Abstract
In China, traditional Chinese medicines (TCMs) have been used in clinical applications for thousands of years. The successful hyphenation of high-Performance liquid chromatography (HPLC) and mass spectrometry (MS) has been applied widely in TCMs and biological samples analysis. Undoubtedly, HPLC/MS technique has facilitated the understanding of the treatment mechanism of TCMs. We reviewed more than 350 published papers within the last 5 years on HPLC/MS in the analysis of TCMs. The present review focused on the applications of HPLC/MS in the component analysis, metabolites analysis, and pharmacokinetics of TCMs etc. 50% of the literature is related to the component analysis of TCMs, which show that this field is the most populär type of research. In the metabolites analysis, HPLC coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry has been demonstrated to be the powerful tool for the characterization of structural features and fragmentation behavior patterns. This paper presented a brief overview of the applications of HPLC/MS in the analysis of TCMs. HPLC/MS in the fingerprint analysis is reviewed elsewhere.
Keywords: traditional Chinese medicines (TCMs), HPLC/MS, component analysis, metabolites analysis, pharmacokinetics
Contents
| Abbrevations | Full names |
|---|---|
| 2D-prep-HPLC-DAD | Two dimensional preparative high-performance liquid chromatography-diode array detector |
| ESI-FfICR-MS/MS | Electrospray ionization Fourier-transform ion cyclotron resonance tandem mass spectrometry |
| ESI-FfICR-MS | Electrospray ionization Fourier-transform ion cyclotron resonance mass spectrometry |
| ESI-Msn | Multistage electrospray ionization mass spectrometry |
| GC-MS | Gas chromatography-mass spectrometry |
| HPLC/APCI-MS/MS | High-performance liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry |
| HPLC/APCI-MS | High performance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry |
| HPLC-DAD/APCI-IT-MS | High-performance liquid chromatography-diode array detector/atmospheric pressure chemical ionization ion trap mass spectrometry |
| HPLC/ESI-IT-TOF/MS | High-performance liquid chromatography/electrospray ionization-ion trap-time-of-flight mass spectrometry |
| HPLC/ESI-MS/MS | High-performance liquid chromatography/electrospray ionization tandem mass spectrometry |
| HPLC/ESI-MS | High-performance liquid chromatography/electrospray ionization mass spectrometry |
| HPLC/ESI-Q-TOF-MS/MS | High-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry |
| HPLC/MS/MS | High-performance liquid chromatography/tandem mass spectrometry |
| HPLC/MS | High-performance liquid chromatography/mass spectrometry |
| HPLC-DAD/ESI-MSn | High-performance liquid chromatography-diode array detector/tandem electrospray ionization mass spectrometry |
| HPLC-DAD/MS | High performance liquid chromatography-diode array detector/mass spectrometry |
| HPLC-PDA | High-performance liquid chromatography-photo diode array |
| HPLC/TOF-MS | High performance liquid chromatography/time-of-flight mass spectrometry |
| HPLC/IT-Msn | High-performance liquid chromatograph!ion trap multistage mass spectrometry |
| MALDI-MS | Matrix-assisted laser desorption ionization mass spectrometry |
| Nano-LC-ESVMS | Nano-liquid chromatography-electrospray ionization mass spectrometry |
| TFC-HPLC/MS | Turbulent -flow chromatography-high-performance liquid chromatography/mass spectrometry |
| UPLC/HDMS | Ultra-performance liquid chromatography/high definition mass spectrometry |
| UPLC/MS/MS | Ultra-performance liquid chromatography/tandem mass spectrometry |
| UPLC/MS | Ultra-performance liquid chromatography/mass spectrometry |
| UPLC/Q-TOF/MS | Ultra-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry |
1. Introduction
It is well known that traditional Chinese medicines (TCMs) have been used in clinical practice for thousands of years. The biologically active ingredients of these compounds play a role in their efficacy. However, TCMs comprise a com-plex mixture of different components and the active ingre-dient content is usually very low. Therefore, it is extremely difficult to study TCMs based on their components.
HPLC/MS combining the Separation of components with quantitative analysis or qualitative identification provides an effective means of analyzing complex samples, and has been one of the most significant Chromatographie technolo-gies of the 21st Century. Therefore, HPLC/MS was applied into TCMs research to identify the material basis of TCMs and understand the action mechanism of TCMs.
In the past 5 years, more than 350 papers have been pub-lished in international Journals on HPLC/MS analysis of TCMs, and the tendency is increasing gradually (Figure 1). This trend also reflects the advantages of HPLC/MS in sol-ving complex problems in TCMs. In the past two years, several comprehensive reviews have been published covering the majority of original publications. Yang [1] provided an overview which focused on the phytochemical analysis of
Figure 1.
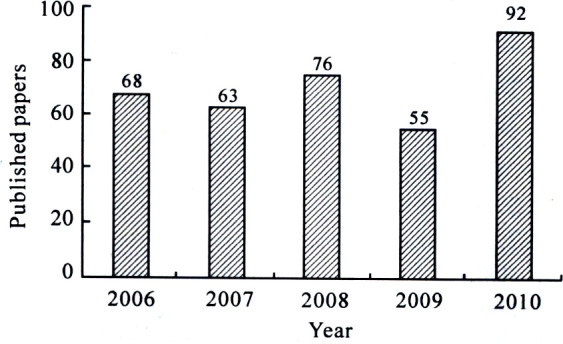
The annual distribution of the published papers on the analysis of TCMs by HPLC/MS
TCMs using HPLC/MS. The review indicated that HPLCI MS technique facilitated the convenient and rapid quality control of traditional medicines and their pharmaceutical preparations. Last year, Gray [2] reviewed the development of HPLC/MS and tandem MS/MS for the analysis of bioactive components and their metabolites of herbal medicines in biological fluids. In 2009, Li [3] and Zhang [4] described recent progress in the chemical analysis of Danshen and Gancao, respectively. Li described various analytical methods and their chromatographic conditions and compared their advantageldisadvantages. Zhang [5] also summarized the newly established methods. Last year, Zhang summarized some of the applications of metabolomics in Special TCMs issues with an emphasis on metabolic biomar-ker discovery. This will facilitate our understanding of the mechanism of action of TCMs formulae and the analysis of Chinese herbal medicines.
In this paper, we reviewed the published papers in international Journals on applications of HPLC/MS in the analysis of TCMs, such as component analysis, metabolites analysis, and pharmacokinetics of TCMs (Table 1).
Table 1.
The distribution of published papers on applications of HPLC/MS in the analysis of TCMs
| TCMs | |||||
|---|---|---|---|---|---|
| Analytical Contents | Active ingredients | Chinese materia medica | TCMs prescription | Others | Total |
| Component analysis | 6 | 109 | 29 | 25 | 169 |
| Metabolites analysis | 20 | 17 | 18 | 4 | 59 |
| Pharmacokinetics | 29 | 9 | 14 | 0 | 52 |
| Quality control | 0 | 40 | 15 | 1 | 56 |
| Synthetic adulterants | 0 | 0 | 11 | 0 | 11 |
| Metabolomics | 1 | 3 | 2 | 0 | 6 |
| Total | 353 | ||||
2. Component analysis of TCMs
50% of the literature is related to the component analysis of TCMs, which show that this field is the most populär type of research. The main focus is on: firstly, the identifica-tion of new compounds and their qualitative and quantitative method development; secondly, establishment of new technology for the rapid and simultaneous determination of multiple similar structural trace components.
2.1. Active ingredients of TCMs
In the analysis of the chemical components of TCMs, HPLC/MS technique is usually used for the Separation and identification of a variety of similar structural compounds, and mass spectrometry is an important qualitative tool.
Jayaprakasam et al. [6] identified five flavonoids (liquiritin, liquiritigenin, isoliquiritigenin, 7, 4’-dihydroxy-flavone, and isoononin) from G. uralensis using nuclear magnetic resonance (NMR) and HPLC/MS. They also tested the potential activity of these isolated pure compounds and glycyrrhizin to inhibit the secretion of eotaxin-1 by human fetal lung fibroblasts (HFL-1). Liquiritigenin, isoliquiritigenin, and 7, 4’-dihydroxyflavone were more effective than liquiritin, isoononin, and glycyrrhizin in sup-pressing eotaxin-1 secretion. Zhao et al. [7] developed an HPLC/ESI-MS/MS method for the Separation, determination, and identification of eight pairs of diastereoisomers of podophyllotoxin and its esters. The method could be used to rapidly identify the purity and monitor the epimerization of 2-H of podophyllotoxin and its analogues from natural products, chemical reactions, and pharmaceutical metabo-lism.
