Abstract
A rapid and simple high performance liquid chromatography (HPLC) method with a UV detection (241 nm) was developed and validated for estimation of eplerenone from spiked human plasma. The analyte and the internal standard (valdecoxib) were extracted with a mixture of dichloromethane and diethyl ether. The chromatographic separation was performed on a HiQSil C-18HS column (250 mm×4.6 mm, 5 μm) with a mobile phase consisting of acetonitrile:water (50:50, v/v) at flow rate of 1 mL/min. The calibration curve was linear in the range 100–3200 ng/mL and the heteroscedasticity was minimized by using weighted least squares regression with weighting factor 1/X.
Keywords: Eplerenone, Liquid–liquid extraction, Weighted regression, HPLC–UV
1. Introduction
Eplerenone is chemically Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, γ-lactone, methyl ester, (7α,11α,17α), an antihypertensive drug that is highly selective aldosterone receptor antagonist (SARA) [1], [2].
A literature search revealed a few quantitative analytical methods for estimation of eplerenone [3], [4]; further, very few methods described the quantification of eplerenone in biological fluids [5], [6]; these methods include LC-MS, which requires expensive instrumentation not available in conventional bioanalytical laboratory. Thus, it was decided to develop a rapid, economical and simple method which was based on liquid–liquid extraction (LLE) for sample preparation and HPLC with UV detection for quantification of eplerenone from spiked human plasma.
2. Experimental
2.1. Equipment and materials
The HPLC system used consisted of a pump PU-2080 plus (Jasco Corporation, Japan) with a Rheodyne loop injector of capacity 100 μL. Detection was carried out by a UV-2075 detector. The data acquisition was done on Borwin chromatography software version 1.50.
Pharmaceutical grade eplerenone and valdecoxib used as an internal standard were kindly supplied as a gift sample from Hetero Drug Laboratories, Hyderabad, India. Blank human plasma was procured as a gift sample from the Arpan Blood Bank, Nashik, India. Plasmas from six sources were mixed thoroughly to get pooled blank plasma. Acetonitrile and water used in analysis were of HPLC grade. Other chemicals used in the study were of AR grade. All chemicals were purchased from SD Fine Chemicals, Mumbai, India. The 0.45 μm Nylon filter papers were purchased from Pall India Pvt. Ltd., Mumbai, India.
2.2. Methods
2.2.1. Preparation of calibration curve (CC) standards and quality control (QC) samples
The stock solution (100 μg/mL) of eplerenone was prepared in methanol and this was then appropriately diluted with methanol to get working standard solutions with concentrations of 2, 4, 8, 16, 32 and 64 μg/mL. Aliquots of 0.95 mL of blank human plasma were spiked with 50 μL of the working standard solutions to get CC standards containing 100, 200, 400, 800, 1600 and 3200 ng/mL of eplerenone. The QC samples were similarly prepared to contain three concentrations [250 ng/mL low quality control (LQC), 1000 ng/mL middle quality control (MQC) and 3000 ng/mL high quality control (HQC)].
2.2.2. Sample preparation
An aliquot of spiked human plasma sample (1 mL) was taken in six different 15 mL stoppered test tubes followed by addition of 50 μL of internal standard working solution (10 μg/mL) and was vortex-mixed for 1 min. To this solution, 5 mL of dichloromethane:diethyl ether (4:6, v/v) was added and the content of the tubes was mixed in an inclined position on a reciprocating shaker at 100 stroke/min for 30 min and centrifuged at 3000 rpm for 10 min to effect phase separation. The supernant organic layer (4 mL) was transferred to another tube and evaporated to dryness under stream of nitrogen. Further, the residue was reconstituted in 250 μL of mobile phase and subjected to chromatographic analysis.
2.2.3. Chromatographic conditions
Chromatographic analysis was performed on a HiQsil C-18HS column (250 mm×4.6 mm, 5 μm), which was preceded by a Hypersil BDS C18 guard column (20 mm×4 mm). The mobile phase consisted of a mixture of acetonitrile:water (50:50, v/v), the flow rate was 1 mL/min and the detection was carried out at 241 nm.
