Abstract
The protein binding of non-steroidal anti-inflammatory drugs flurbiprofen, ketoprofen and etodolac with human serum albumin (HSA) was investigated using indirect chiral high performance liquid chromatography (HPLC) and ultrafiltration techniques. S-(–)-1-(1-naphthyl)-ethylamine (S-NEA) was utilized as chiral derivatization reagent and pre-column derivatization RP-HPLC method was established for the separation and assay of the three pairs of enantiomer. The method had good linear relationship over the investigated concentration range without interference. The average extraction efficiency was higher than 85% in different systems, and the intra-day and inter-day precisions were less than 15%. In serum albumin, the protein binding of etodolac enantiomers showed significant stereoselectivity that the affinity of S-enantiomer was stronger than R-enantiomer, and the stereoselectivity ratio reached 6.06; Flurbiprofen had only weak stereoselectivity in HSA, and ketoprofen had no stereoselectivity at all. Scatchard curves showed that all the three chiral drugs had two types of binding sites in HSA.
Keywords: Protein binding, Non-steroidal anti-inflammatory drugs, Enantiomer, Stereoselectivity, Human serum albumin
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAID) are a class of drugs with the main effects of anti-inflammatory, plus antipyretic, analgesic and anti-rheumatism effect. The common mechanisms of NSAID are inhibiting cyclooxygenase or 5-lipoxidase and reducing the biosynthesis of prostaglandin (PG) to achieve the anti-inflammatory effect. The chemical structure types of NSAID are various, most of which are chiral drugs. Except a few drugs such as naproxen, most chiral NSAID used in clinical are racemes. However, even in the early study of chiral NSAID, it had been found that the chiral NSAID enantiomers differed in pharmacodynamics [1], [2], such as 2-aryl-propionic acid (2-APA) drugs, of which it was the S=enantiomer that inhibited the cyclooxygenase. The stereoselectivity of NSAID in the drug metabolism is observed mainly in the chiral inversion, glucuronidation and oxidation [3], [4], [5]. Most of the 2-APA NSAID mainly undergo the one-way conversion in in vivo, which is from R- to S-enantiomer. In recent years, it has been found that some 2-APA drugs demonstrate two-way choice in some species [6].
Some reports have shown that the protein binding of chiral NSAID has stereoselectivity [7], [8], [9]. For ibuprofen, it is mainly the R-enantiomer that binds with human serum albumin (HSA) concentration-dependently, and the two enantiomers can be mutually replaced. For carprofen, the R-enantiomer is apt to bind with HSA, whereas fenoprofen almost has no stereoselectivity. As NSAID could interact with plasma protein extensively and the low hepatic uptake rate, small changes in drug protein binding rate as well as differences of protein binding between the enantiomers could significantly affect the in vivo disposition process of enantiomers of chiral drug [10]. Therefore, for these chiral drugs with high protein binding rate, respective determination of the enantiomers' protein binding rate as well as the studies of interaction between enantiomers on the level of the protein binding are necessary.
In this work, we investigated the protein binding of acidic chiral NSAID flurbiprofen, ketoprofen and etodolac with HSA. S-(–)-1-(1-naphthyl)-ethylamine (S-NEA) was utilized as chiral derivatization reagent and pre-column derivatization RP-HPLC method was developed for the separation and quantification of the three chiral drugs. Different derivatization conditions were investigated to obtain an optimum separation method. This work established a foundation for the study of their stereoselective protein binding.
2. Experimental
2.1. Apparatus and reagents
HPLC was performed on an Agilent 1100 system consisting of G1311A pump, Aglient Zorbax C18 (250 mm×4.6 mm, 5 μm) column, G1315A (DAD) UV detector, manual injector and Chem-Stations software (Agilent, USA). Eppendorf refrigerate centrifuge (Eppendorf, Germany). Microcon centrifugal system (Millipore, USA). Electrothermal constant temperature oscillation flume (Medical Constant Temperature Corp., Shanghai, China).