2.2. Active parts of TCMs
Compared with the traditional plant chemical “purification-identification” research mode, the HPLC/MS method has shown high efficiency in the Separation, identification and determination analysis of non-volatile components of TCMs, especially in micro and trace component analyses. Furthermore, some of the ingredients not identified by traditional methods have been found and their structures have been rapidly identified by HPLC/MS. 65% of the research topics in the international literature are related to the component analysis of TCMs, particularly Chinese herbal extracts. The classification distribution according to the structure of components is shown in Figure 2.
Figure 2.
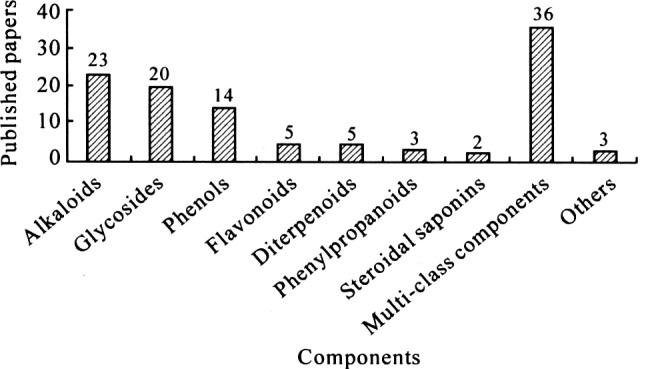
The distribution of the published papers on the component analysis of TCMs by HPLC/MS
2.2.1. Alkaloids
Alkaloids are one of the most important classes of compounds in natural products with biological activity. They are also used as indicators of active ingredients or toxic components in TCMs. Due to the varied structures of alkaloids, HPLC/MS is the most important technique in the qualitative and quantitative analysis of alkaloids, and its application has been very extensive.
Aconitine is an important toxic alkaloid and has been widely studied. Wang et al. [8] developed a MALDI-MS method and semi-qualitatively profiled the alkaloids in the Chinese herbal medicine Fuzi. Liu et al. [9] developed an HPLC/ESI-MS/MS method to separate and identify 32 ac-onitum lipo-alkaloids (LDAs) from three herbs of Aconi-tum genus. Yue et al. [10] studied aconitine-type alkaloids in the Chinese herb Aconitum carmichaeli by HPLC/ESI-MS/MS and ESI-FTICR-MS in positive ion mode. 111 compounds were identified including 11 monoester-diterpe-noid alkaloids (MDA), 10 diesterditerpenoid alkaloids (DDA) and 81 lipo-alkaloids.
There is a rather Special category in the HPLC/MS literature that combines HPLC/MS technique and receptor affinity chromatography or cell affinity screening technology to screen the active ingredients in TCMs. Wang et al. [11] developed an online analytical method that combined alpha
(lA)-adrenoceptor (alpha(lA)AR) cell membrane chro-matography (alpha(lA)AR-CMC) with HPLC/MS for the identification of active ingredients from Radix caulophylli acting on the human alpha(lA)AR. Jong et al. [12] pres-ented an HPLC/MS methodology for the screening of ace-tylcholinesterase (AChE) inhibitors in a crude extract of Narcissus cv “Bridal Crown” bulbs. Yuan et al. [13] cou-pled cell affinity screening (CAS) with HPLC/MS to screen the bioactive compounds related to cardiovascular diseases from the alkaloid extract derived from Aconitum szecheny-ianum Gay.
Zhou et al. [14] developed an HPLC/ESI-Q-TOF-MS/ MS method to investigate the primary steroidal alkaloids in the extracts of eight major Fritillaria species. 41 steroidal alkaloids were selectively identified according to their MS/ MS data and logical fragmentation pathways. Alali et al. [15] used both HPLC/MS and HPLC-PDA techniques to investigate the alkaloid rieh fraction of Colchicum braehy-phyllum Boiss. & Haussk. ex Boiss. (Colchicaceae). The spectral data of the compounds were not matched with that of the compounds isolated previously from this species or with any other colchicinoid; hence the new compounds should be pursued further.
2.2.2. Sugar and glycosides
In the study of sugar and glycosides, HPLC/MS technique showed good qualitative ability for isolating and identifying structural similar glycosides simultaneously, and provided a reliable basis for identification of different sources of Chinese herbal medicines. Zhou et al. [16] used HPLC/ ESI-Q-TOF-MS/MS in positive mode to investigate the fragmentation behavior of four sulfur-containing iridoid glucosides isolated from Paederia scandens and to elueidate the main fragmentation pathways of these compounds. Lee et al. [17] developed an HPLC/ESI-Q-TOF-MS/MS method in negative-ionization mode to determine 12 intact glucosinolates-glucoiberin, glucocheirolin, progoitrin, sinigrin, epiprogoitrin, glucoraphenin, sinaibin, gluconapin, glucosibarin, glucotropaeolin, glucoerucin, and gluconas-turtiin in 10 traditional Chinese herbs. Analysis of the glu-cosinolates provided scientific evidence enabling differentia-tion of three pairs of easily confused plants. Kite et al. [18] studied the major flavonoids in fruits and seeds of Styphnolobium japonicum (L.) Schott (syn. Sophora ja-ponica L) by HPLC/MS and other spectroscopic techniques, and found two previously unreported kaempferol glycosides.
Zhang et al. [19] developed an HPLC/ESI-MS/MS method to simultaneously identify and quantify 6 predomi-nant steroidal saponins in the rhizomes of Paris polyphylla var. yunnanensis and P. polyphylla var. chinensis, which are the qualified plants of “Chonglou” in Chinese. Dong et al. [20] established an ESI-FTICR-MS/MS method to investigate the isomers paeoniflorin and albiflorin in the extracts of the TCMs Paeonia lactiflora Pall. Qi et al. [21] developed a method of HPLC/ESI-Q-TOF-MS/MS to char-acterize ten major pregnane glycosides including one novel compound auriculoside IV from the roots of Cynanchum auriculatum Royle ex Wight when there were no reference compounds available. Xie et al. [22] used UPLC/Q-TOF/ MS and multivariate statistical analysis to analyze 5 medici-nal Panax herbs including Panax ginseng (Chinese ginseng), P. notoginseng (Sanchi), P. japonicus (Rhizoma Panacis Majoris), P. quinquefolium L. (American ginseng), and P. ginseng (Korean ginseng). Results indicated that the proposed method is applicable in the differentiation of complex samples that share similar chemical ingredients.
2.2.3. Phenols
Phenolic compounds are the main antioxidant ingredients in many medicinal plants. Analysis and identification of phenolic compounds are important in the research of screening antioxidant components in TCMs.
Han et al. [23] reported 40 phenolic compounds from Artemisia annua using HPLC-DAD/ESI-MS”. C-glycosyl flavonoids were reported from A. annua for the first time and were found to be a new type of main ingredient, and may be responsible for its antioxidant and antiviral activity. Quinic aeid derivatives were also found to be major ingredients of A. annua. Liu et al. [24] used HPLC-DAD/ESI-MS” in negative ion mode to analyze 11 phenolic aeids isolated from Danshen. Lee et al. [25] developed HPLC/PDA with confirmation of analyte identity by negative-ion ESI-MS/ MS for determination of honokiol and magnolol in Hou Po (Magnolia officinalis). Hu et al. [26] used microwave-assisted extraction (MAE) and nano-LC-ESI/MS to determine and identify the chlorogenic aeid (CA) in Honeysuckle.
2.2.4. Flavonoids
Wang et al. [27] established an HPLC-DAD-MS/MS method for screening and structural identification of the main ingredients in the crude extract of Fructus aurantii Immaturus, and 5 components were preliminarily identified as neoeriocitrin, narirutin, naringin, hesperidin and neo-hesperidin according to their UV and mass spectra. Han et al. [28] developed a bioactive lead compound screening System, composed of high-speed counter-current chroma-tography and HPLC/ESI-Q-TOF-MS/MS. They sueeeeded in discovering apoptosis inducers from gamboge, the resin of Garcinia hanburyi. Furthermore, gambogenic aeid was identified as the lead compound. Zhao et al. [29] established an off-line 2-D RPLC/RPLC-Q/TOF/MS method for the Separation of components in Dalbergia odorifera T. Chen. (Jiangxiang). In total, 637 peaks were separated in 114 fractions from the extraction of Jiangxiang. In addi-tion, 19 flavonoids were tentatively identified from 114 fractions with Q-TOF/MS. The results showed the Separation power of this two dimensional liquid chromatography System.
