ||||
| No | 1937 (98.27) | 533 (98.89) | 1117 (98.41) | 228 (99.56) | ||
| Yes | 34 (1.73) | 6 (1.11) | 18 (1.59) | 1 (0.44) | ||
| Smoking status | 0.0653 | 0.0791 | ||||
| Non-smoker | 1016 (60.23) | 243 (59.41) | 629 (56.92) | 135 (59.47) | ||
| Ex-smoker | 301 (17.84) | 91 (22.25) | 205 (18.55) | 51 (22.47) | ||
| Current smoker | 370 (21.93) | 75 (18.34) | 271 (24.52) | 41 (18.06) | ||
| ECOG_index | 0.0987 | 0.794 | ||||
| 0 | 1815 (86.59) | 510 (85.57) | 983 (87.53) | 200 (88.5) | ||
| 1 | 259 (12.36) | 73 (12.25) | 124 (11.04) | 24 (10.62) | ||
| 2 | 22 (1.05) | 13 (2.18) | 16 (1.42) | 2 (0.88) | ||
| Fuhrman grade | <0.0001 | 0.8018 | ||||
| 1 & 2 | 1347 (63.9) | 316 (52.93) | 703 (61.4) | 139 (60.7) | ||
| 3 | 706 (33.49) | 233 (39.03) | 424 (37.03) | 85 (37.12) | ||
| 4 | 55 (2.61) | 48 (8.04) | 18 (1.57) | 5 (2.18) | ||
| Sarcomatoid differentiation | <0.0001 | 0.7765 | ||||
| No | 1387 (98.86) | 332 (94.05) | 1137 (99.3) | 227 (99.13) | ||
| Yes | 16 (1.14) | 21 (5.95) | 8 (0.7) | 2 (0.87) | ||
| Tumor size | 31.4 (30.5–32.3) | 43.3 (41.1–45.7) | <0.0001 | 30.9 (29.9–32) | 32 (29.9–34.3) | 0.4049 |
Table 2.
Baseline description of renal cell carcinoma patients with or without lymphovascular.
| Pre-propensity cohort | Post-propensity cohort | |||||
|---|---|---|---|---|---|---|
| LVI (−) | LVI (+) | P value | LVI (−) | LVI (+) | P value | |
| Age (years) | 56 ± 12.6 | 58 ± 12.1 | 0.0885 | 56.5 ± 12.8 | 59.2 ± 11.6 | 0.1064 |
| BMI | 24.6 ± 3.3 | 24.3 ± 3.2 | 0.3293 | 24.6 ± 3.5 | 24.5 ± 3.3 | 0.7841 |
| Sex | 0.7192 | 0.595 | ||||
| Male | 2486 (71.75) | 85 (70.25) | 246 (71.3) | 47 (68.12) | ||
| Female | 979 (28.25) | 36 (29.75) | 99 (28.7) | 22 (31.88) | ||
| DM | 0.8164 | 0.6425 | ||||
| No | 2667 (83.95) | 100 (84.75) | 281 (81.45) | 57 (83.82) | ||
| Yes | 510 (16.05) | 18 (15.25) | 64 (18.55) | 11 (16.18) | ||
| HTN | 0.0903 | 0.1687 | ||||
| No | 1889 (60.03) | 80 (67.8) | 206 (60.23) | 47 (69.12) | ||
| Yes | 1258 (39.97) | 38 (32.2) | 136 (39.77) | 21 (30.88) | ||
| CKD | 0.0871 | 0.2068 | ||||
| No | 2476 (97.56) | 117 (100) | 335 (97.67) | 67 (100) | ||
| Yes | 62 (2.44) | 0 (0) | 8 (2.33) | 0 (0) | ||
| Smoking status | 0.0359 | 0.6264 | ||||
| Non-smoker | 1742 (65.64) | 45 (52.94) | 190 (56.38) | 34 (50) | ||
| Ex-smoker | 377 (14.2) | 19 (22.35) | 70 (20.77) | 16 (23.53) | ||
| Current smoker | 535 (20.16) | 21 (24.71) | 77 (22.85) | 18 (26.47) | ||
| ECOG_index | 0.0027 | 0.8813 | ||||
| 0 | 2299 (72.5) | 89 (87.25) | 286 (84.37) | 59 (86.76) | ||
| 1 | 576 (18.16) | 11 (10.78) | 47 (13.86) | 8 (11.76) | ||
| >=2 | 296 (9.33) | 2 (1.96) | 6 (1.77) | 1 (1.47) | ||
| Fuhrman grade | <0.0001 | 0.4718 | ||||
| 1 & 2 | 2038 (62.21) | 37 (34.58) | 120 (34.78) | 21 (30.43) | ||
| 3 | 1123 (34.28) | 58 (54.21) | 187 (54.2) | 37 (53.62) | ||
| 4 | 115 (3.51) | 12 (11.21) | 38 (11.01) | 11 (15.94) | ||
| Sarcomatoid differentiation | 0.0081 | 0.5178 | ||||
| No | 2358 (98.54) | 71 (94.67) | 331 (95.94) | 65 (94.2) | ||
| Yes | 35 (1.46) | 4 (5.33) | 14 (4.06) | 4 (5.8) | ||
| Tumor size | 33.1 (32.2–34) | 59 (53.7–64.9) | <0.0001 | 58.8 (56.1–61.7) | 58.7 (52.8–65.3) | 0.9812 |
Invasion according to pre- and post-propensity matching.
