Abstract
Vidangadi churna is a popular Ayurvedic formulation described in the chapter Krimicikitsa of the Ayurvedic literature Cakradatta for the treatment of Krimiroga. The preparation is a composite mixture of the fine powder of fruits of Vidang (Embelia ribs), glandular trichomes of the fruits of Kamala (Mallotus philippensis), mature fruits of Harde (Terminalia chebula), Saindhava and Yavakshara. The use of reversed phase C18 column eluted with gradient mobile phase of acetonitrile and water enabled the efficient separation of the chemical markers in 22 min. Validation of the method was performed in order to demonstrate its selectivity, accuracy, precision, repeatability and recovery. All calibration curves showed good linear correlation coefficients (r2>0.995) within the tested ranges. Three markers in Vidangadi churna were quantified with respect to Embelin (0.647%, w/w), Rottlerin (4.419%, w/w), and Ellagic acid (0.459%, w/w). Intra-and inter-day RSDs of retention times and peak areas were less than 3.12%. The recoveries were between 99.66% and 102.33%. In conclusion, a method has been developed for the simultaneous quantification of three markers in Vidangadi churna. The RP-HPLC method was simple, precise and accurate and can be used for the quality control of the raw materials as well as formulations.
Keywords: Vidangadi churna, Embelin, Rottlerin, Ellagic acid, RP-HPLC
1. Introduction
Standardization and analysis of the chemical markers of the ayurvedic and other poly herbal formulations is always difficult. Quantitative determination of chemical markers of each ingredient in the poly herbal preparation required optimal separation techniques by which these markers are separated with the highest resolution and the least interferences from each other. For botanicals and herbal preparations, there is a requirement for scientific evidence and clinical validation with chemical standardization, biological assays, animal models and clinical trials [1]. Herbal medicine has been enjoying renaissance among the customers throughout the world. However, one of the impediments in the acceptance of the ayurvedic medicines is the lack of standard quality control profiles. The quality of herbal medicine, i.e. the profile of the constituents in the final product, has implication in efficacy and safety. Due to the complex nature and inherent variability of the chemical constituents of plant-based drugs, it is difficult to establish quality control parameters. Modern analytical techniques are increasing to overcome these problems [2], [3]. Separation, identification and determination of chemical components are very difficult for such polyherbal formulations [4]. The advances in chromatographic separation techniques made it possible to quantify the chemical constituents in a mixture with comparatively little clean-up [5]. Particularly, methods using gradient elution high performance liquid chromatography (HPLC) with reversed phase columns are most commonly applied for the analysis of multiple constituents present in medicinal plants and herbal preparations [6].
Vidangadi churna is a well known Ayurvedic preparation described in the chapter krimicikitsa of the ayurvedic literature cakradatta for the treatment of krimiroga [7]. It consists of the mixture of the fine powder of the fruits of Vidang (Embelia ribes Burm., F. Myrsinaceae), glandular trichomes of the fruits of Kamala (Mallotus philippensis Muell., F. Euphorbiaceae), mature fruits of Harde (Terminalia chebula Retz. F. Combretaceae), Saindhava (Rock salt) and Yavakshara (water soluble ash of the grains of Hordium vulgare Linn., F. Gramineae).
In the present investigation, we have developed a simple, optimized and validated HPLC method for the standardization of Vidangadi churna. Three chemical markers were selected for quantification, and one was from each medicinal herb used as raw materials, Embelin for Vidang, Rottlerin for Kamala and Ellagic acid for Harde. These markers are responsible for the physiological action of the respective plants [8]. The method was validated on the basis of its selectivity, linearity, precision, accuracy, limit of detection (LOD) and limit of quantification (LOQ) according to the International Conference on Harmonization (ICH) requirements. Profiles of the individual ingredients of Vidangadi churna were also recorded and the markers present in them were quantified.
2. Experimental
2.1. Materials
Vidangadi churna and its individual components were procured from a local market in Ahmedabad city, Gujarat and authenticated by comparison with herbarium specimens [9], [10], [11]. Embelin was isolated according to the method of Indian Herbal Pharmacopoeia [12] while Rottlerin was isolated according to the method described by Khorana and Motiwala [13]. The isolated markers were identified by FTIR, MASS and 1H NMR spectroscopy. Ellagic acid was obtained from Sigma-Aldrich (St. Louis, Mo, USA). HPLC grade acetonitrile, water and methanol were obtained from Merck (Darmstadt, Germany).
