Abstract
The total volatile components were extracted from safflower by ultrasonic-assisted solvent extraction (USE) and their chemical constituents were analyzed by gas chromatography–mass spectrometry (GC–MS) to provide scientific basis for the quality control of safflower. Five different solvents (diethyl ether, ethanol, ethyl acetate, dichloromethane and acetone) were used and compared in terms of number of volatile components extracted and the peak areas of these components in TIC. The results showed that USE could be used as an efficient and rapid method for extracting the volatile components from safflower. It also could be found that the number of components in the TIC of ethyl acetate extract was more than that in the TIC of other solvent ones. Meanwhile, the volatile components of safflower from Xinjiang Autonomous Region and Henan Province of China were different in chemical components and relative contents. It could be concluded that both the extraction solvents and geographical origin of safflower are responsible for these differences. The experimental results also indicated that USE/GC–MS is a simple, rapid and effective method to analyze the volatile oil components of safflower.
Keywords: Safflower, Ultrasonic solvent extraction, Gas chromatography–mass spectrometry (GC–MS)
1. Introduction
Safflower (Carthamus tinctorius L.) belongs to the family Compositae. It is a perennial, broad leaf oil seed and medicinal crop [1] and is widely cultivated in agricultural production system of Asia, Europe, Australia and America as a source of high-quality vegetable and industrial oil [2]. In many oriental countries, safflower is used as a food colorant, dye and herbal medicine all the time [3]. There is a great quantity of polyunsaturated fatty acids in safflower edible oil cultivars. Therefore, it is also used as a feed for livestock [4]. Safflower seeds have been clinically used in Korea as herbal medicine to promote bone formation and prevent osteoporosis for a long time. There are some studies on anti-oxidative compounds from safflower, describing their activity in scavenging free radical species [5], and some researches also indicated that the major safflower seed antioxidants, i.e. serotonin derivatives, could inhibit low-density lipoprotein (LDL) oxidation and atherosclerosis in apolipoprotein E-deficient mice [6], [7], [8], [9], [10].
Essential oils are one of the most important compounds in traditional Chinese medicines (TCMs). Many technologies can be used to extract volatile components of TCMs, such as hydro-distillation (HD), micro-simultaneous distillation extraction (MSDE) [11], [12], vacuum headspace (VHS) [12], supercritical fluid extraction (SFE) [13], [14], thermal desorption (TD) [15], [16], extraction with organic solvents [17], [18], solid-phase micro-extraction (SPME) [19] and ultrasonic-assisted solvent extraction (USE) [20]. Among these methods, USE has more advantages, for example, high extraction efficiency, low equipment cost and ease operation, little or no sample preparation and low extraction temperatures. GC–MS is one of the most widespread analytical techniques in many scientific fields owing to its high sensitivity, low detection limit, rapid identification and being capable of simultaneously analyzing a number of ingredients in analytes; therefore, it is the best appropriate to analyzing the volatile components of safflower.
In the present study, USE was used to extract the volatile components from safflower using five different solvents (diethyl ether, ethanol, ethyl acetate, dichloromethane and acetone), and then these volatile components were identified by GC–MS technique. And also the differences in flavor composition among safflower samples from Xinjiang and Henan of China were investigated.
2. Experimental
2.1. Materials
Sample no. 1 was safflower from Xinjiang Autonomous Region and sample no. 2 was from Henan Province of China. The samples were ground and sieved into particles of 0.25 mm in diameter, and then they were dried at 60 °C for 6 h before use. The organic solvents used in the experiment included diethyl ether, ethanol, ethyl acetate, dichloromethane and acetone, which were extra pure and obtained from Merck Co. (Darmschadt, Germany).