In the RCI group, 120 patients were matched with 600 patients without RCI. In the LVI group, 61 patients were matched with 305 patients without LVI. The median follow-up durations in patients with RCI and without RCI were 39 (interquartile range [IQR]: 16–72) and 31 (IQR: 12–60) months, respectively (Tables 1 and 2). The median follow-up durations in patients with LVI and without LVI were 37 (IQR: 13–68) and 34 (IQR: 11–62) months, respectively.
Impact of RCI on oncological outcomes
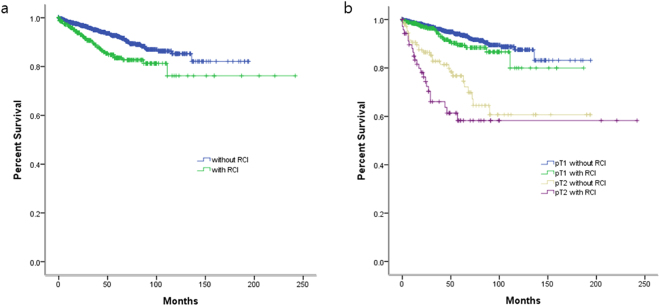
Among all patients evaluated for RCI, recurrence was observed in 209 (7.6%) total patients including 75 (12.4%) patients with RCI and 134 (6.3%) patients without RCI. The 5-year recurrence-free survival rates were 83.5% and 92.4% in patients with and without RCI, respectively (log rank test, p < 0.001, Fig. 1a. When patients were stratified into 4 groups based on RCI and tumor stage (pT1 and pT2), the 5-year recurrence-free survival rates were 89.4% in pT1 patients with RCI versus 93.7% in those without RCI (log rank test, p < 0.001) and 58.3% in pT2 patients with RCI versus 76.7% those without RCI (log rank test, p = 0.088) (Fig. 1b).
Figure 1.
(a) Kaplan-Meier survival curve of localized RCC according to renal capsular invasion (RCI). Recurrence-free survival rate of patients with RCI and without RCI were 83.5% and 92.4% (p < 0.001, log rank test). (b) Patients with RCI in pT1 and pT2 were correlated with cancer recurrence, respectively (p < 0.001, p = 0.088, log rank test).
In separate Cox hazards analysis for recurrence, a non-adjusted univariate (H.R: 2.154, C.I: 1.588–2.923; model 1), and a multivariate adjusted analysis (H.R: 1.668, C.I: 1.060–2.626; model 2), RCI was significantly associated with an increased risk of recurrence (Table 3). In the propensity score matching analysis, RCI was significantly associated with an increased risk of recurrence (H.R: 2.130, C.I: 1.201–3.777) in model 3, and this association remained significant (H.R: 2.057, C.I: 1.146–3.693) in model 4, which combined propensity score matching with adjusting for various potential prognostic factors (Table 3).
Table 3.