2.2. Instrumentation
Analysis was performed on a Shimadzu LC-20AD HPLC system equipped with an online degaser DGU-20 As, an Rheodyne 7725 injection valve furnished with 20 μL loop, an SPD-M20A photodiode array detector and a Class-VP software. Separation was carried out using a Phenomenex column (250 mm×4.6 mm i.d., 5 μm pore size). The column was maintained at 27 °C throughout analysis and the UV detector was set at 298 nm.
2.3. Sample preparation
500 mg powder of Vidangadi churna and 100 mg powder of its three ingredients, Vidang, Kamala and Harde, were extracted three times with 100 mL methanol. The extracts were combined and concentrated at reduced temperature (50 °C) on a rotary evaporator (Equitron rotevar, Medica Instrument Mfg. Co.) to 100 mL. Prior to use, all samples were filtered through a 0.45 μm nylon membrane filter.
2.4. Calibration
The contents of the markers were determined using a calibration curve established with six dilutions of each standard, at concentrations ranging from 10 to 60 μg/mL. Each concentration was measured in triplicate. The corresponding peak areas were plotted against the concentrations of the markers injected. Peak identification was achieved by comparison of both the retention time (Rt) and UV absorption spectrum with those obtained for standards. The reference substances employed to construct the calibration curves were Embelin, Rottlerin and Ellagic acid.
2.5. Validation parameter
The method was validated according to ICH guideline for linearity, precision, accuracy, selectivity, LOD and LOQ [14]. Selectivity was checked using an extract of Vidangadi churna and a mixture of standards in order to optimize separation and detection. Linearity of the method was performed by analyzing a standard solution of markers by the method in the concentration range of 10–60 μg/mL. The accuracy of the proposed method was determined by a recovery study, carried out by adding standard markers in the Vidangadi churna extract. The samples were spiked with three different amounts of standard compounds prior to extraction. The spiked samples were extracted in triplicate and analyzed under the previously established optimal conditions. The obtained average contents of the target compounds were used to calculate the spike recoveries. Precision was determined by repeatability and inter-day and inter-day reproducibility experiments. A standard solution containing three markers was injected six times; Vidangadi churna was also extracted six times to evaluate the repeatability of the extraction process. The mean amount and standard deviation (SD) value of each constitute were calculated. The LOD and LOQ of marker compounds were calculated at signal-to-noise ratio of approximately 3:1 and 10:1 respectively.
3. Results and discussion
3.1. Optimization of HPLC chromatographic conditions
Optimum chromatographic conditions were obtained after running different mobile phases with a reversed phase C18 column. Water was preferred over methanol as a mobile phase because its use resulted in improved separation. Many different gradient systems of mobile phases were tried to achieve the best separation of peaks. Selecting 298 nm as the detection wavelength resulted in an acceptable response and enable the detection of all three compounds used in this study. The temperature of column was maintained at 27 °C throughout analysis. An HPLC fingerprint for Vidangadi churna was developed. Elution was carried out at a flow rate of 0.5 mL/min with acetonitrile as solvent A and water as solvent B using gradient elution in 0–2 min with 50% A, 2–4 min with 50–60% A, 4–6 min with 60% A, 6–8 min with 60–65% A, 8–10 min with 65–40% A, 10–13 min with 40–35% A, 13–17 min with 35–30% A and 17–22 min with 30–10% A. Each run was followed by a 10 min wash with 10% acetonitrile and an equilibration period of 15 min.