Ketoprofen and flurbiprofen were provided by Zhejiang Jiuzhou Pharmaceutical Co.,Ltd., while Etodolac was kindly provided by Ningbo Medical Science and Technology Research Co.,Ltd. (S)-(–)-α-(1-Naphthyl) ethylamine (S-NEA), 1-hydroxybenzotriazole (HOBT) and 1-(3-Dimethylamine propyl)-3-ethyl-carbimide (EDC) were purchased from Sigma-Aldrich. Methanol and acetonitrile are of chromatographic grade. Water used is redistilled water. All the other reagents used were of analytical grade unless otherwise indicated.
2.2. Methods
2.2.1. Determination of flurbiprofen enantiomers
-
(1)
Chromatographic conditions
Mobile phase: acetonitrile–KH2PO4 (pH 4.5, 0.01M) (70:30, v/v); flow rate: 0.80 mL/min; detection wavelength: 250 nm; injection volume: 20 μL; column temperature: room temperature.
-
(2)
Sample preparation and pre-column derivatization.
150 μL protein sample (pre-incubating at 37 °C for 10 min) was added to 10 mL centrifuge tube. Then the internal standard ketoprofen and 100 μL of 1 M sulphuric acid were added and vortically mixed. Afterward, 2 mL dichloromethane was added and vortically mixed for 3 min, following by centrifugation for 10 min (3000g), and then the lower organic layer was then transferred into a 5 mL centrifuge tube, followed by vacuum drying. Whereafter, 100 μL of 0.8% triethylamine-dichloromethane and 100 μL of 2% thionyl chloride-dichloromethane (freshly prepared) were added to the residue, vortically mixing, and air-tightly reacting for 2 h at 30 °C. After vacuum drying 100 μL S-NEA dichloromethane (2.5 mg/mL, freshly prepared) was added to the reaction solution. And then the reaction continued for 0.5 h at 30 °C. After vacuum drying the residue was dissolved by the mobile phase.
2.2.2. Determination of ketoprofen enantiomers
-
(1)
Chromatographic conditions
Mobile phase: acetonitrile–KH2PO4 (pH 4.5, 0.01 M) (60:40, v/v); flow rate: 0.80 mL/min; detection wavelength: 250 nm; injection volume: 20 μL; column temperature: room temperature.
-
(2)
Sample preparation and pre-column derivatization
The procedure of the sample preparation was the same with “2.2.1 (2)” and the internal standard was flurbiprofen.
2.2.3. Determination of etodolac enantiomer
-
(1)
Chromatographic conditions
Mobile phase: methanol–KH2PO4 (pH 4.5, 0.01M) (88:12, v/v); flow rate: 0.80 mL/min; detection wavelength: 278 nm; injection volume: 20 μL; Column temperature: room temperature.
-
(2)
Sample preparation and pre-column derivatization
150 μL protein sample (pre-incubating at 37 °C for 10 min) was added to a 10 mL centrifuge tube. Then 100 μL of 1 M sulphuric acid was added and mixed by vortexing. Afterward, 2 mL dichloromethane was added and vortically mixed for 3 min, following by centrifugation for 10 min (3000g), and then the lower organic layer was then transferred into a 5 mL centrifuge tube, followed by vacuum drying. Thereafter, 50 μL HOBT (2.5 mg/mL, including 1% pyridine), 100 μL EDC (2.5 mg/mL) and 50 μL S-NEA (5 mg/mL) were added to the residue (All the above solutions were freshly prepared with dichloromethane), mixed by vortexing, and air-tightly reacted for 2 h at 30 °C. After vacuum drying the residue was dissolved by the mobile phase.
2.2.4. HSA binding with the drug enantiomers
-
(1)
Nonspecific adsorption between drugs and the ultrafilter
Drugs were added to a 700 μL Sorenson phosphate buffer (pre-incubating for 10 min at 37 °C). After vortically mixing, 500 μL phosphate buffer was taken into the ultrafilter, centrifuging for 5 min at 37 °C (2000g), and 150 μL ultrafiltrate was got. The ultrafiltrate and the phosphate buffer without ultrafiltration were analyzed by HPLC without derivatization.
where Aultrafiltrate is the peak area of the drug in the ultrafiltrate and APBS is the peak area of the drug in phosphate buffer. -
(2)
Chromatographic conditions for the determination of flurbiprofen and ketoprofen
Mobile phase: acetonitrile–KH2PO4 (pH 4.5, 0.01 M) (60:40, v/v); flow rate: 0.80 mL/min; detection wavelength: 250 nm; injection volume: 20 μL; column temperature: room temperature.