Adjusted hazard ratios of disease recurrence according to analysis model.
| H.R | 95% C.I | P-value | ||
|---|---|---|---|---|
| Renal capsule invasion | Model1a | 2.154 | 1.588–2.923 | <0.0001 |
| Model2b | 1.668 | 1.060–2.626 | 0.027 | |
| Model3c | 2.130 | 1.201–3.777 | 0.0097 | |
| Model4d | 2.057 | 1.146–3.693 | 0.0156 | |
| Lymphovascular invasion | Model1a | 3.642 | 2.438–5.440 | <0.0001 |
| Model2b | 1.687 | 0.938–3.034 | 0.0809 | |
| Model3c | 1.242 | 0.693–2.227 | 0.4676 | |
| Model4d | 1.285 | 0.709–2.328 | 0.4082 |
aNot adjusted.
bAdjusted for tumor size, Fuhrman grade, sarcomatoid differentiation, age, sex, DM, HTN, ECOG index, BMI, smoking status, and CKD.
cPS matching for tumor size, Fuhrman grade and sarcomatoid differentiation.
dPS matching and adjusted for age, sex, DM, HTN, ECOG index, BMI, smoking status, and CKD.
Impact of LVI on oncological outcomes
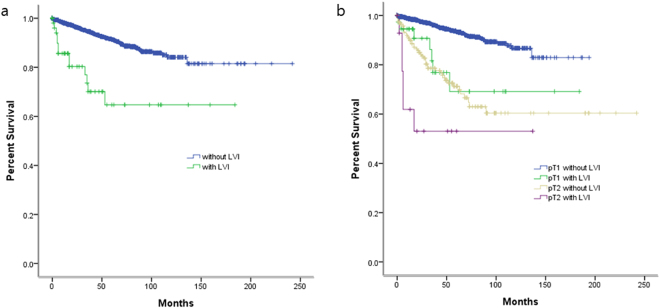
When evaluating patients for LVI, recurrence was observed in 236 (15.2%) patients including 29 (24.0%) patients with LVI and 207 (6.0%) patients without LVI. The 5-year recurrence-free survival rates were 64.7% and 91.3% in patients with and without LVI, respectively (log rank test, p < 0.001, Fig. 2a). When the patients were stratified into 4 groups based on the LVI and stage (pT1 and pT2), the 5-year recurrence-free survival rates were 69.2% in pT1 patients with LVI versus 93.4% in those without LVI (log rank test, p < 0.001) and 53.1% in pT2 patients with LVI versus 71.3% those without LVI (log rank test, p = 0.026) (Fig. 2b).
Figure 2.
(a) Kaplan-Meier survival curve of localized RCC according to lymphovascular invasion (LVI). Recurrence-free survival rate of patients with LVI and without LVI were 64.7% and 91.3% (p < 0.001, log rank test). Patients without LVI were correlated with a higher recurrence-free survival rate (p < 0.001, log rank test). (b) Patients without RCI in pT1 and pT2 were correlated with cancer recurrence, respectively (p < 0.001, p = 0.026, log rank test).
In separate Cox hazards analysis for recurrence, LVI was significantly associated with an increased risk of recurrence only in non-adjusted univariate analysis (H.R: 3.642, C.I: 2.438–5.440; model 1), and not in multivariate adjusted analysis (H.R: 1.687, C.I: 0.938–3.034; model 2) (Table 3). In the PS matching analysis, LVI was not significantly associated with an increased risk of recurrence (H.R: 1.242, C.I: 0.69–2.227) in model 3, and this association remained nonsignificant (H.R: 1.285, C.I: 0.709–2.328) in model 4, which combined PS matching with adjusting for various potential prognostic factors (Table 3).
Predictive factors for LVI
Table 4 shows the logistic regression model that predicted LVI in patients who underwent curative surgery for RCC. The odds ratio of LVI increased significantly with poor pathologic parameters. RCCs with large size, higher Fuhrman grade, sarcomatoid differentiation, and smoking were more likely to have LVI. High ECOG performance status score were inverse relation with LVI.
Table 4.
Predictive factors for LVI in patients who underwent curative surgery for RCC.