2.2.5. Terpenes
Yang et al. [30] developed an HPLC/PDA/ESI-MS/MS method for the rapid analysis of germacrane sesquiterpene lactones in the aerial part of E. lindleyanum. 9 germacrane sesquiterpene lactones were identified by a comparison of their characteristic data on HPLC and MS analyses with those obtained from reference compounds. Liu et al. [31] established a UPLC/Q-TOF-MS method for analysis of protostane triterpenoids in Alisma orientalis (Sam.) Juzep. A total of 20 protostane triterpenoids including 19 known compounds and a new compound were well separated within 7 min. Inbaraj et al. [32] developed an HPLC-DAD/ APCI-IT-MS method for qualitative and quantitative analysis of carotenoids in fruits of Lycium barbarum Linnaeus. Huang etal. [33] used an HPLC/APCI-MS method for the determination of Chlorophylls and their derivatives in Gy-nostemma pentaphyllum Makino, a traditional Chinese herb possessing vital biological activities.
2.2.6. Phenylpropanoids
Ahn et al. [34] developed an HPLC-DAD/ESI-MS” method for the simultaneous determination of 9 coumarin compounds in the Korean medicinal herb, Cham-Dang-Gui, the dried root of Angelica gigas (Umbelliferae). Xie et al. [35] used HPLC-DAD/ESI-MS/MS to analyze the active coumarin components in Radix angelicae dahuricae (AE), and 10 coumarins have been identified. Five of them including xanthotoxol, osthenol, oxypeucedanin hydrate, bya-kangelicin and imperatorin were deemed as target ingredi-ents for the preparative Isolation through a 2D-prep-HPLC-DAD System.
2.2.7. Steroid saponins
Huang et al. [36] first reported P-sitosterol, stigmasterol and ergosterol coexisting in A. roxburghii herbs which were simultaneously identified and determined by an HPLC/APCI-MS method. Liu et al. [37] used UPLC/ESI-Q-TOF/ MS to analyze the toad Bufo bufo gargarizans Cantor (toad skin). A total of 39 bufadienolides were screened out.
2.2.8. Multi-class components
In the application of HPLC/MS technique for the analysis of multi-class components from Chinese herbal medicines, many types of components can be analyzed and identified by HPLC due to its powerful Separation ability. Don et al. [38] used HPLC/MS/MS to simultaneously separate and identify 6 main polyphenolic ingredients and four major abietane-type diterpenes from the dried rhizome of Salvia miltiorrhiza Bunge (Danshen) by comparing their retention time, MS and MS2 data with those obtained from the authentic compounds. Huang et al. [39] also identified 15 major bioactive ingredients from the dried seeds of Oleaceae plants (Forsythiae fructus) by HPLC/MS. Kao et al. [40] developed an HPLC/ESI-Q-TOF/MS method to determine saponins and flavonoids in Gynostemma pentaphyllum (Thunb.) Makino.
2.3. The prescriptions of TCMs
The prescriptions of TCMs including the traditional prescription and the modern prescription is more complicated than the Single herb medicine in components. The contents of the TCMs components may be changed during the prepa-ration process or new compounds may be generated due to their interaction. Therefore, HPLC/MS has been widely used in Chinese prescription composition analysis due to its rapid and efficient isolation and identification capabilities.
2.3.1. Traditional prescriptions
As a rapid qualitative analytical technique, HPLC/MS was used by Liu for complex high-throughput screening of sam-ples, which combined an off-line two-dimensional liquid chromatography, and HPLC-DAD/MS was used to analyze Chinese herbal formulas including Qixuebingzhi Formula, an efficient Chinese herbal formula for treating atheroscle-rosis. The medium-and low-polar extracts (MLPE) of the Chinese herbal formulas were separated and implemented in the production of semi-purified mixture libraries. Several bioactive compounds were quickly identified from this li-brary through the screening and dereplication process [41]. Wen et al. [42] developed microdialysis coupled with HPLC-DAD/MS to study the interaction of a prescription of Danggui Buxue Decoction (CPDBD) with proteins, and 8 compounds were identified which possessed potential activities. Wang et al. [43] developed HPLC-DAD/ESI-MSn to identify and characterize the flavonoids in a Chinese for-mulated preparation, Longdan Xiegan Decoction (LXD). In total, 51 flavonoids were characterized. Yan et al. [44] used UPLC/Q-TOF/MS for the global detection of aconi-tum alkaloids in Yin Chen Si Ni Tang.
2.3.2. Modern prescriptions
Zheng et al. [45] developed a diagnostic fragment-ion-based extension strategy (DFIBES) and HPLC/ESI-IT-TOF/MS method, and more than 30 ginsenosides and 20 lignans have been rapidly detected and identified from Shengmai Injection. Zhang et al. [46] used HPLC/TOF-MS and HPLC/IT-MS” for screening and identification of multi-components in TCMs, and 33 ingredients from Qing-kailing Injection were identified. This study is expected to provide an effective and reliable pattern for the compre-hensive and systematic characterization of TCMs.
2.4. Others
Han etal. [47,48] developed a UPLC-MS/MS method for the simultaneous determination of 5 type B trichothecenes and 6 aflatoxins Bi, B2, G], G2, Mi and M2 in TCMs. Li-au et al. [49] used an integrated method combining super -critical fluid extraction (SFE) with HPLC/APCI-MS/MS to quantify aflatoxins (AFs) in Zizyphi Fructus (fruits of Zizyphus jujube), a traditional Chinese medicine.
3. Metabolites analysis of TCMs
HPLC/MS technique combining high Performance liquid chromatography which has powerful Separation capacity with mass spectrometry detection which has unique struc-tural analysis capacity, has unparalleled high sensitivity and selectivity. This technique is a fast, trace, specific and accurate analytical tool and is one of the most effective methods for identification of metabolites, and has become a powerful analytical tool in the metabolic research of TCMs. One of the notable features of domestic and international research is that the active ingredients and active metabolites were characterized by studying the composition and metabolic products in the body of the prescription or extract.
30% of the literature reported the application of HPLC/ MS in the analysis of metabolites of Chinese herbal medici-nal ingredients. In the past 5 years, the use of HPLC/MS in the analysis of metabolites of Chinese herbal medicinal ingredients included the following aspects: (1) identification of metabolites; (2) determination of plasma concentra-tions of metabolites; (3) analysis of the metabolic pathways of TCMs and metabolic processes based on the metabolites; (4) analysis of the relationship between the metabolites and metabolic enzymes; (5) analysis of metabolites by the side effects of Chinese medicines and pharmacological mecha-nisms.
3.1. Metabolites analysis of active ingredients of TCMs
Figure 3 showed that alkaloids and flavonoids were the major components of TCMs evaluated in metabolites analysis. Psotova et al. [50] identified dihydrosanguinarine (DHSA) as a metabolite of sanguinarine (SA) in rats using HPLC/ESI-MS. Mitragynine is the primary active alkaloid extracted from the leaves of Mitragyna speciosa Korth, a plant that originates in South-East Asia and is commonly known as kratom in Thailand. Lu [51] developed HPLC/ ESI-MS/MS to determine an ultra-trace amount of mitragynine in human urine. Beyer et al. [52] used an HPLC/ESI-MS/MS System (MRM mode) for quantification of the phenalkylamines ephedrine, pseudoephedrine, norephed-rine, norpseudoephedrine, methylephedrine, methylpseud-oephedrine, cathinone, mescaline, synephrine (oxedrine), and methcathinone in plasma. Wang et al. [53] studied the metabolism of triptolide by cytochrome P450s in human and rat liver microsomes. All the products were identified as mono-hydroxylated triptolides by HPLC/MS. Strzelecki et al. [54] used an HPLC/MS/MS method to identify aconi-tine, the main toxin of Aconitum napellus in the blood of a 54-year-old man. This study showed that this technique has broad application potential in the field of forensic science.
Figure 3.
