Abstract
A new method for the determination of arecoline in Semen Arecae decoction pieces by microchip capillary electrophoresis with contactless conductivity detection (MCE-CCD) was proposed. The effects of various electrophoretic operating parameters on the analysis of arecoline were studied. Under the optimal conditions, arecoline was rapidly separated and detected in 1 min with good linearity over the concentration range of 20–1500 μM (r2=0.9991) and the detection limit of 5 μM (S/N=3). The method was used for the analysis of arecoline satisfactorily with a recovery of 96.8–104%.
Keywords: Microchip capillary electrophoresis, Contactless conductivity detection, Arecoline, Semen Arecae
1. Introduction
Areca catechu L., belonging to the family Palmae (or Arecaceae), native to Malaysia, widely cultivated in Indonesia, Sri Lanka, Hainan province, Guangdong province, Yunnan province and other places in Southeast Asia, is one of the most widely used South-China medicine resources. Its dry and mature nut, namely Semen Arecae in traditional Chinese medicine (TCM) has antiparasitic, antifungal, antiviral effects, can dissolve food stagnation, move the Qi and promote urination. It has been used for tapeworm infestation, abdominal distension, diarrhea, edema with high medicinal values. The main ingredient known for Semen Arecae is arecoline, which is considered as the effective constituent. In the previous literature, many studies on the quality control of Semen Arecae focused on the extraction and isolation technology of arecoline and determination on its contents. The main extraction methods of arecoline in Semen Arecae were supercritical-CO2 fluid extraction, ultrasonic extraction, and liquid–solid extraction and the determination of arecoline was performed by high performance liquid chromatography (HPLC) with ultraviolet (UV) detection [1] or mass spectrometry (MS) detection [2] and capillary electrophoresis (CE) with ultraviolet (UV) detection [3]. There are no reports for the analysis of arecoline in Semen Arecae by microchip capillary electrophoresis (MCE).
MCE is a modern analytical technology rapidly developed in recent years. With the advantages of its high degree of integration, remarkable sensitivity, high resolution, rapid analysis, low reagent consumption and very cheap running, it has been applied in chemistry, biology, life sciences [4], pharmaceutical analysis [5], environmental analysis [6], clinical diagnosis [7], food security [8], and so forth.
In this study, a homemade microchip capillary electrophoresis system consisted of a high voltage supplier [9], a contactless conductivity detection (CCD) [10] and a thin microchip was developed. The system is successfully applied to detecting arecoline in Semen Arecae decoction pieces on the microchip.
Electrodes of the CCD did not contact with the solution inside the microchip capillary directly, so there was no special and troublesome interface required. It was only needed to push the microchip capillary through both the tubular electrodes, thus, electrode contamination could be avoided effectively, and its installation and manipulation were extremely easy and convenient. Now the CCD has been employed to the analysis of inorganic anions, cations and some organic compounds, especially amino acids [11], and alkaloids [12].
2. Materials and methods
2.1. Chemicals
Arecoline hydrobromide standard sample was obtained from National Institutes for Food and Drug Control (Beijing, China). 2-(N-morpholino) ethanesulfonic acid (MES) was purchased from AMRES Co., Hong Kong. 2-Amino-2-(hydroxymethyl)-1,3-propanediol (Tris) was from Sigma (St. Louis, MO). β-Cyclodextrin (β-CD) was purchased from Sinopharm Group Chemical Reagent Co. Ltd. (Shanghai, China). Sodium dodecyl sulfate (SDS) was obtained from Shanghai Sangon Biological Engineering Technology & Services Co. Ltd. (Shanghai, China). Semen Arecae decoction pieces were obtained from Sri Lanka, Hainan province, and National South-China Medicine Plantation of Guangdong Food and Drug Vocational College (Guangzhou, China), and authenticated by Professor Yue-Wen Cai, Guangdong Traditional Chinese Medicine Institute (Guangzhou, China). All the other chemicals were from Guangzhou Chemical Reagent Co. (Guangzhou, China) and were all of analytical grade purity.