-
(3)
Chromatographic conditions for the determination of etodolac
Mobile phase: methanol–KH2PO4 (pH 4.5, 0.01 M) (70:30, v/v); Flow rate: 0.80 mL/min; Detection wavelength: 278 nm; Injection volume: 20 μL; Column temperature: room temperature.
-
(4)
Experiments of binding with HSA
Appropriate amount HSA (40 mg/mL in the Sorenson phosphate buffer) was added to make the final concentration of HSA 0.8% in the incubation medium. After 10 min pre-incubation at 37 °C, drugs were added and vortically mixed, and then the incubation system was placed in the constant temperature oscillation device, incubating for 15 min. After the drugs bound completely with plasma protein, 500 μL HSA was taken into the ultrafilter, centrifuging for 5 min at 37 °C (10 000 rpm) and 150 μL ultrafiltrate was obtained. Afterward, the disposal of the ultrafiltrate was the same with “2.2.1 (2)”. Meanwhile, 150 μL HSA without ultrafiltration was parallelly operated according to the same method.
3. Results and discussion
3.1. Determination of the three pairs of enantiomer
3.1.1. Determination of flurbiprofen enantiomers
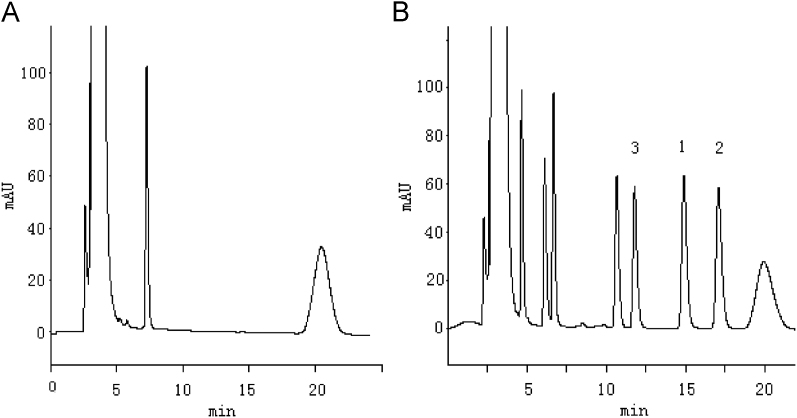
Under the optimized chromatographic conditions, the resolutions between flurbiprofen enantiomers and between flurbiprofen and the internal standard were good, while the retention time was moderate, and there was no interference peak in all sample peak position (Fig. 1). Therefore, this method has good specificity.
Figure 1.
HPLC chromatograms of flurbiprofen enantiomers. (A) blank plasma; (B) blank plasma spiked with racemic flurbiprofen and ketoprofen. Peaks: 1, S-flurbiprofen; 2, R-flurbiprofen; 3, R-ketoprofen (I.S).
Under the above determination conditions, the method exhibited excellent linear relationship with the concentration of flurbiprofen enantiomers in the range of 1.0–50 μg/mL. The limits of detection (LOD) and quantitation (LOQ) were 303 ng/mL and 1.0 μg/mL, respectively. The regression equations are shown as follows: YS=1.5897C+0.0751, r=0.999; YR=1.6045C+0.0501, r=0.999(1.0–50 μg/mL).
The results of recovery and precision of flurbiprofen enantiomers are shown in Table 1.
Table 1.
Recovery and precision of flurbiprofen enantiomers (n=5, ).