2.2. Extraction procedure
The volatile components of safflower were extracted by USE in an ultrasound cleaning bath as described in literature [21]. Safflower samples were pulverized to uniform powder. The headspace vial was charged with 0.2 g of safflower. 2 mL of different solvents (diethyl ether, ethanol, ethyl acetate, dichloromethane and acetone) were used as the extraction solvent. Sonication was held for 120 min. After sonication, the whole organic extract was cooled at 4 °C. Then the organic layer was introduced in centrifuge tube, and centrifuged at 3500 rpm for 3 min. The resulting extract was dried, concentrated under the low temperature in the water bath and stored at 4 °C in dark place before GC–MS analysis. The obtained average volatile oil yield varied from 4.35% to 13.85%. The sample of 1 μL was used for GC–MS analysis.
2.3. GC–MS analysis
GC–MS (Finnigan TRACE MASS) was used. Volatiles were separated using a capillary column (DB-5MS quartz capillary chromatographic column, 30 m×0.25 μm×0.25 mm). The carrier gas was high-purity helium at a constant flow of 1.0 mL/min and the volume of injection was 2.0 μL. The injector temperature was 270 °C. Oven temperature programming was as follows: initiated at 80 °C, held for 1 min, and then rose at 5.0 °C/min to 250 °C and held for 20 min. Ionization source temperature was kept at 200 °C. Mass spectra were taken at 70 eV and solvent delay 1.5 min. Mass range was in a range of m/z 35–650 amu.
2.4. Statistical treatment of the essential oil
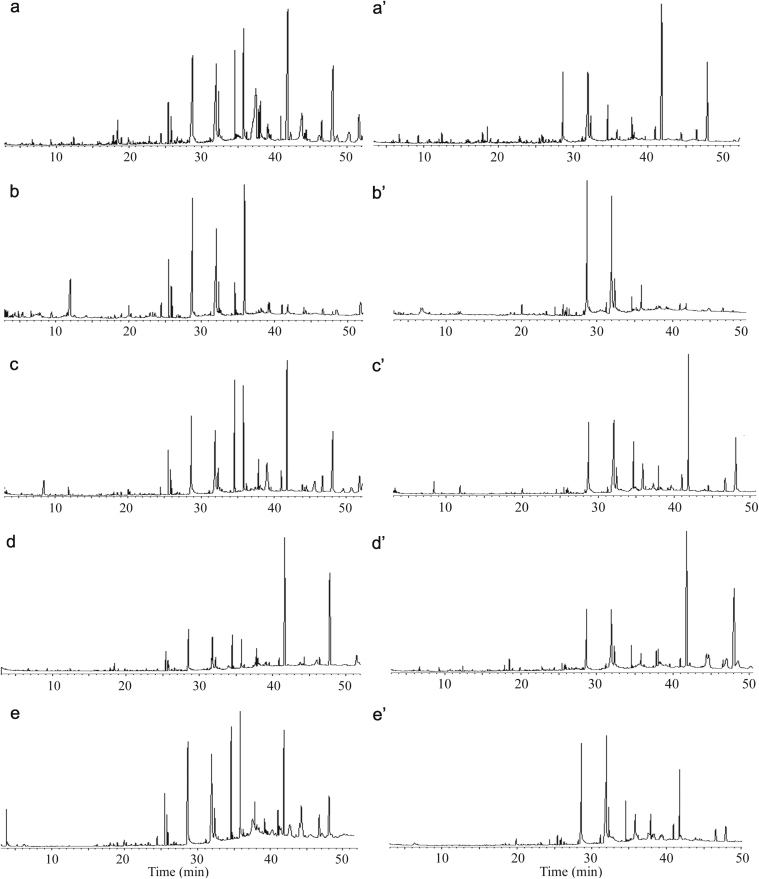
Typical GC–MS total ion chromatograms (TIC) of the volatile oil fraction extracted from safflower samples are shown in Fig. 1. Fig. 1 shows the results of samples from Xinjiang (No. 1) and Henan (No. 2) extracted using diethyl ether, ethanol, ethyl acetate, dichloromethane and acetone. MS data were compared with those in the Xcalibur NIST library to identify the peaks of TIC and determine compositions of the volatile components. Meanwhile, the relative content of each volatile compound was calculated by a ratio of the peak area of each component to total area of all peaks in TIC. And these results are listed in Table 1.