2.2.4. Calibration studies
All calibration standards were analyzed in six replicates and the data of concentrations and corresponding area ratios of eplerenone and internal standard were subjected to unweighted and weighted least squares linear regression. The equations generated from these regression analyses were used to calculate the interpolated concentrations of the CC standards and the % relative error (% RE) was calculated for each CC standard. The calibration model that resulted in minimum total % RE for interpolated concentrations of the CC standards was selected as the one that gave the least error.
2.2.5. Validation
The developed method was validated as per the recommendations of US-FDA Guidance for Industry: Bioanalytical Methods Validation [7]. Selectivity was studied at the lower limit of quantification (LLOQ) of 100 ng/mL by comparing blank responses of plasma from six different sources with peak areas afforded by the LLOQ samples.
Accuracy was estimated as the mean RE while the precision was measured in terms of RSD. The recovery of the extraction procedure was calculated by comparing the peak areas of the processed QC samples to those of corresponding standard dilutions. Stability of eplerenone in human plasma was evaluated under various conditions viz. three freeze–thaw cycles, stability at −20 °C for 30 days and stability at room temperature for 6 h. The amount of the drug in the stability samples was found out and the % nominal and % relative standard deviation of the determination were calculated.
3. Results and discussion
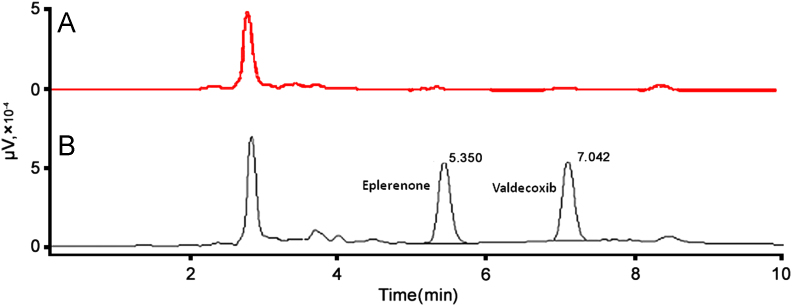
Chromatographic conditions were selected after several trials on different columns using mobile phases comprising of acetonitrile and water in different proportions. The HiQSil C-18 column and mobile phase of acetonitrile:water (50:50, v/v) gave adequate resolution and satisfactory peak shapes for eplerenone and internal standard. The detection wavelength of 241 nm was chosen because it was the λmax of eplerenone. When liquid–liquid extraction was performed using different solvents like diethyl ether, dichloromethane, ethylacetate, chloroform and toluene, it was found that both the drug and internal standard were comparably extracted with dichloromethane. The extraction recovery for eplerenone was 80% while that for valdecoxib was 82%. A mixture of dichloromethane and diethyl ether is less polar and lighter than dichloromethane; it is less likely to coextract interferents and is easily transferable. Thus, it was decided to try a mixture of dichloromethane and diethyl ether as the extracting solvent. During optimization experiments, dichloromethane:diethyl ether (4:6, v/v) gave satisfactory recovery of eplerenone and valdecoxib. During the calibration experiments it was revealed that the standard deviation of area ratios of CC standards increased with concentration indicating a need for weighted regression. Thus, weighted linear regression with weighing factor of 1/X was selected as the calibration model which resulted in the equation Y=0.0012X+0.0030. The area ratios of calibration experiments are depicted in Table 1. During validation studies it was found that the peak areas for the LLOQ samples were more than five times the blank responses obtained using six different plasma sources as can be seen in Table 2. This proved that the method was selective at the LLOQ of 100 ng/mL. The chromatogram of blank plasma extract shown in Fig. 1(A) shows the lack of significant interference at the retention times of eplerenone and internal standard. The representative chromatogram of MQC sample is shown in Fig. 1(B). The US-FDA Guidance requires that the RE should be within ±15%, while the RSD should be less than 15%. The results of assay precision, accuracy and extraction efficiency are shown in Table 3. The results of stability evaluation of eplerenone are presented in Table 4. Analysis of LQC and HQC samples subsequent to various stability cycles viz. three freeze–thaw cycles, stability at −20 °C for 30 days and stability at room temperature for 6 h indicated that eplerenone was stable in human plasma under these conditions.
Table 1.
Area ratios from calibration experiments.
| CC no. | Amount of drug (ng/mL) | Area ratio (mean±SD, n=6) |
|---|---|---|
| CC-1 | 100 | 0.12±0.01 |
| CC-2 | 200 | 0.23±0.01 |
| CC-3 | 400 | 0.49±0.02 |
| CC-4 | 800 | 0.89±0.07 |
| CC-5 | 1600 | 1.95±0.11 |
| CC-6 | 3200 | 3.70±0.35 |
Table 2.
