Abstract
A new simple spectrophotometric method was developed for the simultaneous determination of drugs with interfering spectra in binary mixtures without previous separation. The new method is based on a simple modification for the ratio subtraction method. This modification enabled wider range of application. The proposed ratio difference method was applied for the determination of brimonidine and timolol in laboratory prepared mixtures with mean percentage recoveries 100.40±2.29 and 101.23±1.30 respectively, and in their pharmaceutical formulation with mean percentage recoveries 101.08±0.44 and 100.66±0.52 respectively. The suggested ratio difference method was validated according to USP guidelines and can be applied for routine quality control testing.
Keywords: Spectrophotometry, Ratio difference, Binary mixtures, Brimonidine, Timolol
1. Introduction
Several manipulations were performed on the raw overlapping spectral data to enable mixture resolution, for example, using different order derivatives [1], [2], [3], [4], [5], [6], derivatives of the ratio spectrum [7], [8], [9], [10] and ratio subtraction technique [11].
A new method ratio difference has been developed having the advantages of minimal data processing and wider range of application. The method was applied for the analysis of a mixture of brimonidine tartarate (Br) and timolol maleate (Ti) recently introduced into the markets.
2. Theory of the ratio difference method
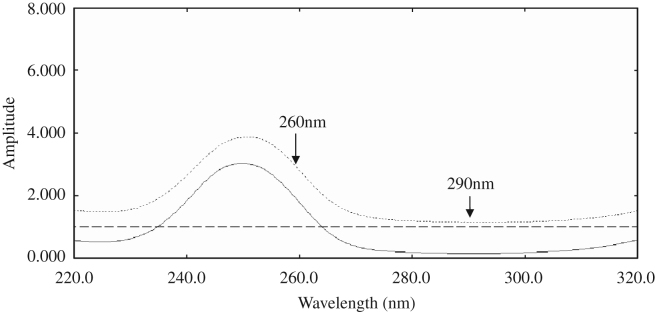
Upon dividing the absorption spectrum of a compound by a spectrum of the same compound, a straight line of constant amplitude (parallel to the baseline) will result. However, upon dividing the absorption spectrum of a compound by the absorption spectrum of another compound, a new spectrum (ratio spectrum) will result (Figure 1, Figure 2).
Figure 1.
The ratio spectrum of 20 μg/mL Br (——), 50 μg/mL Ti (- - -) and a mixture containing 20 μg/mL Br and 50 μg/mL Ti (⋯) using a divisor of 50 μg/mL Ti in distilled water.
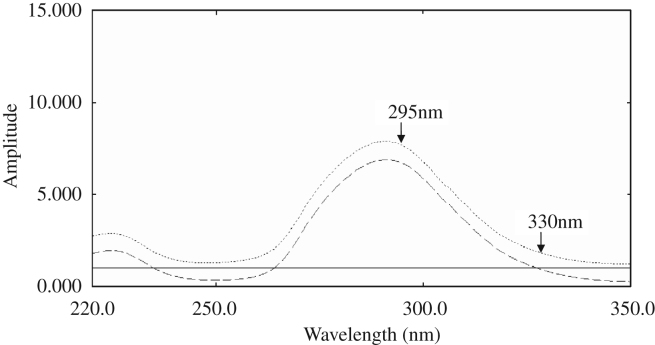
Figure 2.
The ratio spectrum of 20 μg/mL Br (——), 50 μg/mL Ti (- - -) and a mixture containing 20 μg/mL Br and 50 μg/mL Ti (⋯) using a divisor of 20 μg/mL Br in distilled water.
Mathematically it can be explained as follows:
In the ratio spectrum of a laboratory mixture of X and Y divided by a divisor Y′
| (1) |
| (2) |
where P1 and P2 are the amplitudes of the mixture spectrum at λ1 and λ2, respectively. P1X and P2X are the amplitudes of X at λ1 and λ2, respectively, and K is the constant resulting from Y/Y′.
| (3) |
So the component Y will be completely cancelled and the difference will represent the X component only.
Component X in a binary mixture can be determined from a calibration curve that relates the difference in amplitudes (ΔP1−2) in the ratio spectrum at λ1 and λ2 using a certain concentration of Y as a divisor to the corresponding concentration of X. Similarly component Y can be obtained by using a certain concentration of X as a divisor.
3. Materials and method
SHIMADZU UV-1601 dual beam UV–visible spectrophotometer (Kyoto/Japan), Br and Ti reference standards (kindly supplied by Sigma Pharmaceutiacal Co. Cairo, Egypt), stock standard solution of 0.1 mg/mL Br and 0.1 mg/mL Ti in distilled water.