2.2. Preparation of the electrophoretic running buffers
All the binary acid–base buffers consisted of acid (A) and base (B). Stock solutions of A and B were prepared at the concentration of 0.1 M, and then appropriate quantities of A and B were diluted with an appropriate amount of redistilled water and mixed to obtain various electrophoretic running buffers according to its desired concentrations and ratios.
2.3. Preparation of standard solutions
A stock solution of arecoline hydrobromide standard sample was prepared at the concentration of 10.00 mM and appropriately diluted with an appropriate amount of the running buffer to obtain a series of working standard solutions for calibration curves.
2.4. Preparation of sample solutions
Semen Arecae decoction pieces were ground into powder at 200 mesh sieve before use. Accurately weighted 2 g powder was extracted with 30 mL redistilled water by continuously refluxing for 30 min, repeating twice. The extracted solution was combined, and then diluted with an appropriate amount of the running buffer to obtain a series of sample solutions.
All stock solutions and buffer solutions were prepared with redistilled water, and filtered through a 0.22 μm membrane filter before being loaded to the inlet and outlet reservoirs and all solutions were stored at 4 °C and left at room temperature before use.
2.5. Electrophoresis system and procedures
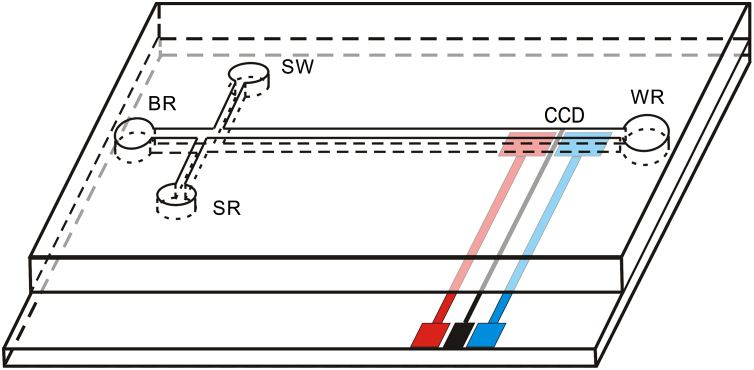
The homemade microchip capillary electrophoresis system consisted of a high voltage supplier, a contactless conductivity detector and a thin microchip. The high voltage supplier made of piezoelectric ceramics [13] was used to provide a separation voltage of 0–5 kV and an injection voltage of 0–500 V, consecutively. The contactless conductivity detector provided three waveforms (sine, square, and triangle) with oscillation frequency of 0–300 kHz and oscillation voltage of 0–300 V (in Vp–p). The detector was connected to a personal computer with an A/D converter (model PCL-711B, EVOC, Taiwan). The polymethylmethacrylate (PMMA) microchip consisted of double-T crossed channels and four reservoirs, including a four-way injection cross (connected to the four reservoirs). A schematic of the PMMA microfluidic devices is shown in Fig. 1.
Figure 1.
PMMA microfluidic-CCD schematic. BR: buffer reservoir; WR: waste reservoir; SR: sample reservoir; SW: sample waste; CCD: contactless conductivity detector. Separation channels dimension: 30 μm in top width, 100 μm in bottom width, 30 μm in depth, 44 mm in length and 43 mm in effective length. Electrodes dimension: 25 μm Pt wire working electrodes.
The channels and four reservoirs of the new PMMA microchip were flushed with 30% ethanol, 1 M nitric acid, 0.1 M sodium hydroxide aqueous solution, and redistilled water for 10, 10, 30, and 30 min, respectively. Before use, the channels and four reservoirs were flushed again with 0.1 M sodium hydroxide aqueous solution, redistilled water, and the electrophoretic running buffer solution for 10, 5, and 20 min, respectively. Reservoirs including buffer reservoir (BR), waste reservoir (WR) and sample waste reservoir (SW) (Fig. 1) were filled with the electrophoretic running buffer solution, while sample reservoir (SR) (Fig. 1) was filled with the sample solution. The injection was performed by applying an injection potential of 200 V between SR and SW for 20 s to facilitate the filling of the injection channel for the first time, but the subsequent injection for 10 s. These operations could transport the sample into the separation channel through the intersection. Separations were done by switching the high voltage and applying a separation potential of 2.5 kV between BR and WR (Fig. 1). The samples were successfully injected, separated and detected within 1 min.