| Sample | Spiked amount (μg/mL) | Absolute recovery (%) |
Precision (%) |
||||
|---|---|---|---|---|---|---|---|
| Intra-day |
Inter-day |
||||||
| S | R | S | R | S | R | ||
| HSA | 1.0 | 88.11±3.72 | 90.25±3.74 | 5.48 | 5.87 | 6.97 | 5.74 |
| 5.0 | 91.48±2.79 | 92.75±2.14 | 3.01 | 2.88 | 5.43 | 6.01 | |
| 50 | 88.44±2.76 | 89.01±2.86 | 2.12 | 3.01 | 2.78 | 3.47 | |
3.1.2. Determination of ketoprofen enantiomers
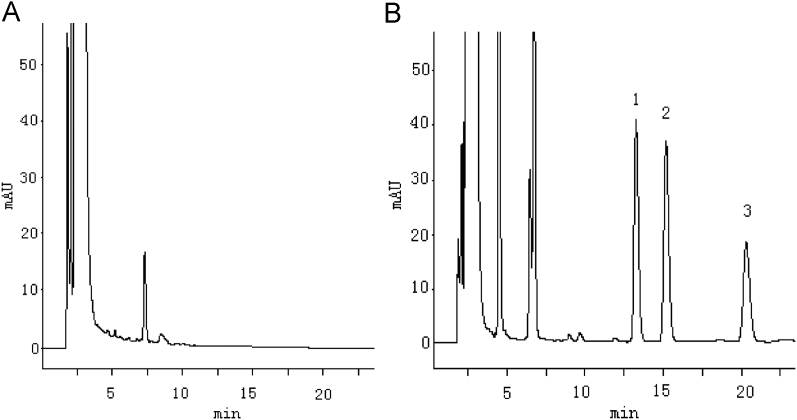
Under the optimized chromatographic conditions, the resolutions between ketoprofen enantiomers and between ketoprofen and the internal standard were good, while the retention time was moderate, and there were no interference peaks in all sample peak position (Fig. 2).
Figure 2.
HPLC chromatograms of ketoprofen enantiomers. (A) Blank plasma; (B) blank plasma spiked with racemic ketoprofen and flurbiprofen. Peaks: 1, S-ketoprofen; 2, R-ketoprofen; 3, S-flurbiprofen (I.S).
The signals responded linearly to the ketoprofen enantiomers' concentration in the range of 1.0–50 μg/mL. The LOD and LOQ were 303 and 1.0 μg/mL, respectively. The regression equations are shown as follows: YS=0.1267C+0.0186, r=0.999; YR=0.1309C+0.0161, r=0.999(1.0–50 μg/mL).
The results of recovery and precision of ketoprofen enantiomers are shown in Table 2.
Table 2.
Recovery and precision of ketoprofen enantiomers (n=5, ).
| Sample | Spiked amount (μg/mL) | Absolute recovery (%) |
Precision (%) |
||||
|---|---|---|---|---|---|---|---|
| Intra-day |
Inter-day |
||||||
| S | R | S | R | S | R | ||
| HSA | 1.0 | 91.44±2.98 | 92.43±3.58 | 4.22 | 3.76 | 5.85 | 6.01 |
| 5.0 | 90.01±3.45 | 91.91±3.11 | 4.12 | 3.32 | 3.10 | 4.03 | |
| 50 | 88.46±1.79 | 88.65±1.81 | 1.14 | 0.87 | 2.61 | 2.57 | |
3.1.3. Determination results of etodolac enantiomers
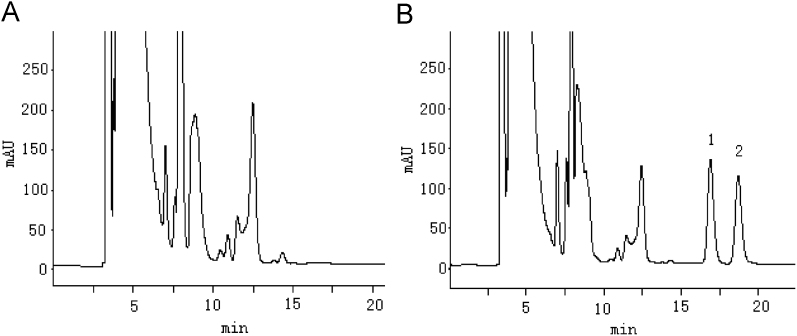
The chromatograms of etodolac enantiomers are shown in Fig. 3. The signals showed excellent linear relationship with the concentration of etodolac enantiomers in the range of 1.0–50 μg/mL. The LOD and LOQ were 303 and 1.0 μg/mL, respectively. The regression equations are shown as follows: YS=129.10C+115.24, r=0.999; YR=124.25C+128.54, r=0.999(1.0–50 μg/mL).
Figure 3.
HPLC chromatograms of etodolac enantiomers. (A) Blank plasma; (B) blank plasma spiked with racemic etodolac. Peaks: 1, S-etodolac; 2, R-etodolac.