| H.R. (95% CI) | p | |
|---|---|---|
| Age | 1.013 (0.998, 1.028) | 0.0939 |
| BMI | 0.973 (0.918, 1.032) | 0.3656 |
| Sex | 0.8344 | |
| Male | 0.958 (0.643, 1.428) | |
| Female | 1 (ref.) | |
| DM | 0.5873 | |
| No | 1 (ref.) | |
| Yes | 0.866 (0.514, 1.457) | |
| HTN | 0.0198 | |
| No | 1 (ref.) | |
| Yes | 0.611 (0.404, 0.925) | |
| CKD | 0.2154 | |
| No | 1 (ref.) | |
| Yes | 0.17 (0.01, 2.802) | |
| Smoking status | 0.022 | |
| Non-smoker | 1 (ref.) | |
| Ex-smoker | 1.884 (1.055, 3.367) | |
| Current smoker | 2.131 (1.169, 3.886) | |
| ECOG_status | 0.0044 | |
| 0 | 1 (ref.) | |
| 1 | 0.49 (0.26, 0.922) | |
| >= 2 | 0.159 (0.039, 0.651) | |
| Fuhrman grade | <0.0001 | |
| 1 & 2 | 1 (ref.) | |
| 3 | 2.859 (1.875, 4.359) | |
| 4 | 5.646 (2.859, 11.146) | |
| Sarcomatoid differentiation | 0.0149 | |
| No | 1 (ref.) | |
| Yes | 3.746 (1.294, 10.845) | |
| Tumor size (Per 1 cm) | 5.332 (3.651, 7.787) | <0.0001 |
Discussion
The main findings of this study for patients with localized clear cell RCC are as follows. (1) Patients with RCI were more likely to develop recurrence of RCC independent of confounding variables. RCI could be an important risk factor for recurrence in localized RCC. (2) The association of LVI with the risk of recurrence was significant only in univariate analysis. (3) After adjusting for confounding factors, LVI did not influence the recurrence of RCC following curative surgery.
The renal capsule is a tough fibrous layer surrounding the kidney and is covered in a thick layer of perinephric adipose tissue. These fibrous layers could provide protection from tumor seeding or spreading to adjacent tissue. Capsular invasion by the tumor would be early step to spread, which reflects tumor aggressiveness. Several studies have investigated the prognostic significance of RCI, but there are substantial inconsistencies regarding the association between RCI and oncological outcomes2–6,25. These studies had a relatively small sample size (the sample size in largest study was 653 patients), and the number of oncological events such as recurrence or death was small, in accordance with small cohort size. Therefore, the statistical value in previous studies is limited due to the small event number in a small cohort.
Moreover, many of these previous studies included various histological types of RCC such as clear cell, papillary, and chromophobe. The enrollment of patients with heterogeneous RCC subtypes could have resulted in bias and discordances26. The positive rate of RCI in previous studies that included heterogeneous histological types showed a wide range between 21.6% and 37.5%2–6,25 compared to the current study, which could also have affected the outcomes of the analysis. Therefore, the role of RCI as a prognostic factor remains controversial due to the small number of heterogeneous RCCs in the cohort.
To overcome the limitations of previous studies, the current study was conducted based on a large number of homogenous RCCs in the cohort, and demonstrated the significant impact of RCI on recurrence.
Campbell et al.27, found that RCI was detected more frequently in RCC patients with positive Cav-1 expression compared to those with negative Cav-1 expression. This study suggested that Caveolin-1 (Cav-1) promoted cell invasion in RCC cell lines and was a powerful predictor of metastasis in patients with clinically confirmed disease, which supports the relevance of RCI for prognosis in the current study by presenting a molecular biological mechanism.
LVI is thought to be associated with a predisposition toward recurrence or metastasis, because metastasis starts with malignant cells accessing the circulation through the blood or lymphatic vessels7,28. LVI is an important prognostic factor in other urinary tract malignancies such as bladder29 and upper urinary tract10 cancers. However, as in RCI, there are conflicting results regarding the prognostic significance of LVI for RCC. Some studies suggested that LVI influences the oncological outcome of RCC13–15,19,21,30, whereas others did not16–18,20.
Most previous studies regarding the prognostic significance of LVI have a few weak points. These analyses were based on relatively small cohorts and did not independently examine those with localized (≤pT2) and locally advanced (≥pT3a) disease, nor did it discriminate among histologic subtypes, even though the prognosis of RCC patients varies according to stage and histologic subtype. The positive rate of LVI in previous studies was relatively high (11.0% in Katz et al.18, 14.3% in Belsante et al.13, 18.6% in Kroeger et al.30) compared to the current study, which may be due to the heterogeneity in the previous studies, especially since they included patients with advanced stage RCC and positive nodes which increased the LVI detection rate.
A relatively large study (n = 833) conducted by Sorbellini21 suggested that LVI was associated with recurrence in clear cell RCC patients. Although Sorbellini’s study analyzed a relatively large sample cohort of clear cell RCC, disease recurrence was noted only in 72 patients, including those with advanced RCC. A recently reported study in patients with organ-confined RCC13 suggested that LVI is an independent predictor of recurrence and disease-specific survival. This study showed only 15 cases of recurrence in the pT1-2 patient group (n = 333). This small number led to a broader confidence interval (from 1.7 to 92.7), and consequently statistical reliability was not high.