3.2. Quantification of markers present in Vidangadi churna
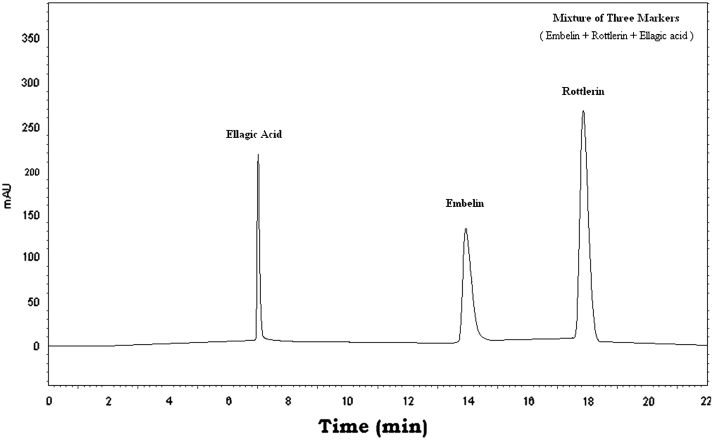
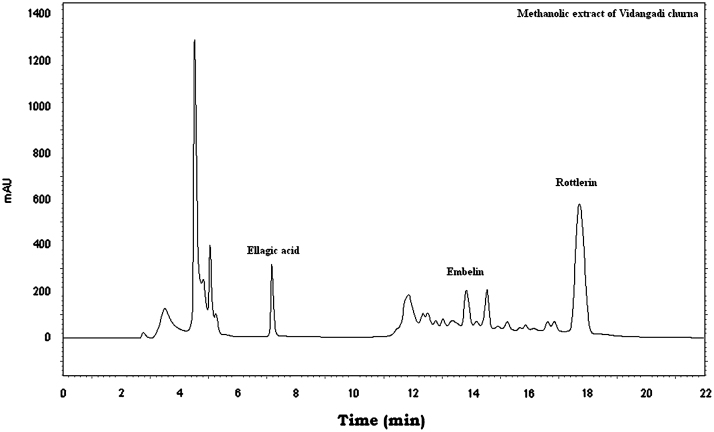
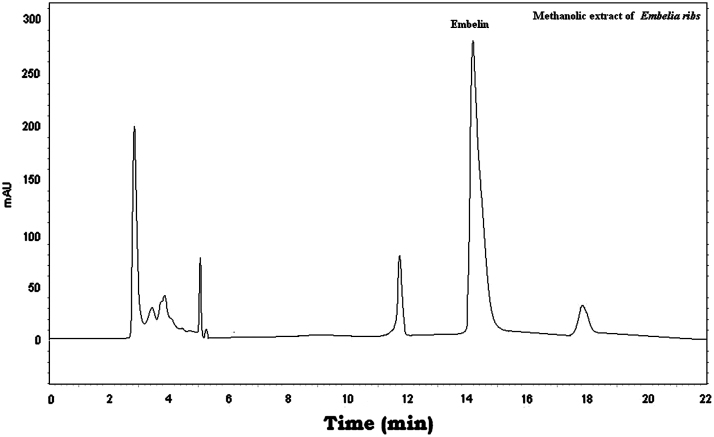
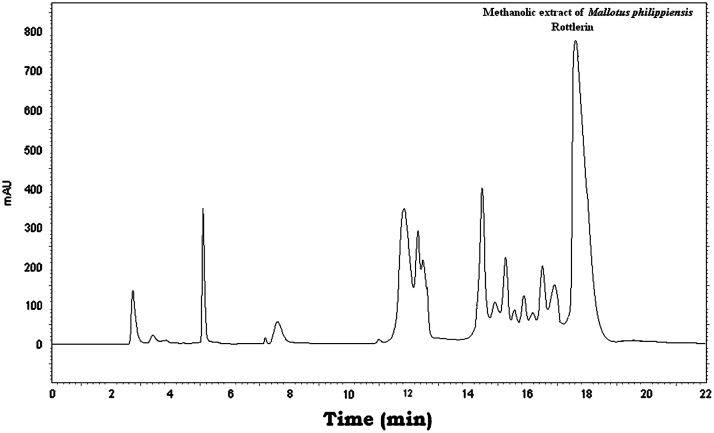
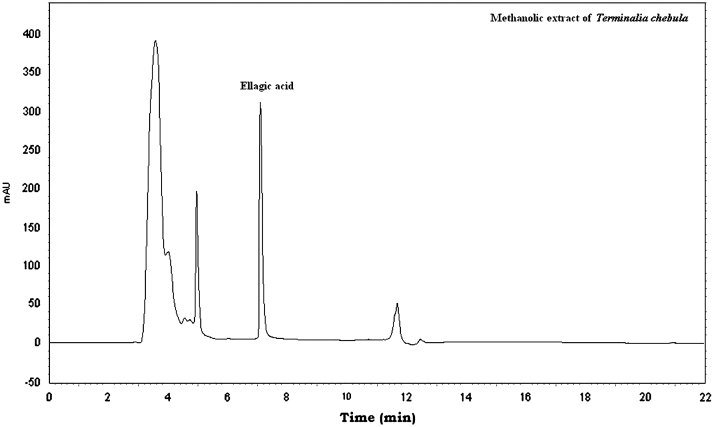
The three markers were found in Vidangadi churna and they were quantified with respect to Embelin (0.647%, w/w), Rottlerin (4.419%, w/w), and Ellagic acid (0.459%, w/w). The chromatograms of a mixture of Embelin, Rottlerin and Ellagic acid (Fig. 1) and Vidangadi churna (Fig. 2) showed complete separation of the three markers. The chromatograms of the individual components of Vidangadi churna, Vidang (Fig. 3), Kamala (Fig. 4) and Harde (Fig. 5), were obtained. The ingredients were also quantified with respect to the following standards. Vidang contained Embelin (3.126%, w/w), Kamala contained Rottlerin (21.215%, w/w) while Harde contained Ellagic acid (2.218%, w/w). The results obtained are shown in Table 1.