The recovery and precision results of etodolac enantiomers are shown in Table 3.
Table 3.
Recovery and precision of etodolac enantiomers (n=5, mean±SD).
| Sample | Spiked amount (μg/mL) | Absolute recovery (%) |
Precision (%) |
||||
|---|---|---|---|---|---|---|---|
| Intra-day |
Inter-day |
||||||
| S | R | S | R | S | R | ||
| HSA | 1.0 | 91.92±4.98 | 90.21±4.81 | 8.08 | 6.87 | 7.92 | 6.84 |
| 5.0 | 89.45±4.13 | 88.27±3.89 | 5.21 | 4.89 | 5.43 | 5.02 | |
| 50 | 90.71±3.86 | 89.75±3.11 | 3.89 | 4.07 | 2.88 | 3.15 | |
3.2. Protein binding of the drug enantiomers
3.2.1. Nonspecific adsorption between drugs and the ultrafilter
The nonspecific adsorption of flurbiprofen, ketoprofen and etodolac at different concentrations with ultrafilter is shown in Table 4. All the nonspecific adsorption rates of flurbiprofen, ketoprofen and etodolac are less than 5%, indicating that the ultrafilter can be used for the determination of these drugs' protein binding.
Table 4.
Nonspecific adsorption of chiral drugs with ultrafilter (n=3).
| Spiked amount (μg/mL) | P (Flubiprofen) (%) | P (Ketoprofen) (%) | P (Etodolac) (%) |
|---|---|---|---|
| 1.0 | 2.78 | 3.77 | 4.21 |
| 5.0 | 1.72 | 3.86 | 2.15 |
| 50 | 1.41 | 2.95 | 2.01 |
| (%) | 1.97 | 3.53 | 2.79 |
3.2.2. Drug enantiomers binding with HSA
As acidic drugs, flurbiprofen, ketoprofen and etodolac mainly bind with albumin in plasma. Being different from basic drugs, acidic drugs seldom bind with other proteins such as acid glycoprotein in plasma.
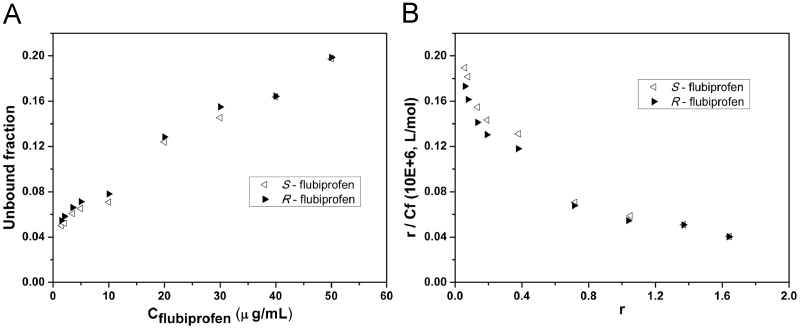
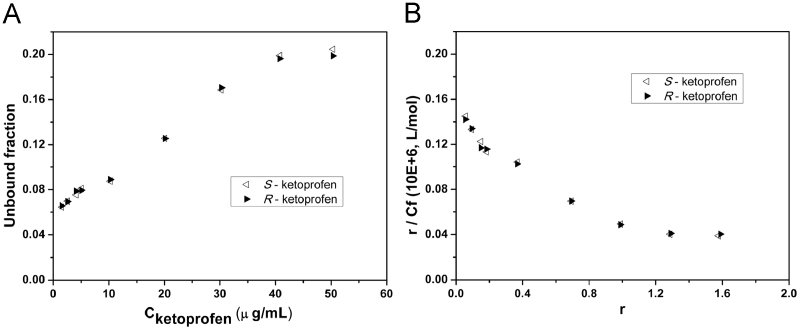
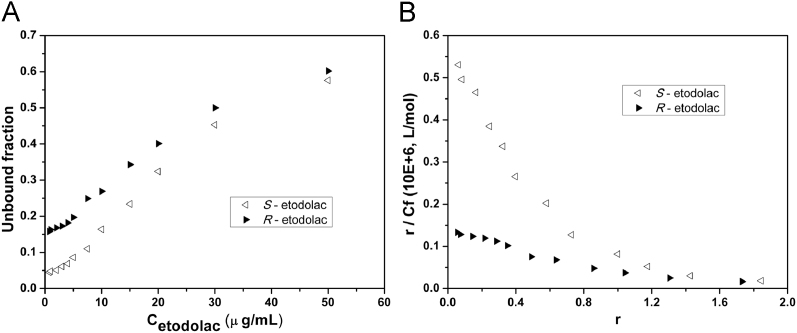
In human serum albumin, all the binding rate of flurbiprofen, ketoprofen and etodolac at the low concentrations is up to 95%. Furthermore, the trend of the HSA binding rate with concentration is similar to that in plasma protein, indicating that albumin affinity site is the main binding sites. In albumin, flurbiprofen shows a weak stereoselectivity that the protein binding rate of S-enantiomer is slightly higher than R-enantiomer (Fig. 4). However, the stereoselectivity of ketoprofen and etodolac in HSA is the same with that in plasma (Figure 5, Figure 6).