In the current study with a large event size (230 cases of recurrence among patients with or without LVI), unadjusted analysis showed that LVI was a significant prognostic factor for recurrence. However, after adjusting for confounding variables by multivariate Cox regression and propensity score matching, LVI was not a significant prognostic factor for recurrence in localized clear cell RCC. Our hazard ratios show a narrow CI width (Table 3) in comparison with the previous study13.
LVI was associated with worse pathologic features, so the positive association between LVI and recurrence in univariate analysis might be due to overall pathologic features. Patients with worse pathology (larger tumor size, higher grade, and sarcomatoid differentiation) were more likely to show positive LVI.
The current study has several strengths and weaknesses. This study is a non-randomized, retrospective study, which inherently could not be representative of general RCC patients. Our study was analyzed without central pathology review despite the retrospective multi-institutional nature of the study. Nonetheless, it has included a large number of localized clear cell RCC patients retrieved from an observational longitudinal multi-institutional database of Korean RCC patients. Moreover, our study was conducted through different statistical models. First, Cox proportional hazard regression analysis was performed to evaluate the relationship between recurrence and CI or LVI. In multivariate Cox proportional hazard regression analysis, the hazard ratio was calculated with or without adjusting confounding variables. Second, propensity score matched analysis was performed. In the propensity score matched analysis, the uneven distribution of known prognostic factors including tumor stage, Fuhrman’s grade, and sarcomatoid differentiation were balanced between the two groups (with or without CI or LVI). On the other hand, one disadvantage of propensity score matching is that it only accounts for observed covariates31. To compensate for this disadvantage and to assess the relationships between other confounding variables, the adjusted hazard ratio was calculated by combining propensity score matched analysis. These complementary statistical methods provide solid evidence for the prognostic significance of CI and LVI.
Conclusion
After adjusting for confounding variables with statistical models using Cox regression and propensity score matching analysis, this study showed that RCI in localized clear cell RCC can provide clinically important prognostic information based on the association between RCI and RCC recurrence. On the other hand, LVI did not influence the recurrence of localized clear cell RCC following curative surgery.
Acknowledgements
This study was supported by a grant from the Korean Urological Oncology Society 2015 research fund, Korea. We also acknowledge the Korean Renal Cell Carcinoma Study Group COHORT investigators and coordinators who collected data used in this study.
Author Contributions
U.S.H. was involved in data collection and management, analyzed the data, and wrote and edited the manuscript. K.W.L. and J.J. were analyzed the data and edited the manuscript. S.S.B., C.K., J.C., E.C.H., Y.J.K., T.G.K. and S.H.K. were involved in data collection and edited the manuscript. S.H.H was involved in project development, data collection and management, and wrote and edited the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-25182-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/11/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- 1.Wallen EM, Pruthi RS, Joyce GF, Wise M. Kidney cancer. J. Urol. 2007;177:2006–2019. doi: 10.1016/j.juro.2007.01.126. [DOI] [PubMed] [Google Scholar]
- 2.Klatte T, et al. Prognostic relevance of capsular involvement and collecting system invasion in stage I and II renal cell carcinoma. BJU Int. 2007;99:821–824. doi: 10.1111/j.1464-410X.2006.06729.x. [DOI] [PubMed] [Google Scholar]
- 3.May M, et al. Evaluation of renicapsular involvement in Stages I and II renal cell carcinoma from the morphological and prognostic point of view. Urol. Oncol. 2010;28:274–279. doi: 10.1016/j.urolonc.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Cho HJ, et al. Prognostic value of capsular invasion for localized clear-cell renal cell carcinoma. Eur. Urol. 2009;56:1006–1012. doi: 10.1016/j.eururo.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Süer E, Ergün G, Baltaci S, Bedük Y. Does renal capsular invasion have any prognostic value in localized renal cell carcinoma? J. Urol. 2008;180:68–71. doi: 10.1016/j.juro.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Rouach Y, et al. Capsular involvement in patients undergoing partial nephrectomy for localized renal cell carcinoma: an adverse pathological finding? BJU Int. 2010;105:616–619. doi: 10.1111/j.1464-410X.2009.08797.x. [DOI] [PubMed] [Google Scholar]