Before the second cycle, the microchannels were treated sequentially with 0.1 M sodium hydroxide for 5 min, redistilled water for 10 min and then flushed with the running buffer solution. Before closing time every day the microchip channels and reservoirs were filled with redistilled water so as to prevent clogging of the microchannels.
3. Results and discussion
3.1. The optimization of the operating parameters
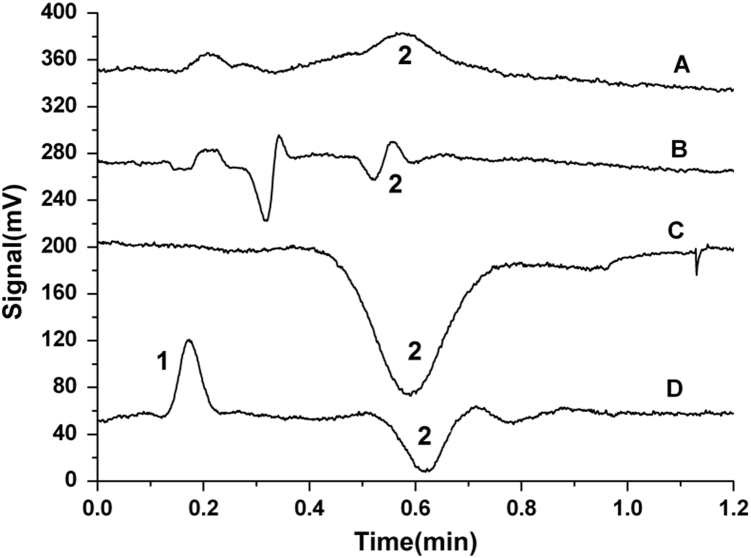
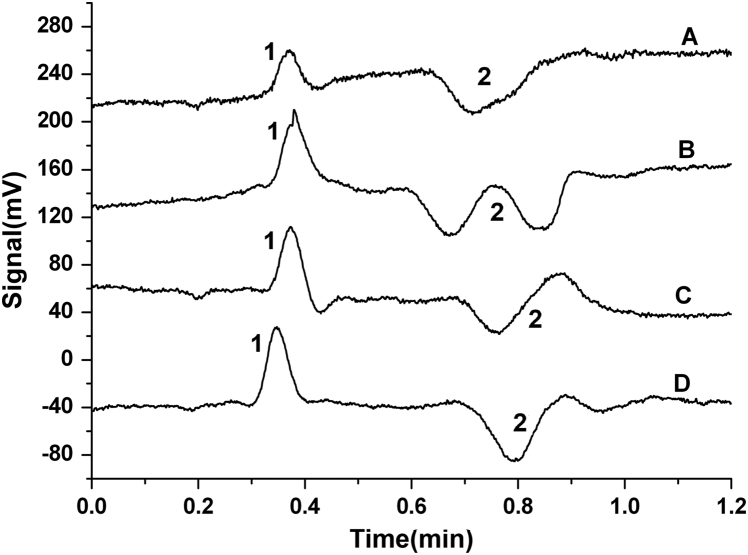
The basic principle of CCD is based on the difference between the sample zone and the background electrolytes. Therefore, the characteristic of the selected electrophoretic running buffers will greatly affect the response sensitivity of CCD [14]. In order to obtain a satisfactory separation and detection of arecoline in Semen Arecae, different electrophoretic running buffers were researched (Fig. 2) including a series of Tris–H3BO3, MES–His, Tris–citric acid, acetic acid–sodium acetate, Tris–citric acid, etc. Besides, the influences of ratios and concentrations of the binary acid–base buffers were researched and a variety of additives, e.g., methanol, acetone, β-cyclodextrin (β-CD), and sodium dodecyl sulfate (SDS) were also studied (Fig. 3). The experimental results showed that the binary buffer of 2 mM acetic acid and 2 mM sodium acetate at pH 4.76 without the extra additive was the best buffer in this experiment.