Figure 1.
GC–MS total ion chromatograms of essential oil in safflower from Xinjiang (No.1, a–e) and Henan (No. 2, a′–e′) using five different extraction solvents: a, a′, diethyl ether; b, b′, ethanol; c, c′, ethyl acetate; d, d′, dichloromethane; e, e′, acetone.
Table 1.
Compositions of the volatile component in safflower from Xinjiang (No. 1) and Henan (No. 2) using five different extraction solvents.
| No. | Retention time | Name of compounds | Molecular weight | Molecular formula | Content (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 |
No. 2 |
|||||||||||||
| a | b | c | d | e | a | b | c | d | e | |||||
| 1 | 11.97 | 5-hydroxymethyl-2-Furancarboxaldehyde | 126 | C6H6O3 | – | 8.41 | 0.66 | – | – | – | – | 0.9 | – | – |
| 2 | 18.49 | 2,4-bis(1,1-dimethylethyl)- phenol | 206 | C14H22O | 1.05 | – | – | – | – | 1.46 | – | – | 1.07 | – |
| 3 | 24.48 | Tetradecanoic acid | 228 | C14H28O2 | – | 1.48 | 0.72 | – | – | – | – | – | – | – |
| 4 | 25.8 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 296 | C20H40O | 0.81 | 2.12 | 1.43 | – | 1.58 | – | – | – | – | – |
| 5 | 25.97 | 6,10,14-trimethyl-2-Pentadecanone | 268 | C18H36O | – | 0.83 | – | – | 0.57 | – | – | – | – | – |
| 6 | 28.79 | n-Hexadecanoic acid | 256 | C16H32O2 | 11.3 | 24.9 | 11 | 8 | 12.2 | 13.1 | 31.7 | 15.2 | 10.9 | 17.9 |
| 7 | 31.94 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid methyl ester | 292 | C19H32O2 | 5.14 | 9.49 | 3.84 | 5.68 | 4.61 | 14.6 | 30.3 | – | 12.6 | – |
| 8 | 32.02 | (Z,Z)-9,12-Octadecadienoic acid | 280 | C18H32O2 | 4.81 | 13.2 | 6.19 | – | 8.2 | – | – | 17.4 | 2.14 | 22.2 |
| 9 | 32.4 | Octadecanoic acid | 284 | C18H36O2 | 2.8 | 2.97 | 1.6 | 1.31 | 2.03 | – | 3.73 | 2.08 | – | 2.91 |
| 10 | 34.59 | Heneicosane | 296 | C21H44 | 3.04 | 2.19 | 5.71 | 3.3 | 5.3 | 2.6 | 1.41 | 3.9 | 1.52 | 2.77 |
| 11 | 35.83 | 7,9-Docosanedione | 338 | C22H42O2 | 5.47 | 11.4 | 7.18 | 3.64 | 8.14 | – | 4.2 | 4.38 | 1.31 | 2.96 |
| 12 | 37.83 | Pentacosane | 352 | C25H52 | – | – | 1.61 | 1.74 | 1.34 | 1.95 | – | 1.92 | – | 2.07 |
| 13 | 38.11 | 2-hydroxy-1-(hydroxymethyl)ethyl hexadecanoic acid ester | 330 | C19H38O4 | 1.97 | – | – | – | – | – | – | – | 2.05 | – |
| 14 | 39.13 | Octacosane | 394 | C28H58 | – | – | 6.41 | – | – | – | – | – | – | – |
| 15 | 41.86 | Nonacosane | 408 | C29H60 | 10.1 | 1.62 | 13.6 | 22.3 | 8.33 | 19.9 | 1.35 | 20.5 | 18.5 | 8.48 |
| 16 | 45.64 | Stigmasta-5,22-dien-3-ol | 412 | C29H48O | – | – | 2.93 | – | – | – | – | – | – | – |
| 17 | 46.57 | γ-Sitosterol | 414 | C29H50O | 1.58 | – | 2.02 | – | 2.5 | 1.95 | – | 2.35 | – | 2.1 |
| 18 | 47.87 | Dotriacontane | 450 | C32H66 | – | – | – | 28.1 | – | 19.9 | – | – | – | 2.97 |
| 19 | 48.13 | Nonacosanol | 424 | C29H60O | 10.8 | – | 10.1 | – | 6.38 | – | – | 12.5 | 18.5 | – |
| 20 | 50.33 | β-Amyrin | 426 | C30H50O | 2.03 | – | – | – | – | – | – | – | – | – |
a, diethyl ether; b, ethanol; c, ethyl acetate; d, dichloromethane; e, acetone.