- 7.Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clin. Exp. Med. 2006;6:145–149. doi: 10.1007/s10238-006-0117-4. [DOI] [PubMed] [Google Scholar]
- 8.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Delahunt B, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 2013;37:1490–1504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi E, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J. Clin. Oncol. 2009;27:612–618. doi: 10.1200/JCO.2008.17.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim SB, et al. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis. Colon Rectum. 2010;53:377–384. doi: 10.1007/DCR.0b013e3181cf8ae5. [DOI] [PubMed] [Google Scholar]
- 12.Rakha EA, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118:3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 13.Belsante M, et al. Lymphovascular invasion in clear cell renal cell carcinoma–association with disease-free and cancer-specific survival. Urol. Oncol. 2014;32:30.e23–38. doi: 10.1016/j.urolonc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Dall’Oglio MF, et al. Microvascular tumour invasion in renal cell carcinoma: the most important prognostic factor. BJU Int. 2007;100:552–555. doi: 10.1111/j.1464-410X.2007.07015.x. [DOI] [PubMed] [Google Scholar]
- 15.Dall’Oglio MF, et al. Microvascular tumor invasion, tumor size and Fuhrman grade: a pathological triad for prognostic evaluation of renal cell carcinoma. J. Urol. 2007;178:425–428. doi: 10.1016/j.juro.2007.03.128. [DOI] [PubMed] [Google Scholar]
- 16.Horiguchi A, et al. Intratumoral lymphatics and lymphatic invasion are associated with tumor aggressiveness and poor prognosis in renal cell carcinoma. Urology. 2008;71:928–932. doi: 10.1016/j.urology.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 17.Ishimura T, et al. Clinicopathological features of recurrence after radical surgery for nonmetastatic renal cell carcinoma. Int. J. Clin. Oncol. 2004;9:369–372. doi: 10.1007/s10147-004-0409-1. [DOI] [PubMed] [Google Scholar]
- 18.Katz MD, et al. The role of lymphovascular space invasion in renal cell carcinoma as a prognostic marker of survival after curative resection. Urol. Oncol. 2011;29:738–744. doi: 10.1016/j.urolonc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Madbouly K, Al-Qahtani SM, Ghazwani Y, Al-Shaibani S, Mansi MK. Microvascular tumor invasion: prognostic significance in low-stage renal cell carcinoma. Urology. 2007;69:670–674. doi: 10.1016/j.urology.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Sevinç, M., Kirkali, Z., Yörükoğlu, K., Mungan, U. & Sade, M. Prognostic significance of microvascular invasion in localized renal cell carcinoma. Eur. Urol. 38, 728–733, doi:20370 (2000). [DOI] [PubMed]
- 21.Sorbellini M, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J. Urol. 2005;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 22.Byun SS, et al. The establishment of KORCC (KOrean Renal Cell Carcinoma) database. Investig Clin Urol. 2016;57:50–57. doi: 10.4111/icu.2016.57.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Compton CC. The American Joint Committee on Cancer: the7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs G, et al. The Heidelberg classification of renal cell tumours. J. Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Jeong IG, et al. Prognostic implication of capsular invasion without perinephric fat infiltration in localized renal cell carcinoma. Urology. 2006;67:709–712. doi: 10.1016/j.urology.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Campbell L, et al. Caveolin-1 in renal cell carcinoma promotes tumour cell invasion, and in co-operation with pERK predicts metastases in patients with clinically confined disease. J. Transl. Med. 2013;11:255. doi: 10.1186/1479-5876-11-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson N, Storr S, Zhang S, Martin S. Lymphovascular invasion: assessment and prognostic impact in melanoma and breast cancer. Histol. Histopathol. 2015;30:1001–1009. doi: 10.14670/HH-11-615. [DOI] [PubMed] [Google Scholar]
- 29.Shariat SF, et al. International validation of the prognostic value of lymphovascular invasion in patients treated with radical cystectomy. BJU Int. 2010;105:1402–1412. doi: 10.1111/j.1464-410X.2010.09217.x. [DOI] [PubMed] [Google Scholar]
- 30.Kroeger N, et al. Prognostic value of microvascular invasion in predicting the cancer specific survival and risk of metastatic disease in renal cell carcinoma: a multicenter investigation. J. Urol. 2012;187:418–423. doi: 10.1016/j.juro.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Garrido MM, et al. Methods for constructing and assessing propensity scores. Health Serv. Res. 2014;49:1701–1720. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]