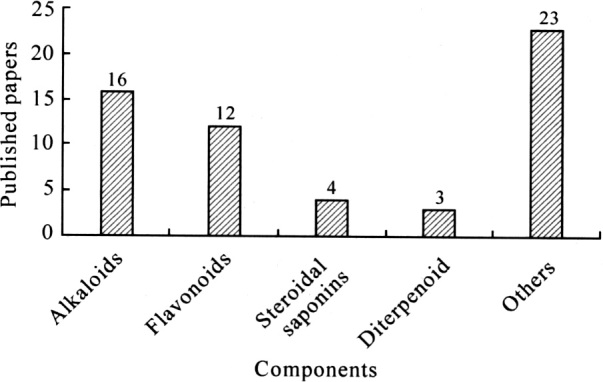
The distribution of the published papers on the metabolites analysis of TCMs by HPLC/MS
3.2. Metabolites analysis of Chinese materia medica
Kaneko et al. [55] developed a simple and sensitive meth-od for measuring four types of Aconitum alkaloids (aconi-tine, hypaconitine, jesaconitine and mesaconitine) by HPLC/ESI-TOF/MS. This method is applicable in clinical and forensic toxicology. Kontrimaviciute [56] developed an HPLC/ESI-MS method for the determination of ibogaine and noribogaine in human plasma and whole blood. The method was successfully used in the analysis of poisoning involving Tabernanthe iboga root.
The domestic researchers have shown interest in: (1) the distribution of TCMs in tissue and metabolism; (2) screen-ing the active ingredients by determination of the distribution of TCMs in tissue and the metabolic products; (3) the pharmacological mechanism of TCMs.
Wang et al. [57] studied the tissue distribution and ex-cretion of resveratrol in urine and bile in rats after intragas-tric administration of Polygonum cuspidatum extract using HPLC/MS/MS. In that paper, serum chemistry and com-bined HPLC/DAD-MS techniques were used to study the constituents of Huangbai-Zhimu herb-pair (HBZMHP) extract absorbed into rat serum after oral administration.
Ma et al. [58] studied rat serum after oral administration of HBZMHP extract by HPLC/DAD-MS techniques. A total of nine characteristic HPLC peaks in the TIC chro-matograms were identified as magnoflorine (1), menisper-ine (2), palmatine (3), berberine (4), timosaponin N or timosaponin El (5), timosaponin D (6), timosaponin 13111, anemarsaponin C or xilingsaponin B (7) timosaponin BIII (8) and timosaponin AIII (9). Ni et al. [59] developed UPLC/Q-TOF/MS and the MetaboLynx (TM) Software combined with mass defect filtering (MDF) to provide unique high throughput capabilities for the study of drug metabolism. They have screened and identified the constituents absorbed and metabolized in studies of G. lon-gituba extract after oral administration in rats. The results showed that 21 parent components of G. longituba extract were absorbed into the rat blood circulation and a total of 80 metabolites of 9 parent compounds were tentatively detected. This work suggests that the integrative metabolism approach make a useful template for drug metabolism research in TCMs. Li et al. [60] used HPLC/MS to deter-mine the active ingredients of Epimedium brevicornum Maxim and its metabolites. Four active ingredients of Epimedium were found in the blood circulation of kidney-defi-cient rats and two of their metabolites in urine. The meta-bonomic approach is a potentially powerful tool to analyze the material basis and mechanism of action. In drug metab-olism research, Guo et al. [61] developed UPLC/Q-TOF/ MS with automated data analysis Software (MetaboLynx (TM)) for fast analysis of the metabolic profile of fla-vonoids in Abelmoschus manihot.
3.3. Metabolites analysis of the prescriptions of TCMs
18 articles on the metabolites analysis of prescriptions of TCMs were reported within 59 articles. Li et al. [62] developed an HPLC/MS/MS-based method to study the multiple active licorice flavonoids (including liquiritin apioside, liquiritin, liquiritigenin, isoliquiritin apioside, isoliquiritin, and isoliquiritigenin) in rat plasma following an oral dose of Xiaochaihu Tang. Zhao et al. [63] developed a UPLC/Q-TOF/MS method for urinary metabonomics to study the mechanism involved after treatment of blood stasis using the TCMs prescription Xindi Soft Capsules. Lü et al. [64] simultaneously determined scoparone, capillarisin, rhein, and emodin in rat urine after oral administration of Yinchenhao Decoction preparation by UPLC/Q-TOF/MS.
3.4. Metabolites analysis of others
Zhang et al. [65] used HPLC/MS/MS to investigate the chemical components of PHY906 and its metabolites in the plasma of a patient with metastatic colorectal cancer (mCRC) treated with irinotecan and PHY906. The find-ings demonstrated that HPLC/MS/MS was an effective and reliable method for studying the parent chemicals of the Chinese herbal medicine PHY906 and its metabolites in this patient.
4. Pharmacokinetics of TCMs
In the pharmacokinetics research of TCMs, 76% of the studies reported in the literature used the HPLC/MS/MS method, and 24% of the studies in the literature used the HPLC/ESI-MS method. Xiong et al. [66] developed a UPLC/MS-MS method for the simultaneous determination of harpagoside and cinnamic acid in rat plasma and success-fully applied this to the pharmacokinetic study of harpagoside and cinnamic acid in rats after oral administration of Yanyan tablets, a compound traditional Chinese medicine.
4.1. Pharmacokinetics of active ingredients of TCMs
Figure 4 showed that alkaloids, saponins and flavonoids were the major components of TCMs evaluated in pharmacokinetics analysis. Alkaloids included oxymatrine, vincris-tine, cepharanthine, dauricine and peimine. Guilhaumou et al. [67] developed an HPLC/MS/MS method for the quantification of vincristine in plasma in order to investigate the pharmacokinetics in a pediatric population. Hao et al. [68] determined cepharanthine in human plasma using HPLC/MS/MS. Xin et al. [69] developed an on-line TFC-HPLC/MS method. This method was successfully applied in the pharmacokinetic study of verticine, verticinone and isoverticine, the chemical markers of Fritillaria thun-bergii, after oral administration of a total steroidal alkaloid extract of F. thunbergü in rats.
Figure 4.
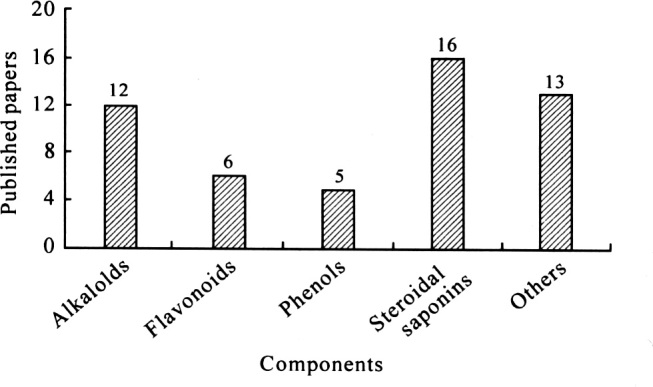
The distribution of the published papers on the pharmacokinetics of TCMs by HPLC/MS
The literature on saponins includes ginsenoside, baicalin, astragaloside IV, mangiferin and gastrodin. Li et al. [70] established an HPLC/ESI-MS method for the simultaneous determination of Panax notoginsenoside Ri, ginsenoside Rg1, Rd, Re and Rbi in rat plasma. The pharmacokinetic platform was successfully applied to the pharmacokinetic study of a multiple-constituent traditional Chinese medicine, total Panax notoginsenoside (Xuesaitong Injection). Kim et al. [71] used an HPLC/MS/MS method to deter-mine the pharmacokinetics of baicalein, baicalin, wogonin and oroxylin A after intravenous administration of Scutel-lariae radix extract to male Sprague-Dawley rats. Su-ryawanshia et al. [72] developed an HPLC/MS/MS method for the simultaneous estimation of two bioactive markers, mangiferin and amarogentin along with three other components, amaroswerin, sweroside and swertiamarin in plasma after intravenous administration of a herbal preparation in male Sprague-Dawley (SD) rats.
The literature on flavonoids includes tanshinone, tanshi-none IIA, silibinin, quercetin, apigenin, and genistein. Some reports include the pharmacokinetics of triptolide, bi-lobalide and paeonol. Xie et al. [73] developed an HPLC/ MS/MS method for the simultaneous determination of ginkgolides (includes ginkgolide C for the first time) and bilobalide in rat plasma following intravenous administration of Ginkgo biloba extract. Xie et al. [74] used an HPLC/Q-TOF/MS technique to compare the pharmacokinetic behavior and metabolic profile in rats following oral administration of the pure paeonol alone and an herbal preparation “Qingfu Guanjieshu” (QFGJS) containing paeonol. The results indicated that other components in QFGJS could effectively influence the pharmacokinetic behavior and metabolic profile of paeonol in rats.