3. Results and discussion
In recent years, USE has been used to isolate bioactive compounds from plant materials using organic solvents, for example, aroma compounds from aromatic plants and foods at room temperature, and volatile compounds from TCMs [20], [22], [23], [24]. The USE method has many advantages as mentioned above, and specially can make sample matrix efficiently contact with solvent. Mechanical action, thermal action and acoustic cavitations all have direct effect on the efficiency of ultrasonic extraction [25]. But acoustic cavitation is the most significant factor. Owing to the action of ultrasound irradiation, micro-bubbles will be formed when the negative pressure is high enough. Once the bubbles are generated, they will grow during the period of negative pressure and will be compressed during the period of positive pressure. The expansion and compression actions can cause constant pulsating or violent collapsing of micro-bubbles. When the collapse occurs near solid surface it can cause shockwave that passes through the solvent. Therefore, it can damage the cell walls to facilitate the release of contents [26]. The main advantages of USE method are high extraction efficiency, low equipmental cost, ease operation, little or no sample preparation and low extraction temperature. Hence, ultrasonic assistance is used more and more in analytical chemistry, and applied to different steps in the analytical process, particularly in sample preparation [27], [28].
3.1. Comparison of the extraction solvents
In order to compare the extraction ability of different solvents, the number of components extracted from safflower and the peak areas of these components in TIC were investigated. Because the polarity, molecular weight(MW) and viscosity of solvents have an important effect on their extraction actions, several kinds of extraction solvents were selected and tested, such as diethyl ether (MW=74.12 g/mol, dipole moment=1.15 D), ethanol (MW=46.07 g/mol, dipole moment=1.69 D), ethyl acetate (MW=88.105 g/mol, dipole moment=1.78 D), dichloromethane (MW=84.93 g/mol, dipole moment=1.14 D) and acetone (MW=58.08 g/mol, dipole moment=2.91 D). The results are listed in Table 1. The results showed that not only the components extracted by different solvents from the same sample were different, but also the components extracted by the same solvents from the samples of different geographical origins were different. Meanwhile, it also could be found that the number of components in the TIC of ethyl acetate extract was more than that in the TIC of other solvent extracts. The main reason may be that owing to the action of acoustic cavitations mechanism, the efficiency of bubble formation is different in extraction solvents with different viscosities. Therefore, viscosity of the solvent plays the major role in the efficiency of bubble formation.
3.2. Characterization of the volatile components
The chemical composition of the volatile components of safflower obtained by the USE method was determined by GC–MS. As shown in Table 1, n-Hexadecanoic acid, Heneicosane and Nonacosane were found in all samples. 7,9-Docosanedione was found in nine samples. Octadecanoic acid and (Z,Z,Z)-9,12,15-Octadecatrienoic acid methyl ester were found in eight samples. Pentacosane and Nonacosanol were found in six samples. (Z,Z)-9,12-Octadecadienoic acid was found in five samples. These compounds represent the constituents found with the highest frequency. Furthermore, the samples from Xinjiang showed the presence of tetradecanoic acid, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, and 6,10,14-trimethyl-2-pentadecanone. These components were only present in Xinjiang safflower, but not found in samples from Henan. Safflower samples from these two cultivation sites had some flavor peculiarities due to these unusual components. On the basis of these results, safflower samples from Xinjiang and Henan could be rapidly identified according to differences of these components. It is important to note that although the extraction procedure for the samples is the same the extraction solvents are different. It could be concluded that both geographical origin of safflower and the extraction solvents are responsible for these differences. In summary, it can be said that the true nature of safflower aroma probably involves contributions from most of the separated and identified components in this study.