Figure 2.
Microchip capillary electrophotograms of arecoline in different electrophoretic running buffers. 1: Arecoline; 2: background. A: Tris–H3BO3; B: MES–His; C: Tris–citric acid; D: acetic acid–sodium acetate.
Figure 3.
Microchip capillary electrophotograms of arecoline in HAc–NaAc buffer with different additives. 1: Arecoline; 2: background. A: Methanol; B: acetone; C: β-CD; D: no additive.
The applied excitation voltage is one of the operating parameters that affect the peak height and the signal-to-noise ratio. It was found that when the excitation voltage varied from 20 V to 40 V(in Vp–p), the peak height and the signal-to-noise ratio steadily increased and then seemed to be stable, and when the excitation voltages were higher than 70 V, the peak height and the signal-to-noise ratio continued to descend. However, the excitation voltage over 100 V induced the peak distortion and the baseline drift due to Joule heating. Therefore, we chose 60 V as the suitable excitation voltage for this experiment.
The separation voltage is the crucial operating parameter affecting migration time, baseline noise, signal-to-noise ratio, and reproducibility. It was found that the migration time decreased with the increase of separation voltage but the baseline noise also increased and the signal-to-noise ratio descended slowly. The peak height increased steadily upon raising the separation voltage between 0.5 kV and 2.5 kV, and then the signals seemed to be stable. To obtain a reproducible detection with high sensitivity, 2.5 kV was selected as the optimal separation voltage in the following experiments.
The injection time can control the volume of the sample plug, which also greatly affects the separation efficiency of arecoline. The effect of injection time varied from 5 s to 20 s was investigated in this experiment. As expected, the peak currents increased with the increase of injection time from 5 s to 10 s. However, when injection time was above 10 s, peak currents became constant but background noise was increased and peak shapes became broad. We took into account the detection sensitivity, background noise, and peak shapes, and chose 10 s as the suitable injection time for the detection of arecoline.
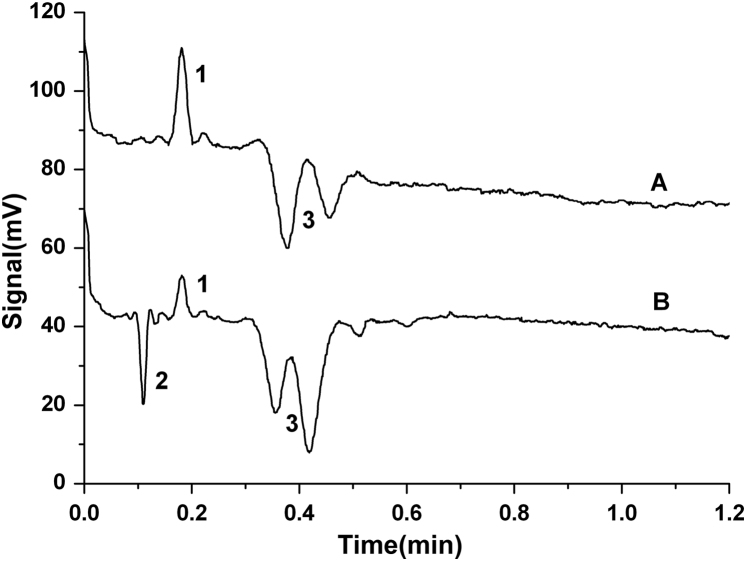
Under the optimal conditions, the typical microchip capillary electrophotograms of arecoline(A) and sample(B) are shown in Fig. 4.
Figure 4.