4.2. Pharmacokinetics of Chinese materia medica
In the analysis of the pharmacokinetics of Chinese materia medica, Coptis chinensis, baikal skullcap root, ginseng berry, Salvia miltiorrhiza and Schisandra chinensis were the major materia medica. Feng et al. [75] developed a sensitive, rapid and selective HPLC/MS/MS method for the simultaneous determination of baicalin, baicalein, wogonin, berberine, palmatine and jatrorrhizine in Scutel-laria-Coptis herb couple in rat plasma after oral administra-tion of Yiqing Capsules and Gegen-Qinlian Tablets in rats. Wang et al. [76] used an HPLC/ESI-MS method for the simultaneous quantification of four active schisandra lignans (schisandrin, schisantherin A, deoxyshisandrin and gamma-schisandrin) from a traditional Chinese medicine Schisandra chinensis (Wuweizi) in rat plasma.
Due to the complexity of Chinese medicines, generally only one, two or three components were measured as an index of the quality of Chinese materia medica. Therefore, to comprehensively analyze both the Contents and pharmacokinetics of the various components of Chinese materia medica is a great challenge.
4.3. Pharmacokinetics of the prescriptions of TCMs
In the pharmacokinetics of the prescriptions of TCMs, sap-onins are the major research point. The following prescriptions including saponins were studied, such as Epimedium Decoction, Shenmai Injection, Gushudan, Zishen Pills, Tangminling Pills, Shuanghuanglian Oral Liquid, Xi-aochaihu Tang, Luxiancao Decoction, Huanglianjiedu Decoction and Dachengqi Decoction. Zhu et al. [77] com-pared the pharmacokinetics of baicalin and wogonoside in rats following oral administration of Xiaochaihu Tang (Mi-nor Radix Bupleuri Decoction) and Radix scutellariae extract using an HPLC/MS method.
5. Other analysis
5.1. Quality control
Generally, one or two active ingredients in TCMs were em-ployed for evaluating the quality of TCMs. In 2006, Ye and colleagues [78] developed a new strategy combining qualitative HPLC/MS analysis and quantitative HPLC to deter-mine major bufadienolides for the global quality control of ChanSu crude drug. Last year, Liu et al. [79] established an HPLC analytical method for the quantitation of the diester-alkaloids content in the decoctions. They also inves-tigated the components and content of alkaloids in these decoctions by semi-quantitative ESI-MS. Zhao et al. [80] developed an HPLC/APCI-MS method for the qualitative and quantitative analysis of steroids, as well as for the quality control of Polyporus umbellatus. In the same year, Han et al. [81] developed a reliable isotope dilution method for the simultaneous determination of fumonisins’Bi, B2 and B3 in TCMs by UPLC/MS-MS.
Han and Ye [82] reported an HPLC/MS method for the quality control of Shuanghuanglian Oral Liquid in 2006. This will be a comprehensive quality control method of this commonly used herbal preparation. Wang and coworkers [83] developed an HPLC-MS/MS method employing both positive and negative electrospray ionization for the simultaneous determination of the nine identified compounds in the raw herbs and products of Si Wu Tang (SWT). The study proved it is a sensitive and rapid quantification ap-proach and is a useful method in the quality control of raw herbs and products of SWT.
5.2. Analysis of synthetic adulterants
Adulteration of herbal remedies with undeclared synthetic drugs is a common problem, which may potentially cause serious adverse effects. Jung et al. [84] and Vidal et al. [85] studied the metabolites of “Lida Dai Dai Hua Capsules”, a weight loss product of Chinese origin. The central nervous System drug sibutramine was identified as an additive in this recipe, and the dosage was far beyond the pro-visions of the German national drug dose administration.
Reepmeyer and colleagues [86] analyzed a herbal dietary Supplement which can enhance sexual function. They developed an HPLC/MS and a hydrolytic technique for the detection and structure elucidation of a novel synthetic vardenafil designer drug added illegally to a “natural” herbal dietary Supplement. One year later, the same group analyzed and detected a new sidenaf il analogue as an adulterant in a herbal dietary Supplement using HPLC with photodiode array and mass spectral detection [87].
5.3. Metabonomics study
Because metabonomics are usually used in analytical tech-nology, with the development of analytical technology, metabonomics are now widely applied.
Last year, Ma and coworkers [88] studied the metabolic profile of plasma and kidney tissue from rats treated with Morning Glory Seed (MGS) using a UPLC/MS metabo-nomic approach. Their results were helpful in understand-ing the clinical diagnosis of TCMs-induced nephrotoxicity. Wang and colleagues [89] explored the thyroxine-and reserpine-induced changes in the metabolic profiles of rat urine and the therapeutic effect of Liu Wei Di Huang Pills employing UPLC/HDMS. Gu et al. [90] carried out a comprehensive metabonomic method, in combination with fingerprint analysis and target analysis to determine poten-tial mechanisms of berberine action in the treatment of patients with type 2 diabetes and dyslipidemia.
6. Condusion
With the development of HPLC/MS techniques, more and more TCMs and their in vivo analytes have been investiga-ted. HPLC/MS techniques become the first choice for the determination of targets in biological fluids such as blood, plasma and urine. With the high resolution, high reproduc-ibility and high selectivity of UPLC and MSn, UPLC/Q-TOF/MS has been demonstrated to be powerful tools for the characterization of low-abundance targets in complex samples. Some of peaks can be characterized directly online by comparing the retention time, UV spectra, and frag-mentation information with the reference. During the dis-covery process of novel compounds, it is important to dif-ferentiate novel from known compounds in crude extracts before starting a time-consuming process of purification.
However, until now, there is no universal mass database available, because the fragment information of ESI and APCI is easily affected by ionization modes and HPLC con-ditions. It is necessary to establish universal database with the help of the reference substances development. Another limitation of HPLC/MS is that the peak capacity of an HPLC column is limited. Therefore, HPLC/MS in the qualitative study of TCMs is not as mature as GC-MS.