Bank responses and LLOQ peak areas for eplerenone.
| Sr. no. | Blank response (μV) | Peak areas at LLOQ (μV) |
|---|---|---|
| 1 | 3978 | 43,254 |
| 2 | 4025 | 55,462 |
| 3 | 4825 | 60,415 |
| 4 | 3746 | 53,944 |
| 5 | 4482 | 57,169 |
| 6 | 3248 | 51,543 |
Figure 1.
HPLC chromatograms of (A) blank plasma extract and (B) eplerenone and internal standard (valdecoxib) extracted from plasma.
Table 3.
Results of accuracy and precision studies for eplerenone.
| Level | Concentration added (ng/mL) | Intra-day (n=5) |
Inter-day (n=5) |
Recovery (n=5) | ||||
|---|---|---|---|---|---|---|---|---|
| Meanconcentration found (ng/mL) | RE (%) | RSD (%) | Meanconcentration found (ng/mL) | RE (%) | RSD (%) | |||
| LQC | 250 | 255.87 | 2.35 | 6.28 | 255.58 | 2.23 | 5.45 | 80.75 |
| MQC | 1000 | 947.48 | −5.25 | 8.97 | 976.19 | −2.38 | 5.80 | 81.23 |
| HQC | 3000 | 3141.81 | 4.73 | 6.01 | 3070.23 | 2.34 | 5.79 | 74.35 |
| IS | – | – | – | – | – | – | – | 82.20 |
Table 4.
Results of stability studies for eplerenone.
| QC level | Stability at RT | Stability at −20 °C | Freeze–thaw stability | |||
|---|---|---|---|---|---|---|
| % Nominal | RSD (%) | % Nominal | RSD (%) | % Nominal | RSD (%) | |
| LQC | 102.23 | 4.97 | 104.93 | 5.03 | 103.34 | 3.87 |
| HQC | 104.58 | 3.46 | 105.21 | 6.01 | 98.45 | 4.75 |
Acknowledgment
Authors are thankful to the trustees of Bhujbal Knowledge City, Nashik, for providing analytical facilities and to the Hetero Drug Laboratories, Hyderabad, for providing gift samples of eplerenone and valdecoxib.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Spetrus J.A., Tooely J., Jones P. Expanding the outcomes in clinical trils of heart failure: the quality of life and economic components of EPHESUS (eplerenone's neurohormonal efficiency and survival study) Am. Heart J. 2002;143(4):636–642. doi: 10.1067/mhj.2002.120775. [DOI] [PubMed] [Google Scholar]
- 2.Delyani J.A., Rocha R., Cook C.S. Eplerenone: a selective aldosterone receptor antagonist (SARA) Cardiovasc. Ther. 2001;19(3):185–200. doi: 10.1111/j.1527-3466.2001.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 3.Rane V.P., Patil K.R., Sangshetti J.N. Stability-indicating RP-HPLC method for analysis of eplerenone in the bulk drug and in pharmaceutical dosage form. Acta Chromatogr. 2009;21(4):619–629. [Google Scholar]
- 4.Sonawane S., Gide P. An experimental design approach for the forced degradation studies and development of a stability-indicating method for eplerenone in tablets. J. Liq. Chromatogr. Relat. Technol. 2011;34(17):2020–2031. [Google Scholar]
- 5.Zhang J.Y., Fast D.M., Breau A.P. Development and validation of a liquid chromatography-tandem mass spectrometric assay for eplerenone and its hydrolyzed metabolite in human plasma. J. Chromatogr. B. 2003;787(2):333–344. doi: 10.1016/s1570-0232(02)00964-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J.Y., Fast D.M., Breau A.P. A validated SPE–LC/MS assay for eplerenone and its hydrolyzed metabolite in human urine. J. Pharm. Biomed. Anal. 2003;31(1):103–115. doi: 10.1016/s0731-7085(02)00595-2. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services, Food and Drug Administration, Guidance for Industry, Bioanalytical Method Validation, 〈http://www.fda.gov/cder/guidance/index.htm〉 (visited on 16.11.11).