Figure 4.
The binding of flubiprofen enantiomers to HSA. (A) Unbound fraction of flubiprofen enantiomers at various initial concentrations. (B) Scatchard plots for the protein binding of flubiprofen enantiomer.
Figure 5.
The binding of ketoprofen enantiomers to HSA. (A) Unbound fraction of ketoprofen enantiomers at various initial concentrations. (B) Scatchard plots for the protein binding of ketoprofen enantiomer.
Figure 6.
The binding of etodolac enantiomers to HSA. (A) Unbound fraction of etodolac enantiomers at various initial concentrations. (B) Scatchard plots for the protein binding of etodolac enantiomer.
There are at least two types of binding sites at HSA of the three drugs, viz. high affinity sites and low affinity sites. On the basis of the literature reported [11], [12], [13], we used Eq. (1) for the nonlinear least squares fitting, and the binding constant is shown in Table 5. Ketoprofen enantiomers did not show stereoselectivity because the binding constants of S- and R-enantiomer in the two types of binding sites were the same. Etodolac has a strong chiral recognition (KS/KR=6.06) in the high affinity sites. In the low concentration range, the significant differences of the protein binding rate of the S-enantiomer and R-enantiomer are caused by the binding to the sites. When the drug concentration is higher than 20 μg/mL, etodolac could interact with the low affinity sites and the stereoselectivity is weakened. Generally speaking, such low affinity and high capacity sites are of poor stereoselectivity. As the drug enantiomers' binding data in the experiments are measured in racemes, for the high binding rate drugs, the possible competitive inhibition between enantiomers can affect the accuracy of the binding constant, thereby it could not actually reflect the interaction between the single enantiomer and the protein [14]. Taking into account that the three chiral drugs are commonly used as racemes in clinical, the enantiomers–protein binding measured in the racemes could better explain the actual disposition in in vivo and thus still has important clinical significance.
| (1) |
Table 5.
Binding parameters of enantiomers of flubiprofen, ketopreofen and etodolac in 1% HSA (n=3).
| Enantiomers | Binding parameters |
|||
|---|---|---|---|---|
| K1 (×105) | K2 (×104) | n1 | n2 | |
| S-flubiprofen | 1.040 | 1.151 | 0.955 | 2.698 |
| R-flubiprofen | 1.167 | 1.090 | 0.862 | 3.051 |
| S-ketopreofen | 0.933 | 0.811 | 1.003 | 2.861 |
| R-ketopreofen | 0.935 | 0.884 | 1.021 | 2.767 |
| S-etodolac | 5.301 | 2.341 | 0.875 | 1.983 |
| R-etodolac | 0.874 | 0.664 | 0.823 | 2.307 |
4. Conclusion
In this paper, S-NEA was utilized as chiral derivatization reagent and pre-column derivatization RP-HPLC method was established for the separation of ketoprofen, flurbiprofen and etodolac enantiomers. As acidic drugs, ketoprofen, flurbiprofen and etodolac enantiomers strongly interacted with serum albumin in plasma and showed concentration-dependent. In serum albumin, the protein binding of etodolac enantiomers showed significant stereoselectivity as the affinity of S-enantiomer was stronger than R-enantiomer, and the stereoselectivity ratio reached 6.06; flurbiprofen had only weak stereoselectivity in HSA, while ketoprofen has no stereoselectivity at all. Scatchard curves showed that all the three chiral drugs had two types of binding sites in HSA.