3.1. Procedures
3.1.1. Construction of calibration curves
The zero order spectra of different solutions of Br and Ti were divided by the spectra of 50 μg/mL Ti and 20 μg/mL Br, respectively. The difference in the peak amplitudes ΔP at the ratio spectra was measured at 260 and 290 nm (ΔP260−290 nm) for Br and at 295 and 330 nm (ΔP295−330 nm) for Ti. Calibration graphs relating ΔP at the chosen wavelength couples to the corresponding concentrations of Br and Ti were constructed, and the corresponding regression equations were computed.
3.1.2. Analysis of laboratory prepared mixtures
The zero order spectra of different laboratory prepared mixtures were divided by the spectrum of 50 μg/mL Ti (ΔP260–290 was recorded) and the spectrum of 20 μg/mL Br (ΔP295–330 nm was recorded) for determination of Br and Ti, respectively.
3.1.3. Application of the proposed ratio difference method for the simultaneous determination of Br and Ti in Combigan ophthalmic solution
0.5 mL of the solution was transferred to another 100 mL measuring flask, and the volume was completed with distilled water. The concentrations of Br and Ti were obtained as in Section 3.1.2.
4. Results and discussion
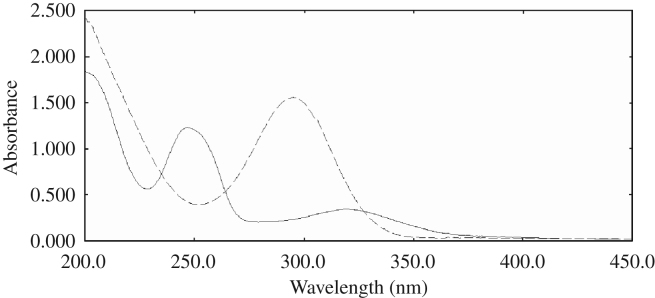
The proposed ratio difference method can be applied for resolving absorption spectra of two components with high degree of overlap as in the case of Br and Ti (Fig. 3), where the application of the direct spectrophotometry failed to determine either of them in their mixture.
Figure 3.
The absorption spectra of 20 μg/mL Br (——) and 50 μg/mL Ti (- - -) in distilled water.
The method comprises two critical steps, the first is the choice of the divisors, the selected divisors should compromise between minimal noise and maximum sensitivity. The divisor concentrations 50 μg/mL Ti and 20 μg/mL Br gave the best results regarding the average percentage recovery when used for the prediction of Br and Ti concentrations, respectively. The second critical step is the choice of the wavelengths at which measurements are recorded. Any two wavelengths can be chosen provided that they exhibit different amplitudes in the ratio spectrum and a good linearity is present at each wavelength individually.
Linear correlation was obtained between the differences in amplitude at 260 and 290 nm (ΔP260–290 nm) for Br and at 295 and 330 nm (ΔP295–330 nm) for Ti, against the corresponding concentrations of Br and Ti, respectively.
The proposed ratio difference method was applied for the determination of brimonidine and timolol in laboratory prepared mixtures (Table 1), and in their pharmaceutical formulations (Table 2). The suggested ratio difference method was validated according to USP guidelines [12] and can be applied for routine quality control testing, as shown in Table 3.
Table 1.
Determination of Br and Ti in laboratory prepared mixtures by the proposed ratio difference method.
| Concentration (μg/mL) |
Recovery (%) |
||
|---|---|---|---|
| Br | Ti | Br ΔP260–290 nm | Ti ΔP295–330 nm |
| 10.00 | 50.00 | 96.52 | 101.06 |
| 20.00 | 20.00 | 100.75 | 101.42 |
| 20.00 | 40.00 | 100.43 | 102.53 |
| 20.00 | 50.00 | 100.82 | 101.23 |
| 25.00 | 30.00 | 103.33 | 102.26 |
| 30.00 | 50.00 | 99.58 | 98.87 |
| Mean | 100.40 | 101.23 | |
| Std | 2.29 | 1.30 | |
| RSD | 2.28 | 1.28 | |
Table 2.
Determination of Br and Ti in Combigan® eye drops by the proposed ratio difference method and application of standard addition technique.
| Product in Combigan® eye dropa | Standard addition |
Recovery (mean±SD, %) |
||||
|---|---|---|---|---|---|---|
| Taken (μg/mL) | Added (μg/mL) | Found (μg/mL) | Recovery (%) | Proposed method | Standard addition | |
| Br | 10.00 | 5.00 | 4.93 | 98.60 | 101.08±0.44 | 100.10±1.41 |
| 10.00 | 10.14 | 101.40 | ||||
| 20.00 | 20.06 | 100.30 | ||||
| Ti | 25.00 | 20.00 | 20.15 | 100.75 | 100.66±0.52 | 100.42±0.68 |
| 25.00 | 24.91 | 99.64 | ||||
| 30.00 | 30.26 | 100.87 | ||||
Combigan® eye drops, batch no. E62201 labeled to contain 2 mg/mL of brimonidine tartarate and 5 mg/mL of timolol.