Typical microchip capillary electrophotograms of arecoline (A) and the sample (B) in the optimal conditions. 1: Arecoline; 2: impurity; 3: background.
3.2. Linearity and detection limit of the analysis
The stock standard solution was appropriately diluted with the optimized running buffer of 2 mM acetic acid and 2 mM sodium acetate (pH=4.76) to obtain a series of working standard solutions. And then the calibration curve was constructed by plotting the peak height (Y) versus the concentration (X) of arecoline. As a result, the correlation coefficient (r2) of 0.9991 indicated preferable linearity over the concentration range of 20–1500 μM, with the regression equation of Y =5.0255×104X+2.0593×103. The limit of detection(LOD) was found to be 5 μM at the signal-to-noise ratio (S/N) of 3.
3.3. Precision
The method precision was assessed using multiple preparations of a single sample. The same Semen Arecae decoction pieces were prepared and analyzed six times in the optimal conditions on the same day. The relative standard deviation (RSD) values obtained for the migration time and the peak height of arecoline were 0.5% and 2.0%, respectively.
3.4. Recovery
To evaluate the accuracy of the method, the recovery test was carried out by measuring the amount of arecoline recovered from the samples which was mixed with the standard sample at high, middle and low concentration levels in three replicates. The results are listed in Table 1.
Table 1.
Results of the recovery test.
| Component | Found (μM) | Added (μM) | Total found (μM) | Recovery (%) | RSD (n=3) (%) |
|---|---|---|---|---|---|
| Arecoline | 30.87 | 24.70 | 56.51 | 104.0 | 1.5 |
| 30.87 | 62.16 | 101.0 | 2.3 | ||
| 37.04 | 66.72 | 96.8 | 1.7 |
The recoveries were between 96.8% and 104%. High recoveries illustrated good accuracy of the method.
3.5. Sample analysis
The developed MCE method was applied to the determination of arecoline in Semen Arecae from three different regions. The contents of the analytes are shown in Table 2, corresponding to the results from the previous literature [15], [16]. The contents of arecoline in Semen Arecae from different regions varied substantially because of different climates and geographical environment.
Table 2.
Results of the content determination of Semen Arecae from three different regions.
| Sample source | Peak height | Found (μM) | Content (%) | Mean±SD (%) | RSD (n=3) (%) |
|---|---|---|---|---|---|
| Sri Lanka | 2,782,718 | 55.33 | 0.4294 | 0.4310±0.0067 | 1.6 |
| 2,840,653 | 56.48 | 0.4383 | |||
| 2,756,271 | 54.80 | 0.4253 | |||
| Hainan | 1,582,765 | 31.45 | 0.2441 | 0.2396±0.0042 | 1.8 |
| 1,549,298 | 30.79 | 0.2389 | |||
| 1,528,811 | 30.38 | 0.2357 | |||
| Guangzhou | 2,592,114 | 51.54 | 0.3999 | 0.4078±0.0085 | 2.1 |
| 2,635,423 | 52.40 | 0.4066 | |||
| 2,701,325 | 53.71 | 0.4168 |
4. Conclusions
In this work, an alternative approach for the rapid analysis of arecoline using PMMA microfluidic-CCD devices was described. The optimal conditions used were 2 mM HAc+2 mM NaAc (pH=4.76) as the running buffer, 60 V for the excitation voltage, 2.5 kV for the separation voltage, and 10 s for the injection. The calibration curve was found to be linear between 20 and 1500 μM with coefficient of determination (r2) of 0.9991 and the detection limit of 5 μM. The method was simple, rapid, reliable, and successfully employed to the quantification of arecoline in Semen Arecae, and it could be used in the quality control of Semen Arecae decoction pieces.
Acknowledgments
Financial support from the National Natural Science Foundation of China (Nos. 20727006, 21075139), Guangdong Provincial Science and Technology Project (2008A030102009), and the Medical Scientific Research Foundation of Guangdong Province (A2012155), are gratefully acknowledged.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Zi-You Cai, Email: ziyoutsai@163.com.