Figure 1.
HPLC chromatogram of the mixture of Embelin, Rotlerin and Ellagic acid.
Figure 2.
HPLC chromatogram of Vidangadi churna.
Figure 3.
HPLC chromatogram of Vidang.
Figure 4.
HPLC chromatogram of Kamala.
Figure 5.
HPLC chromatogram of Harde.
Table 1.
Quantification of Embelin, Rottlerin and Ellagic acid in Vidangadi churna and its ingredients by HPLC method.
| Sample | Amount of Embelina (%, w/w) | Amount of Rottlerina (%, w/w) | Amount of Ellagic acida (%, w/w) |
|---|---|---|---|
| Vidang powder | 3.13±0.01 | – | – |
| Kamala powder | – | 21.25±0.11 | – |
| Harde powder | – | – | 2.22±0.05 |
| Vidangadi churna | 4.15±0.08 | 0.46±0.04 | 0.65±0.01 |
Mean±SD (n=6).
3.3. Method validation for HPLC fingerprinting method
The HPLC method was validated by defining the selectivity, linearity, accuracy, precision, LOD and LOQ. For qualitative purposes, the method was evaluated by taking into account the precision in the retention time and selectivity of marker compounds eluted. A high repeatability in the retention time was obtained for standards and extracts even at high concentrations. For quantitative purpose, linearity, accuracy, precision, LOD and LOQ were evaluated. LOD and LOQ values were 0.12 μg/mL and 0.5 μg/mL for Embelin, 0.45 μg/mL and 1.9 μg/mL for Rottlerin and 0.66 μg/mL and 2.4 μg/mL for Ellagic acid, respectively. Linear correlation was obtained between peak area and concentration of three markers in the range of 10–60 μg/mL. Values of the regression coefficients (r2) of the markers were higher than 0.99, thus confirming the linearity of the methods (Table 2). The high recovery values (95.66–102.33%) indicated a satisfactory accuracy. Relative standard deviation of all the parameters was less than 3.5% for the degree of repeatability, indicating the high repeatability of the developed method (Table 3). The low coefficient of variation values of intra-day and intra-day precision revealed that the method is precise (Table 4). Therefore, this HPLC method can be regarded as selective, accurate and precise.
Table 2.
Regression parameter, linearity, limit of detection (LOD) and limit of quantification (LOQ) of the proposed HPLC method.
| Compound | Concentration range (μg/mL) | Rt (min) a | Regression equation | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| Embelin | 10–60 | 14.10±0.14 | y=42150x−22788 | 0.995 | 0.12 | 0.5 |
| Rottlerin | 10–60 | 17.88±0.03 | y=39764x+91252 | 0.996 | 0.45 | 1.9 |
| Ellagic acid | 10–60 | 7.01±0.06 | y=21019x+33669 | 0.998 | 0.66 | 2.4 |
Mean±SD (n=6).
Table 3.
Repeatability and recovery study for the three markers in Vidangadi churna.
| Compound | Contentsa (mg/g) | Added amount (mg) | Recorded amounta (mg) | Recovery ratea (%) | RSD (%) |
| Embelin | 6.47±0.01 | 3 | 9.34±0.11 | 95.66±1.08 | 1.12 |
| 6 | 12.37±1.10 | 98.33±2.04 | 2.07 | ||
| 9 | 15.37±0.07 | 98.89±0.77 | 0.77 | ||
| Rottlerin | 41.49±0.08 | 20 | 61.69±1.07 | 100.33±0.73 | 0.73 |
| 40 | 80.30±2.04 | 98.55±1.55 | 1.57 | ||
| 60 | 100.57±1.08 | 99.10±1.83 | 1.84 | ||
| Ellagic acid | 4.59±0.04 | 2 | 6.67±0.17 | 102.33±2.30 | 2.24 |
| 4 | 8.62±0.02 | 100.75±0.71 | 0.70 | ||
| 6 | 10.71±1.10 | 101.98±1.08 | 1.06 | ||
Mean±SD (n=3).