4. Conclusion
In this study, we successfully developed a USE/GC–MS method for the determination of volatile components in safflower samples from Xinjiang and Henan of China. It was found that there exist differences between the compositions of the two kinds of samples. This could be due to the differences in the geographical origin of safflower and/or the extraction solvents. Compared with conventional methods, the experimental results demonstrate that USE/GC–MS is a simple, rapid, reliable and low-cost method for the determination of volatile fraction in Chinese herb.
References
- 1.Park S.D., Park K.S., Kim K.J. Effect of sowing time on development of safflower anthracnose disease and degree of resistance in various cultivars. J. Phytopathol. 2005;153(1):48–51. [Google Scholar]
- 2.Radhika K., Sujatha M., Rao T.N. Thidiazuron stimulates adventitious shoot regeneration in different safflower explants. Biol. Plant. 2006;50(2):174–179. [Google Scholar]
- 3.Kim H.J., Bae Y.C., Park R.W. Bone-protecting effect of safflower seeds in ovariectomized rats. Calcif. Tissue Int. 2002;71(1):88–94. doi: 10.1007/s00223-001-1080-4. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal D., Raina S.N. Genotyping safflower (Carthamus tinctorius) cultivars by DNA fingerprints. Euphytica. 2005;146(1–2):67–76. [Google Scholar]
- 5.Kanehira T., Takekoshi S., Nagata H. A novel and potent biological antioxidant, Kinobeon A, from cell culture of safflower. Life Sci. 2003;74(1):87–97. doi: 10.1016/j.lfs.2003.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Cho M.H., Hahn T.R. Purification and characterization of precarthamin decarboxylase from the yellow petals of Carthamus tinctorius L. Arch. Biochem. Biophys. 2000;382(2):238–244. doi: 10.1006/abbi.2000.1984. [DOI] [PubMed] [Google Scholar]
- 7.Koyama N., Kuribayashi K., Seki T. Serotonin derivatives, major safflower (Carthamus tinctorius L.) seed antioxidants, inhibit low-density lipoprotein (LDL) oxidation and atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2006;54(14):4970–4976. doi: 10.1021/jf060254p. [DOI] [PubMed] [Google Scholar]
- 8.Karapanagiotis I., Chryssoulakis Y. Investigation of red natural dyes used in historical objects by HPLC-DAD-MS. Ann. Chim. 2006;96(1–2):75–84. doi: 10.1002/adic.200690008. [DOI] [PubMed] [Google Scholar]
- 9.Hai L.N., Chen Y.G., Liao X.R. Fatty acid comparision of safflower seed oil in different cultivation. J. Yunnan Normal Univ. 2004;24(1):53–54. (in Chinese) [Google Scholar]
- 10.Zhao M.B., Deng X.L., Wang Y.L. Establishment of chromatographic fingerprint and quality assessment of Carthamus tinctorius L. by high performance liquid chromatography. Acta Pharmacol. Sin. 2004;39(3):212–216. (in Chinese) [PubMed] [Google Scholar]
- 11.Rodel W., Petrzika M. Analysis of the volatile components of saffron. J. High-Resolution Chromatogr. 1991;14(11):771–774. [Google Scholar]
- 12.Tarantilis P.A., Polissiou M.G. Isolation and identification of the aroma components from saffron (Crocus sativus) J. Agric. Food Chem. 1997;45(2):459–462. [Google Scholar]
- 13.Lozano P., Delgado D., Gomez D. A non-destructive method to determine the safranal content of saffron (Crocus sativus L.) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography. J. Biochem. Biophys. Methods. 2000;43(1–3):367–378. doi: 10.1016/s0165-022x(00)00090-7. [DOI] [PubMed] [Google Scholar]