Table 3.
Assay validation sheet of the proposed ratio difference method for the determination of Br and Ti.
| Parameter | Br | Ti |
|---|---|---|
| Accuracy (mean±SD) | 100.16±1.32 | 100.17±1.66 |
| Specificity | 100.40±2.29 | 101.23±1.30 |
| Precision | ||
| Repeatabilitya | 100.21±0.73 | 100.06±0.32 |
| Intermediate precisionb | 100.07±0.40 | 100.09±0.39 |
| Linearity | ||
| Slope | 0.0762 | 0.0811 |
| Intercept | 0.1105 | 0.0780 |
| Correlation coefficient r | 0.9999 | 0.9998 |
| Range (μg/mL) | 5–30 | 10–60 |
The intraday (n=3), average of three concentrations (10, 15 and 20 μg/mL) for Br and (20, 30 and 40 μg/mL) for Ti repeated three times within the day.
The interday (n=3), average of three concentrations (10, 15 and 20 μg/mL) for Br and (20, 30 and 40 μg/mL) for Ti repeated three times on three consecutive days.
The suggested ratio difference method showed a wider range of application over the ratio subtraction method; it was able to determine both components in the binary mixture without limitations, whereas the ratio subtraction method [11] failed to determine the component with the more extended spectrum (Br in this case).
Regarding simplicity, the proposed ratio difference method showed minimal data manipulation; instead of applying a certain order derivative in the derivative ratio method [7], [8], [9], [10] or subtraction of a constant and re-multiplication by the divisor in the ratio subtraction method, simply the following step will be calculating ΔP at any two wavelengths in the ratio spectrum.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2012.04.004.
Appendix A. Supplementary materials
Supplementary Material
References
- 1.Altinös S., Tekeli D. Analysis of glimepiride by using derivative UV spectrophotometric method. J. Pharm. Biomed. Anal. 2001;24(3):507–515. doi: 10.1016/s0731-7085(00)00445-3. [DOI] [PubMed] [Google Scholar]
- 2.Bonazzi D., Gotti R., Andrisano V. Analysis of ACE inhibitors in pharmaceutical dosage forms by derivative UV spectroscopy and liquid chromatography. J. Pharm. Biomed. Anal. 1997;16(3):431–438. doi: 10.1016/s0731-7085(97)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Challa B.R., Awen B.Z., Chandu B.R. Method development and validation of monteluksat in human plasma by HPLC coupled with ESI-MS/MS: application to a bioequivalence study. Sci. Pharm. 2010;78:711. doi: 10.3797/scipharm.1002-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erk N. Spectrophotometric analysis of valsartan hydrochloride. Anal. Lett. 2002;35(2):283–302. [Google Scholar]
- 5.Gallego J.M. Lemus, Arroyo J. Pérez. Simultaneous resolution of dexamethasone and polymyxin b by spectrophotometry derivative and multivariate methods. Anal. Lett. 2001;34(8):1265–1283. [Google Scholar]
- 6.Nanda R.K., Pangarkar V.B., Thomas A.B. Simultaneous estimation of Montelukast sodium and Bambuterol hydrochloride in tablets by spectrophotometry. Hind. Antibiot. Bull. 2008;49-50(1-4):29–33. [PubMed] [Google Scholar]
- 7.Darwish H.W., Hassan S.A., Salem M.Y. Three different spectrophotometric methods manipulating ratio spectra for determination of binary mixture of Amlodipine and Atorvastatin. Spectrochim. Acta A. 2011;83:140–148. doi: 10.1016/j.saa.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Hassib S.T., El-Zaher A.A., Fouad M.A. Validated stability-indicating derivative and derivative ratio methods for the determination of some drugs used to alleviate respiratory tract disorders and their degradation products. Drug Test. Anal. 2011;3(5):306–318. doi: 10.1002/dta.258. [DOI] [PubMed] [Google Scholar]
- 9.Metwally F.H., El-Zeany B.A., Darwish H.W. New methods for determination of cinnerizine in mixture with piracetam by spectrodensitometry, spectrophotometry, and liquid chromatography. J. AOAC Int. 2005;88(6):1666–1676. [PubMed] [Google Scholar]
- 10.Salinas F., Nevado J.B., Espinosa M.A. A new spectrophotometric method for quantitative multicomponent analysis resolution of mixtures of salicylic and salicyluric acids. Talanta. 1990;37(3):347–351. doi: 10.1016/0039-9140(90)80065-n. [DOI] [PubMed] [Google Scholar]
- 11.El-Bardicy M.G., Lotfy H.M., El-Sayed M.A. Smart stability-indicating spectrophotometric methods for determination of binary mixtures without prior separation. J. AOAC Int. 2008;91(2):299–310. [PubMed] [Google Scholar]
- 12.United States Pharmacopeia Convention Inc.; Rockville: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material