Zuan-Guang Chen, Email: chenzg@mail.sysu.edu.cn.
References
- 1.Huang J.L. High-performance liquid chromatographic determination of the alkaloids in betel nut. J. Chromatogr. 1989;475:447–450. [Google Scholar]
- 2.Wen K.Q. Determination of arecoline in betel nut foods by high performance liquid chromatography/electrospray mass spectrometry. Chin. Pract. Med. 2008;3(8):12–13. [Google Scholar]
- 3.Yuan W., Lü J.D., Fu X.Y. Separation and quantitative determination of arecoline and arecaidine by capiliary electrophoresis. Chin. J. Anal. Chem. 2000;28(6):749–752. [Google Scholar]
- 4.Hopwood A.J., Hurth C., Yang J.N. Integrated microfluidic system for rapid forensic DNA analysis: sample collection to DNA profile. Anal. Chem. 2010;82:6991–6999. doi: 10.1021/ac101355r. [DOI] [PubMed] [Google Scholar]
- 5.Cai Z.Y., Li Y.C., Chen Z.G. Determination of moroxydine hydrochloride tablets by microfluidic chip. Chin. J. Pharm. Anal. 2011;31(8):1492–1495. [Google Scholar]
- 6.Jokerst J.C., Emory J.M., Henry C.S. Advances in microfluidics for environmental analysis. Analyst. 2012;137:24–34. doi: 10.1039/c1an15368d. [DOI] [PubMed] [Google Scholar]
- 7.Kim M.S., Kim T., Kong S.Y. Breast cancer diagnosis using a microfluidic multiplexed immunohistochemistry platform. PLoS One. 2010;5:e10441. doi: 10.1371/journal.pone.0010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hervas M., Lopez M.A., Escarpa A. Electrochemical microfluidic chips coupled to magnetic bead-based ELISA to control allowable levels of zearalenone in baby foods using simplified calibration. Analyst. 2009;134:2405–2411. doi: 10.1039/b911839j. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z.G., Wang L.S., Mo J.Y. A micro power for micro total analysis system. Chem. J. Chin. Univ. 2004;25(Suppl.):26–27. [Google Scholar]
- 10.Chen Z.G., Li Q.W., Li O.L. A thin cover glass chip for contactless conductivity detection in microchip capillary electrophoresis. Talanta. 2007;71(5):1944–1950. doi: 10.1016/j.talanta.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Zhai H.Y., Wang J.M., Yao X.L. Analysis of glutamic acid in cerebrospinal fluid by capillary electrophoresis with high frequency conductivity detection. Chin. Chem. Lett. 2005;16(2):225–228. [Google Scholar]
- 12.Li Y.C., Cai Z.Y., Wang C. Determination of berberine hydrochloride in berberine hydrochloride tablets by microfluidic chip. J. Chin. Med. Mater. 2011;34(5):802–804. [Google Scholar]
- 13.Chen Z.G., MO J.Y., Yang X.Y. A new capillary electrophoresis apparatus with piezoelectric ceramics high voltage source and amperometric detector. Chin. Chem. Lett. 1999;10(3):231–234. [Google Scholar]
- 14.Wang L.S., Zhang S.F., Dang Z. Running buffers for determination of chromium (VI)/(III), cobalt (II) and zinc (II) in complex matrices by capillary electrophoresis with contactless conductivity detection. Talanta. 2007;72(4):1342–1347. doi: 10.1016/j.talanta.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 15.Lin L., Xu H.H., Deng P.F. A TLC densitometric method for the determination of arecoline content in Semen Arecae from different producing areas. Chin. J. Chin. Mater. Med. 1992;17(8):491–492. [PubMed] [Google Scholar]
- 16.Han Z.P., Huang M.F., Zhu D.M. Study of the impact of pretreatment on arecoline extraction rate. Food Sci. Technol. Chin. 2010;35(8):240–243. [Google Scholar]