From our survey of the literature, the majority of studies only focused on determining the components of TCMs. It is insufficient in the depth of research. Therefore, more efforts should be made to explore the relationship between the effectiveness and components of TCMs by using HPLC/ MS techniques. In addition, most of the authors of the published papers were from universities and research insti-tutes, and very few from pharmaceutical companies. Therefore, it is necessary to strengthen the research coop-eration between the pharmaceutical Company and university or research institute.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30873193 and No. 81073038)
References
- 1.Yang M., Sun J.H., Lu Z.Q. Phytochemical analysis of traditional Chinese medicine using liquid chromatography coupled with mass spec-trometry. J Chromatogr A. 2009;1216(11):2045–2062. doi: 10.1016/j.chroma.2008.08.097. [DOI] [PubMed] [Google Scholar]
- 2.Gray M.I., Chang D., Zhang Y. Development of liquid chromatography/mass spectrometry methods for the quantitative analysis of herbal medicine in biological fluids: a review. Biomed Chromatogr. 2010;24(1):91–103. doi: 10.1002/bmc.1287. [DOI] [PubMed] [Google Scholar]
- 3.Li Y.G., Song L., Liu M. Advancement in analysis of Salviae miltior-rhizae Radix et Rhizoma (Danshen) J Chromatogr A. 2009;1216(11):1941–1953. doi: 10.1016/j.chroma.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q.Y., Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice) J Chromatogr A. 2009;1216(11):1954–1969. doi: 10.1016/j.chroma.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 5.Zhang A.H., Sun H., Wang Z.G. Metabolomics: Towards under-standing traditional Chinese medicine. Planta Medica. 2010;76(17):2026–2035. doi: 10.1055/s-0030-1250542. [DOI] [PubMed] [Google Scholar]
- 6.Jayaprakasam B., Doddaga S., Wang R. Licorice Flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57(3):820–825. doi: 10.1021/jf802601j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L., Tian X., Fan P.C. Separation, determination and identifi-cation of the diastereoisomers of podophyllotoxin and its esters by high-performance liquid chromatography/tandem mass spectrometry. J Chromatogr A. 2008;1210(2):168–177. doi: 10.1016/j.chroma.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Van Der Heijden R., Spijksma G. Alkaloid profiling of the Chinese herbal medicine Fuzi by combination of matrix-assisted laser de-sorption ionization mass spectrometry with liquid chromatography-mass spectrometry. J Chromatogr A. 2009;1216(11):2169–2178. doi: 10.1016/j.chroma.2008.11.077. [DOI] [PubMed] [Google Scholar]
- 9.Liu W.L., Pi Z.F., Wang X.Y. HPLC/ESI-MSn and ESI-MS studies on the Aconitum alkaloids in three Chinese medicinal herbs. J Sep Sci. 2010;33(17–18):2898–2906. doi: 10.1002/jssc.201000285. [DOI] [PubMed] [Google Scholar]
- 10.Yue H., Pi Z.F., Song F.R. Studies on the aconitine-type alkaloids in the roots of Aconitum Carmichaeli Debx. by HPLC/ESIMS/MSn. Talanta. 2009;77(5):1800–1807. doi: 10.1016/j.talanta.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Ren J., Sun M. A combined cell membrane chromatography and online HPLC/MS method for screening compounds from Radix Caulophylli acting on the human alpha(lA)-adrenoceptor. J Pharm Biomed Anal. 2010;51(5):1032–1036. doi: 10.1016/j.jpba.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.De Jong C.F., Derks R.J.E., Bruyneel B. High-Performance liquid chromatography-mass spectrometry-based acetylcholinesterase assay for the screening of inhibitors in natural extracts. J Chromatogr A. 2006;1112(1–2):303–310. doi: 10.1016/j.chroma.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 13.Yuan J.F., Zhang Z.Q., Kang X.Q. LC-MS analysis for the components captured by ECV304 cell from extract of Aconitum szechenyianum Gay. Biomed Chromatogr. 2009;23(4):406–411. doi: 10.1002/bmc.1131. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J.L., Xin G.Z., Shi Z.Q. Characterization and identification of steroidal alkaloids in Fritillaria species using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A. 2010;1217(45):7109–7122. doi: 10.1016/j.chroma.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Alali F.Q., Tahboub Y.R., Al-Daraysih I.S. LC-MS and LC-PDA vs. phytochemical analysis ofColchicum brachyphyllum. Pharmazie. 2008;63(12):860–865. [PubMed] [Google Scholar]
- 16.Zhou Y., Zou X., Liu X. Multistage electrospray ionization mass spectrometric analyses of sulfur-containing iridoid glucosides in Paederia scandens. Rapid Commun Mass Spectrom. 2007;21(8):1375–1385. doi: 10.1002/rcm.2965. [DOI] [PubMed] [Google Scholar]
- 17.Lee K.C., Cheuk M.W., Chan W. Determination of glucosinolates in traditional Chinese herbs by high-performance liquid chromatography and electrospray ionization mass spectrometry. Anal Bioanal Chem. 2006;386(7–8):2225–2232. doi: 10.1007/s00216-006-0882-7. [DOI] [PubMed] [Google Scholar]
- 18.Kite G.C., Veitch N.C., Boaich M.E. Flavonol tetraglycosides from fruits of Styphnolobium japonicum (Leguminosae) and the authentication of Fructus Sophorae and Flos Sophorae. Phytochemistry. 2009;70(6):785–794. doi: 10.1016/j.phytochem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T., Liu H., Liu X.T. Qualitative and quantitative analysis of steroidal saponins in crude extracts from Paris polyphylla var. yunnanensis and P. polyphylla var. chinensis by high Performance liquid chromatography coupled with mass spectrometry. J Pharm Biomed Anal. 2010;51(1):114–124. doi: 10.1016/j.jpba.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Dong H.J., Liu Z.Q., Song F.R. Structural analysis of monoterpene glycosides extracted from Paeonia lactiflora Pall. using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(19):3193–3199. doi: 10.1002/rcm.3203. [DOI] [PubMed] [Google Scholar]
- 21.Qi L.W., GuXJ, Li P. Structural characterization of pregnane glycosides from Cynanchum auriculatum by liquid chromatography on a hybrid ion trap time-of-flight mass spectrometer. Rapid Commun Mass Spectrom. 2009;23(14):2151–2160. doi: 10.1002/rcm.4125. [DOI] [PubMed] [Google Scholar]
- 22.Xie G.X., Plumb R., Su M.M. Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomic research. J Sep Sci. 2008;31(6–7):1015–1026. doi: 10.1002/jssc.200700650. [DOI] [PubMed] [Google Scholar]
- 23.Han J., Ye M., Qiao X. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J Pharm Biomed Anal. 2008;47(3):516–525. doi: 10.1016/j.jpba.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Liu A.H., Guo H., Ye M. Detection, characterization and identification of phenolic acids in Danshen using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J Chromatogr A. 2007;1161(1–2):170–182. doi: 10.1016/j.chroma.2007.05.081. [DOI] [PubMed] [Google Scholar]
- 25.Lee S., Khoo C., Halstead C.W. Liquid Chromatographie determination of honokiol and magnolol in Hou Po (Magnolia officinalis) as the raw herb and dried aqueous extract. J AOAC Int. 2007;90(5):1210–1218. [PubMed] [Google Scholar]
- 26.Hu F.L., DengCH, Liu Y. Quantitative determination of chlorogenic aeid in Honeysuckle using microwave-assisted extraction followed by nano-LC-ESI mass spectrometry. Talanta. 2009;77(4):1299–1303. doi: 10.1016/j.talanta.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Wang C., Pan Y.J., Fan G.R. Application of an efficient strategy based on MAE, HPLC-DAD-MS/MS and HSCCC for the rapid extrac-tion, identification, Separation and purification of flavonoids from Fructus Aurantii Immaturus. Biomed Chromatogr. 2010;24(3):235–244. doi: 10.1002/bmc.1278. [DOI] [PubMed] [Google Scholar]
- 28.Han Q.B., Zhou Y., Feng C. Bioassay guided discovery of apoptosis inducers from gamboge by high-speed counter-current chromatography and high-pressure liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sei. 2009;877(4):401–407. doi: 10.1016/j.jchromb.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y.Y., Guo Z.M., Zhang X.L. Offline 2-D RPLC/RPLC meth-od for Separation of components in Dalbergja odorifera T. Chen. J Sep Sei. 2010;33(9):1224–1230. doi: 10.1002/jssc.200900778. [DOI] [PubMed] [Google Scholar]
- 30.Yang N.Y., Duan J.A., Shang E.X. Analysis of Sesquiterpene Lactones in Eupatorium lindleyanum by HPLC-PDA-ESI-MS/MS. Phytochem Anal. 2010;21(2):144–149. doi: 10.1002/pca.1170. [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Li S.L., Zhou Y. Characterization of protostane triterpenoids in Alisma orientalis by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(11):1514–1522. doi: 10.1002/rcm.4548. [DOI] [PubMed] [Google Scholar]