Acknowledgements
This project was supported by National Major Projects for Science and Technology Development of Ministry Science and Technology of China (2009ZX09304-003).
References
- 1.Suri A., Grundy B.L., Derendorf H. Pharmacokinetics and pharmacodynamics of enantiomers of ibuprofen and flurbiprofen after oral administration. Int. J. Clin. Pharmacol. Ther. 1997;35(1):1–8. [PubMed] [Google Scholar]
- 2.Landoni M.F., Lees P. Pharmacokinetics and pharmacodynamics of ketoprofen enantiomers in calves. Chirality. 1995;7(8):586–597. doi: 10.1002/chir.530070806. [DOI] [PubMed] [Google Scholar]
- 3.Glowka F.K. Stereoselective pharmacokinetics of indobufen from tablets and intramuscular injections in man. Chirality. 2000;12(1):38–42. doi: 10.1002/(SICI)1520-636X(2000)12:1<38::AID-CHIR7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Akira K., Taira T., Hasegawa H. Studies on the stereoselective internal acyl migration of ketoprofen glucuronides using C-13 labeling and nuclear magnetic resonance spectroscopy. Drug. Metabol. Dispos. 1998;26(5):457–464. [PubMed] [Google Scholar]
- 5.Soraci A., Benoit E., Jaussaud P. Enantioselective glucuronidation and subsequent biliary-excretion of carprofen in horses. Am. J. Vet. Res. 1995;56(3):358–361. [PubMed] [Google Scholar]
- 6.Jamali F., Lovlin R., Aberg G. Bi-directional chiral inversion of ketoprofen in CD-1 mice. Chirality. 1997;9(1):29–31. doi: 10.1002/(SICI)1520-636X(1997)9:1<29::AID-CHIR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.Itoh T., Saura Y., Tsuda Y. Stereoselectivity and enantiomer–enantiomer interactions in the binding of ibuprofen to human serum albumin. Chirality. 1997;9(7):643–649. doi: 10.1002/(SICI)1520-636X(1997)9:7<643::AID-CHIR1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Mignot I., Presle N., Lapicque F. Albumin binding sites for etodolac enantiomers. Chirality. 1996;8(3):271–280. doi: 10.1002/(SICI)1520-636X(1996)8:3<271::AID-CHIR7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Dubois N., Muller N., Lapicque F. Stereoselective protein-binding of nonsteroidal antiinflammatory drugs—pharmacological consequences. Therapie. 1993;48(4):335–339. [PubMed] [Google Scholar]
- 10.Itoh T., Maruyama J., Tsuda Y. Stereoselective pharmacokinetics of ibuprofen in rats: Effect of enantiomer–enantiomer interaction in plasma protein binding. Chirality. 1997;9(4):354–361. doi: 10.1002/(SICI)1520-636X(1997)9:4<354::AID-CHIR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Eap C.B., Baumann P. Isoelectric-focusing of alpha-1 acid glycoprotein (orosomucoid) in immobilized pH-gradients with 8 M urea: detection of its desialylated variants using an alkaline phosphatase-linked secondary antibody system. Electrophoresis. 1988;9(10):650–654. doi: 10.1002/elps.1150091005. [DOI] [PubMed] [Google Scholar]
- 12.Lin J.H., Cocchetto D.M., Duggan D.E. Protein-binding as a primary determinant of the clinical pharmacokinetic properties of nonsteroidal antiinflammatory drugs. Clin. Pharmacokinet. 1987;12(6):402–432. doi: 10.2165/00003088-198712060-00002. [DOI] [PubMed] [Google Scholar]
- 13.Morgan D.J., Huang J.L. Effect of plasma-protein binding on kinetics of capillary uptake and efflux. Pharm. Res. 1993;10(2):300–304. doi: 10.1023/a:1018959415963. [DOI] [PubMed] [Google Scholar]
- 14.Vorum H. Reversible ligand binding to human serum albumin. Theoretical and clinical aspects. Dan. Med. Bull. 1999;46(5):379–399. [PubMed] [Google Scholar]