- 14.Zougagh M., Ríos A., Valcárcel M. Determination of total safranal by in situ acid hydrolysis in supercritical fluid media: application to the quality control of commercial saffron. Anal. Chim. Acta. 2006;578(2):117–121. doi: 10.1016/j.aca.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 15.Alonso G.L., Salinas M.R., Esteben-Infants F.J. Determination of safranal from saffron (Crocus sativus L.) by thermal desorption-gas chromatography. J. Agric. Food Chem. 1996;44(1):185–188. [Google Scholar]
- 16.Carmona M., Martínez J., Zalacain A. Analysis of saffron volatile fraction by TD-GC–MS and e-nose. Eur. Food Res. Technol. 2006;223(1):96–101. [Google Scholar]
- 17.Tarantilis P.A., Polissiou M., Manfait M. Separation of picrocrocin, cis–trans-crocins and safranal of saffron using high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. A. 1994;664(1):55–61. doi: 10.1016/0021-9673(94)80628-4. [DOI] [PubMed] [Google Scholar]
- 18.Zarghami N.S., Heinz D.E. Monoterpene aldehydes and isophorone-related compounds of saffron. Phytochemistry. 1971;10(11):2755–2761. [Google Scholar]
- 19.D'Auria M., Mauriello G., Rana G.L. Volatile organic compounds from saffron. Flavour Fragrance J. 2004;19(1):17–23. [Google Scholar]
- 20.Kanakis C.D., Daferera D.J., Tarantilis P.A. Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-cyclohexene-1-carboxaldehyde (HTCC) in Greek saffron. J. Agric. Food Chem. 2004;52(14):4515–4521. doi: 10.1021/jf049808j. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z.Y., Ru S.G., Zhu Q.S. Research on ultrasound-assisted extraction of volatile oil from Patchouli. Chem. Bioeng. 2010;27(6):85–87. (in Chinese) [Google Scholar]
- 22.Pan J., Xia X.X., Liang J. Analysis of pesticide multi-residues in leafy vegetables by ultrasonic solvent extraction and liquid chromatography-tandem mass spectrometry. Ultrason. Sonochem. 2008;15(1):25–32. doi: 10.1016/j.ultsonch.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Jerkovic I., Mastelić J., Marijianović Z. Comparison of hydrodistillation and ultrasonic solvent extraction for the isolation of volatile compounds from two unifloral honeys of Robinia pseudoacacia L. and Castanea sativa L. Ultrason. Sonochem. 2007;14(6):750–756. doi: 10.1016/j.ultsonch.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Alissandrakis E., Daferera D., Tarantilis P.A. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003;82(4):575–582. [Google Scholar]
- 25.Romanik G., Gilgenast E., Przyjazny A. Techniques of preparing plant material for chromatographic separation and analysis. J. Biochem. Biophys. Methods. 2007;70(2):253–261. doi: 10.1016/j.jbbm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y., Zhang F. Ultrasound-assisted extraction of rutin and quercetin from Euonymus alatus (Thunb.) Sieb. Ultrason. Sonochem. 2008;15(4):308–313. doi: 10.1016/j.ultsonch.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Priego-Capote F., de Castro L. Analytical uses of ultrasound—I. Sample preparation. Trends Anal. Chem. 2004;23(9):644–653. [Google Scholar]
- 28.Aydin M.E., Tor A., Özcan S. Determination of selected polychlorinated biphenyls in soil by miniaturised ultrasonic solvent extraction and gas chromatography-mass-selective detection. Anal. Chim. Acta. 2006;577(2):232–237. doi: 10.1016/j.aca.2006.06.050. [DOI] [PubMed] [Google Scholar]