- 32.Inbaraj B.S., Lu H., Hung C.F. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J’ Pharm Biomed Anal. 2008;47(4–5):812–818. doi: 10.1016/j.jpba.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Huang S.C, Hung C.F., Wu W.B. Determination of Chlorophylls and their derivatives in Gynostemma pentaphyllum Makino by liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2008;48(1):105–112. doi: 10.1016/j.jpba.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Ahn M.J., Lee M.K., Kim Y.C. The simultaneous determination of coumarins in Angelica gigas root by high Performance liquid chromatogra-phy-diode array detector coupled with electrospray ionization/mass spectrometry. J Pharm Biomed Anal. 2008;46(2):258–266. doi: 10.1016/j.jpba.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Xie Y., Zhao W.Q., Zhou T.T. An efficient strategy based on MAE, HPLC-DAD-ESI-MS/MS and 2D-prep-HPLC-DAD for the rapid extraction, Separation, identification and purification of five active coumarin components from Radix Angelicae Dahuricae. Phytochem Anal. 2010;21(5):473–482. doi: 10.1002/pca.1222. [DOI] [PubMed] [Google Scholar]
- 36.Huang L.Y., Zhong T.H., Chen T.W. Identification of beta-sitosterol, stigmasterol and ergosterin in A-roxburghii using supercritical fluid extrac-tion followed by liquid chromatography/atmospheric pressure chemical io-neization ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(18):3024–3032. doi: 10.1002/rcm.3181. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y.F., Xiao Y.S., Xue X.Y. Systcmatic screening and characterization of novel bufadienolides from toad skin using ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(5):667–678. doi: 10.1002/rcm.4436. [DOI] [PubMed] [Google Scholar]
- 38.Don M.J., Ko H.C., Yang C.W. Detection of polyphenols and tanshi-nones in commercial Danshen by liquid chromatography with UV and mass spectrometry. J Food Drug Anal. 2006;14(3):254–259. [Google Scholar]
- 39.Huang W.Y., Sheu S.J. Separation and identification of the fifteen constitu-ents in forsythiae fructus. J Food Drug Anal. 2007;15(l):33–39. [Google Scholar]
- 40.Kao T.H., Huang S.C., Inbaraj B.S. Determination of flavonoids and saponins in Gynostemma pentaphyllum (Thunb.) Makino by liquid chro-matography-mass spectrometry. Anal ChimActa. 2008;626(2):200–211. doi: 10.1016/j.aca.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 41.Liu L., Li YFCheng Y.Y. A method for the produetion and characterization of fractionated libraries from Chinese herbal formulas. J Chromatography B Analyt Technol Biomed Life Sei. 2008;862(1–2):196–204. doi: 10.1016/j.jchromb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Wen X.D., Qi L.W., Chen J. Analysis of interaction property of bio-active components in Danggui Buxue Decoction with protein by microdialy-sis coupled with HPLC-DAD-MS. J Chromatogr B Analyt Technol Biomed Life Sei. 2007;852(1–2):598–604. doi: 10.1016/j.jchromb.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Yang L., He Y.Q. Characterization of fifty-one flavonoids in a Chinese herbal prescription Longdan Xiegan Decoction by high-per-formance liquid chromatography coupled to electrospray ionization tandem mass spectrometry and photodiode array detection. Rapid Commun Mass Spectrom. 2008;22(12):1767–1778. doi: 10.1002/rcm.3536. [DOI] [PubMed] [Google Scholar]
- 44.Yan G.L., Sun H., Sun W.J. Rapid and global detection and characterization of aconitum alkaloids in Yin Chen Si Ni Tang, a traditional Chinese medical formula, by ultra Performance liquid chromatography-high resolution mass spectrometry and automated data analysis. J Pharm Biomed Anal. 2010;53(3):421–431. doi: 10.1016/j.jpba.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Zheng C.N., Hao H.P., Wang X. Diagnostic fragment-ion-based ex-tension strategy for rapid screening and identification of serial components of homologous families contained in traditional Chinese medicine prescription using high-resolution LC-ESI-IT-TOF/MS: Shengmai injeetion as an example. J Mass Spectrom. 2009;44(2):230–244. doi: 10.1002/jms.1502. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H.Y., Hu P., Luo G.A. Screening and identification of multi-component in Qingkailing injeetion using combination of liquid chromatog-raphy/time-of-flight mass spectrometry and liquid chromatography/ion trap mass spectrometry. Anal Chim Acta. 2006;577(2):190–200. doi: 10.1016/j.aca.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 47.Han Z., Liu X.S., Ren Y.P. A rapid method with ultra-high-Performance liquid chromatography-tandem mass spectrometry for simultaneous determination of five type B trichothecenes in traditional Chinese medi-cines. J Sep Sei. 2010;33(13):1923–1932. doi: 10.1002/jssc.201000094. [DOI] [PubMed] [Google Scholar]
- 48.Han Z., Zheng Y.L., Luan L.J. An ultra-high-Performance liquid chromatography-tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in traditional Chinese medicines. Anal Chim Acta. 2010;664(2):165–171. doi: 10.1016/j.aca.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Liau B.C., Jong T.T., Lee M.R. Supercritical fluid extraction and quantification of aflatoxins in Zizyphi Fructus by liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(5):667–673. doi: 10.1002/rcm.2870. [DOI] [PubMed] [Google Scholar]
- 50.Psotova J., Klejdus B., Vecera R. A liquid chromatographic-mass spectrometric evidence of dihydrosanguinarine as a first metabolite of sart-guinarine transformation in rat. J Chromatogr B Analyt Technol Biomed Life Sei. 2006;830(1):165–172. doi: 10.1016/j.jchromb.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 51.Lu S.J., Tran B.N., Nelsen J.L. Quantitative analysis of mitragynine in human urine by high Performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sei. 2009;877(24):2499–2505. doi: 10.1016/j.jchromb.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Beyer J., Peters F.T., Kraemer T. Detection and validated quantification of nine herbal phenalkylamines and metheathinone in human blood plasma by LC-MS/MS with electrospray ionization. J Mass Spectrom. 2007;42(2):150–160. doi: 10.1002/jms.1132. [DOI] [PubMed] [Google Scholar]
- 53.Li W., Liu Y., He Y.Q. Characterization of triptolide hydroxylation by cytochrome P450 in human and rat liver microsomes. Xenobiolica. 2008;38(12):1551–1565. doi: 10.1080/00498250802503359. [DOI] [PubMed] [Google Scholar]
- 54.Strzelecki A., Pichon N., Gaulier J.M. Acute toxic herbal intake in a suieide attempt and fatal refractory ventricular arrhythmia. Basic Clin Pharmacol Toxicol. 2010;107(2):698–699. doi: 10.1111/j.1742-7843.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 55.Kaneko R., Hattori S., Furuta S. Sensitive analysis of aconitine, hypaconitine, mesaconitine and jesaconitine in human body fluids and Aconitum tubers by LC/ESI-TOF-MS. J Mass Spectrom. 2006;41(6):810–814. doi: 10.1002/jms.1038. [DOI] [PubMed] [Google Scholar]
- 56.Kontrimaviciute V., Breton H., Mathieu O. Liquid chromatography-electro spray mass spectrometry determination of ibogaine and noribogaine in human plasma and whole blood—Application to a poisoning involving Tabemanthe iboga root. J Chromatogr B Analyt Technol Biomed Life Sei. 2006;843(2):131–141. doi: 10.1016/j.jchromb.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 57.Wang D.G., Xu Y.R., Liu W.Y. Tissue distribution and exeretion of resvera-trol in rat after oral administration of Polygonum cuspidatum extract (PCE) Phytomedicine. 2008;15(10):859–866. doi: 10.1016/j.phymed.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Ma C.H., Fan M.S., Tang Y.H. Identification of major alkaloids and steroidal saponins in rat serum by HPLC-diode array detection-MS/MS following oral administration of Huangbai-Zhimu herb-pair Extract. Biomed Chromatogr. 2008;22(8):835–850. doi: 10.1002/bmc.1000. [DOI] [PubMed] [Google Scholar]
- 59.Ni S.M., Qian D.W., Duan J.A. UPLC-QTOF/MS-based screening and identification of the constituents and their metabolites in rat plasma and urine after oral administration of Glechoma longituba extract. J Chromatogr B Analyt Technol Biomed Life Sei. 2010;878(28):2741–2750. doi: 10.1016/j.jchromb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Li F.M., Lu X.M., Liu H.P. A pharmaco-metabonomic study on the therapeutic basis and metabolic effects of Epimedium brevicornum Maxim, on hydrocortisone-induced rat using UPLC-MS. Biomed Chromatogr. 2007;21(4):397–405. doi: 10.1002/bmc.770. [DOI] [PubMed] [Google Scholar]
- 61.Guo J.M., Shang E.X., Duan J.A. Fast and automated characterization of major constituents in rat biofluid after oral administration of Abelmoschus manihot extract using ultra-performance liquid chromatography/qua-drupole time-of-flight mass spectrometry and MetaboLynx. Rapid Commun Mass Spectrom. 2010;24(4):443–453. doi: 10.1002/rcm.4416. [DOI] [PubMed] [Google Scholar]