Table 4.
Precision of the intra-day and inter-day HPLC measurement for Embelin, Rottlerin and Ellagic acid in Vidangadi churna.
| Compound | Intra-daya |
Inter-dayb |
||
| Contentsc (%, w/w) | RSD (%) | Contentsc (%, w/w) | RSD (%) | |
| Embelin | 0.65±0.01 | 1.54 | 0.64±0.02 | 3.12 |
| Rottlerin | 4.42±0.08 | 1.81 | 4.46±0.10 | 0.22 |
| Ellagic acid | 0.46±0.01 | 2.17 | 0.45±0.01 | 2.21 |
Sample were analyzed six times a day.
Sample were analyzed once a day over six consecutive days.
Mean±SD (n=6).
4. Conclusion
The results indicate that Vidangadi churna contains a number of markers that may be responsible for its therapeutic activity. The developed HPLC method will assist in the standardization of Vidangadi churna using biologically active chemical markers. The developed HPLC method for simultaneous determination of Embelin, Rottlerin and Ellagic acid from Vidangadi churna is accurate, precise, reproducible and repeatable. Vidangadi churna also contains a number of other constituents, which are currently the subject of further investigation, apart from those standards studied. In addition, profiles of the individual components in Vidangadi churna have been recorded as a standardization tool. With the growing demand for herbal drugs and increased belief in the usage of herbal medicine, the development of a standardization tool will help in maintaining the quality of this important Ayurvedic preparation.
Acknowledgments
The authors thank to S.K. Patel College of Pharmaceutical Education & Research (SKPCPER), Ganpat University, Mehsana and for providing facility to carry out this research.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Ong E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B. 2004;812:23–33. doi: 10.1016/j.jchromb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 2.Asokar L.V., Kakkar K.K., Chakra O.J. Publication and Information Directorate; New Delhi: 1992. Glossary of Indian medicinal plants with active principles. p. 122. [Google Scholar]
- 3.Ravishankara M.N., Shrivastava N., Padh H. HPTLC method for the estimation of alkaloids of Cinchona officinalis stems bark and its marketed formulations. Planta Med. 2001;67:294–296. doi: 10.1055/s-2001-11995. [DOI] [PubMed] [Google Scholar]
- 4.Dhalwal K., Biradar Y.S., Rajani M. TLC densitometric method for simultaneous quantification of phyllanthin, hypophyllanthin, gallic acid and ellagic acid in Phyllanthus amarus using HPTLC. J. AOAC Inter. 2006;89:619–623. [PubMed] [Google Scholar]
- 5.Vol. I. Indian Council of Medical Research; New Delhi: 2003. (Quality Standards of Indian Medicinal Plants). pp. 10–50. [Google Scholar]
- 6.Sheu S.J., Li K.L. Liquid chromatographic determination of the constituents in Shao-yao-tang and related chinese herbal preparations. J. High Resol. Chromatogr. 1998;21:569–573. [Google Scholar]
- 7.The Ayurvedic Formulary of India. Part II. first ed. Government of India, Ministry of Health and Family Welfare; New Delhi: 2000. p. 128. [Google Scholar]
- 8.Vol. I, first ed. Government of India, Ministry of Health and Family Welfare; New Delhi: 2001,. (The Ayurvedic Pharmacopoeia of India). pp. 47–48, 55, 123–124. [Google Scholar]
- 9.Vol. IV. Indian Council of Medicinal Research; New Delhi: 2006. (Quality Standard of Indian Medicinal Plants). pp. 130–136. [Google Scholar]
- 10.Mukherjee P.K. Business Horizons; New Delhi: 2005. Quality Control of Herbal Drugs. pp. 741–743. [Google Scholar]
- 11.Vol. I. Indian Council of Medicinal Research; New Delhi: 2003. (Quality Standard of Indian Medicinal Plants). pp. 205–211. [Google Scholar]
- 12.Indian Herbal Pharmacopoeia. Indian Drug Manufacturer's association; Mumbai: 2002. pp. 206–213. [Google Scholar]
- 13.Khorana M.L., Motiwala D.K. Anthelmentic activity of Kamala and its constituents. Indian J. Pharm. 1949;11:37–43. [Google Scholar]
- 14.Guideline on Validation of Analytical Procedure-Methodology. International Conference on Harmonization, Geneva, Switzerland, 1996.