- 62.Li L., Liang S.P., Du F.F. Simultaneous quantification of multiple lic-orice flavonoids in rat plasma. J AmSoc Mass Spectrom. 2007;18(4):778–782. doi: 10.1016/j.jasms.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Zhao X.I., Zhang Y., Meng X.L. Effect of a traditional Chinese med-icine preparation Xindi soft capsule on rat model of acute blood stasis: A urinary metabonomics study based on liquid chromatography-mass spec-trometry. J Chromatogr B Analyt Technol Biomed Life Sei. 2008;873(2):151–158. doi: 10.1016/j.jchromb.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Lü H.T., Sun H., Wang X.I. Simultaneous determination by UPLC-ESI-MS of scoparone, capillarisin, rhein, and emodin in rat urine after oral administration of Yin Chen Hao Tang preparation. J Sep Sei. 2008;31(4):659–666. doi: 10.1002/jssc.200700596. [DOI] [PubMed] [Google Scholar]
- 65.Zhang W., Saif M.W., Dutschman G.E. Identification of chemicals and their metabolites from PHY906, a Chinese medicine formulation, in the plasma of a patient treated with irinotecan and PHY906 using liquid chromatography/tandem mass spectrometry (LC/MS/MS) J Chromatogr A. 2010;1217(37):5785–5793. doi: 10.1016/j.chroma.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong Z.L., Fu Y.H., Li J.J. A UPLC-MS-MS method for quantification of harpagoside and cinnamic aeid in rat plasma and its application to a pharmaeokinetie study after oral administration of Yanyan Tablets. Chro-matographia. 2010;72(1–2):163–169. [Google Scholar]
- 67.Guilhaumou R., Solas C., Rome A. Validation of an electrospray ionization LC/MS/MS method for quantitative analysis of vincristine in human plasma samples. J Chromatogr B Analyt Technol Biomed Life Sei. 2010;878(3–4):423–427. doi: 10.1016/j.jchromb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Hao G.T., Liang H.X., Li Y.Y. Simple, sensitive and rapid HPLC-MS/MS method for the determination of cepharanthine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sei. 2010;878(28):2923–2927. doi: 10.1016/j.jchromb.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 69.Xin G.Z., Zhou J.L., Qi L.W. Turbulent-flow chromatography cou-pled on-line to fast high-Performance liquid chromatography and mass spectrometry for simultaneous determination of verticine, verticinone and isoverticine in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sei. 2010;878(3–4):435–441. doi: 10.1016/j.jchromb.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 70.Li X.Y., Sun J.G., Wang G.J. Simultaneous determination of panax notoginsenoside R1, ginsenoside Rg1, Rd, Re and Rb1 in rat plasma by HPLC/ESI/MS: platform for the pharmaeokinetie evaluation of total panax notoginsenoside, a typical kind of multiple constituent traditional Chinese medicine. Biomed Chromatogr. 2007;21(7):735–746. doi: 10.1002/bmc.813. [DOI] [PubMed] [Google Scholar]
- 71.Kim Y.H., Jeong D.W., Paek I.B. Liquid chromatography with tandem mass spectrometry for the simultaneous determination of baicalein, baica-lin, oroxylin A and wogonin in rat plasma. J Chromatogr B Analyt Technol Biomed Life Sei. 2006;844(2):261–267. doi: 10.1016/j.jchromb.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Suryawanshia S., Asthana R.K., Gupta R.C. Simultaneous estimation of mangiferin and four seeoiridoid glycosides in rat plasma using liquid chromatography tandem mass spectrometry and its application to pharmaeokinetie study of herbal preparation. J Chromatogr B Analyt Technol Biomed Life Sei. 2007;858(1–2):211–219. doi: 10.1016/j.jchromb.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 73.Xie J.S., Ding C.G., Ge Q.H. Simultaneous determination of ginkgol-ides A, B, C and bilobalide in plasma by LC-MS/MS and its application to the pharmaeokinetie study of Ginkgo biloba extract in rats. J Chromatogr B Analyt Technol Biomed Life Sei. 2008;864(1–2):87–94. doi: 10.1016/j.jchromb.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 74.Xie Y., Zhou H., Wong Y.F. Study on the pharmaeokinetics and me-tabolism of paeonol in rats treated with pure paeonol and an herbal preparation containing paeonol by using HPLC-DAD-MS method. J Pharm Biomed Anal. 2008;46(4):748–756. doi: 10.1016/j.jpba.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 75.Feng J., Xu W., Tao X. Simultaneous determination of baicalin, baicalein, wogonin, berberine, palmatine and jatrorrhizine in rat plasma by liquid chromatography-tandem mass spectrometry and application in pharmaeokinetie studies after oral administration of traditional Chinese medicinal preparations containing scutellaria-coptis herb couple. J Pharm Biomed Anal. 2010;53(3):591–598. doi: 10.1016/j.jpba.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Wang B.L., Hu J.P., Tan W. Simultaneous quantification of four ac-tive schisandra lignans from a traditional Chinese medicine Schisandra chinensis(Wuweizi) in rat plasma using liquid chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sei. 2008;865(1–2):114–120. doi: 10.1016/j.jchromb.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Z.Y., Zhao L.A., Liu X.F. Comparative pharmaeokinetics of baicalin and wogonoside by liquid chromatography-mass spectrometry after oral administration of Xiaochaihu Tang and Radix scutellariae extract to rats. J Chromatogr B Analyt Technol Biomed Life Sei. 2010;878(24):2184–2190. doi: 10.1016/j.jchromb.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 78.Ye M., Guo H., Guo H.Z. Simultaneous determination of cytotoxic bufadienolides in the Chinese medicine ChanSu by high-Performance liquid chromatography coupled with photodiode array and mass spectrometry de-tections. J Chromatogr B Analyt Technol Biomed Life Sei. 2006;838(2):86–95. doi: 10.1016/j.jchromb.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 79.Liu W.L., Song F.R., Liu Z.Q. Chemical study on combination Taboo of Radix aconiti with Rhizoma pinelliae, Fructus trichosanthis, Bulbus fritillariae thunbergli, Radix ampelopsis and Rhizoma bletillae. Ada Chim Sin. 2010;68(9):889–896. [Google Scholar]
- 80.Zhao Y.Y., Cheng X.L., Zhang Y.M. Simultaneous determination of eight major steroids from Polyporus umbellatus by high-Performance liquid chromatography coupled with mass spectrometry detections. Biomed Chromatogr. 2010;24(2):222–230. doi: 10.1002/bmc.1277. [DOI] [PubMed] [Google Scholar]
- 81.Han Z., Ren Y.P., Liu X.S. A reliable isotope dilution method for simultaneous determination of fumonisins B1, B2 and B3 in traditional Chinese medicines by ultra-high-Performance liquid chromatography-tan-dem mass spectrometry. J Sep Sei. 2010;33(17–18):2723–2733. doi: 10.1002/jssc.201000423. [DOI] [PubMed] [Google Scholar]
- 82.Han J., Ye M., Guo H. Analysis of multiple constituents in a Chinese herbal preparation Shuang-Huang-Lian oral liquid by HPLC-DAD-ESI-MSn. J Pharm Biomed Anal. 2007;44(2):430–438. doi: 10.1016/j.jpba.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z.J., Wo S.K., Wang L. Simultaneous quantification of active components in the herbs and produets of Si-Wu-Tang by high Performance liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2009;50(2):232–244. doi: 10.1016/j.jpba.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Jung J., Hermanns-Clausen M., Weinmann W. Anorectic sibutramine detected in a Chinese herbal drug for weight loss. Forensic Sei Inte. 2006;161(2–3):221–222. doi: 10.1016/j.forsciint.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 85.Vidal C., Quandte S. Identification of a sibutramine-metabolite in patient urine after intake of a “pure herbal” Chinese slimming produet. Ther Drug Monit. 2006;28(5):690–692. doi: 10.1097/01.ftd.0000245392.33305.b0. [DOI] [PubMed] [Google Scholar]
- 86.Reepmeyer J.C., Woodruff J.T. Use of liquid chromatography-mass spectrometry and a hydrolytic technique for the detection and strueture eluci-dation of a novel synthetic vardenafil designer drug added illegally to a “natural” herbal dietary Supplement. J Chromatogr A. 2006;1125(1):67–75. doi: 10.1016/j.chroma.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 87.Reepmeyer J.C., Woodruff J.T. Use of liquid chromatography-mass spectrometry and a chemical cleavage reaction for the strueture elueidation of a new sildenafil analogue detected as an adulterant in an herbal dietary Supplement. J Pharm Biomed Anal. 2007;44(4):887–893. doi: 10.1016/j.jpba.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Ma C., Bi K.S., Su D. Serum and kidney metabolic changes of rat nephrotoxicity induced by Morning Glory Seed. Food Chem Toxicol. 2010;48(10):2988–2993. doi: 10.1016/j.fct.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 89.Wang P., Sun H., Lv H. Thyroxine and reserpine-induced changes in metabolic profiles of rat urine and the therapeutic effect of Liu Wei Di Huang Wan detected by UPLC-HDMS. J Pharm and Biomed Anal. 2010;53(3):631–645. doi: 10.1016/j.jpba.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 90.Gu Y., Zhang Y.F., Shi X.Z. Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta. 2010;81(3):766–772. doi: 10.1016/j.talanta.2010.01.015. [DOI] [PubMed] [Google Scholar]


