Abstract
A comparison of the volatile compounds in Rhizomes Curcumae (Ezhu) and Radix Curcumae (Yujin) was undertaken using gas chromatography–mass spectrometry (GC–MS). Ultrasonic extraction and GC–MS methods were developed for the simultaneous determination of five sesquiterpenes, namely, α-pinene, β-elemene, curcumol, germacrone and curdione, in Ezhu and Yunjin. Good linearity (r>0.999) and high inter-day precision were observed over the investigated concentration ranges. The validated method was successfully used for the simultaneous determination of five sesquiterpenes in Ezhu and Yujin. The quantitative method can be effectively used to evaluate and monitor the quality of Chinese curcuma in clinical use.
Keywords: Gas chromatography–mass spectrometry, Rhizomes Curcumae, Radix Curcumae, Volatile compounds, Quality control
1. Introduction
Traditional Chinese medicines (TCMs) are invaluable drug resources due to their high pharmacological activities, low toxicity and rare complications. TCMs have been used in the clinical therapy of many diseases for thousands of years [1]. Rhizomes Curcumae and Radix Curcumae belong to the Zingiberaceae family. The rhizomes of three species of Curcuma, namely, Curcuma phaeocaulis Val., Curcuma kwangsiensis S.G. Lee et C.F. Liang and Curcuma wenyujin Y.H. Chen et C. Ling, are used as a remedy, Ezhu, to alleviate blood stasis and pain [2]. The radix of three species of Curcuma, namely, C. wenyujin Y.H. Chen et C. Ling, Curcuma longa L. and C. kwangsiensis S.G. Lee et C.F. Ling or C. phaeocaulis Val., are used as a remedy, Yujin, to invigorate circulation, reduce stasis and inhibit inflammation [2]. Ezhu and Yujin are commonly used as TCMs in China because of their different medicinal properties. These differences are likely to be associated with their qualitative and quantitative constituents. The main bioactive constituents of Ezhu and Yujin consist of essential oils and curcumin. Until now, many reports on Ezhu and Yujin have focused on the role of curcumin [3], [4], [5]. While the essential oils contained remain poorly studied, they are known to have strong pharmacological bioactivities, such as anti-tumor [6] and anti-viral activities [7], and β-elemene, curcumol, germacrone and curdione are thought to be the principal biologically active ingredients.
To evaluate the quality of Curcumae based TCMs, gas chromatography–flame ionization detection (GC–FID) [8], [9], [10], gas chromatography–mass spectrometry (GC–MS) [11], [12], [13] and high-performance liquid chromatography (HPLC) [14], [15], [16] have been used to quantify the sesquiterpenes contained in Ezhu and Yujin. However, all previous studies have focused on the identification of components and the determination of their relative amounts without the use of standards. Therefore, the results are not appropriate for the purpose of quality control. GC–MS offers a powerful tool for the identification and quantification of chemical components in essential oils.
Some conventional extraction methods such as hydrodistillation, steam distillation (SD), reflux extraction and Soxhlet extraction have been developed for extracting the quality control markers such as curdione, curcumol and germacrone in TCMs [17], [18]. However, problems of low extraction efficiency, lengthy protocols and toxic solvent residues in the extract may be encountered using these methods. These shortcomings have led to the development of new techniques such as ultrasonic extraction (UE) in essential oil extraction, which typically use less solvent and time. In the present study, GC–MS and UE were developed for the simultaneous determination of five volatile compounds, namely, α-pinene, β-elemene, curcumol, germacrone and curdione, in Ezhu and Yujin.
To the best of our knowledge, this is the first example of a systematic and comparative study on the volatile oils in Ezhu and Yujin. The analysis of essential oils can help to provide a scientific basis for appropriate use of Ezhu and Yujin. The simple method demonstrated should be of significant use in controlling the quality of these medicines.
2. Experimental
2.1. Reagents and materials
Ezhu and Yujin were separately purchased from Sichuan Province, Guangdong Province and Chinese medicine stores (Leiyunshang), and were authenticated. The samples were ground to a fine power in a high-speed rotary cutting mill and sieve (40 mesh) and stored in a zip lock bag until analysis. n-Hexane (analytical grade) and n-tridecane (purity>99%), used as an internal standard, were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). α-pinene, β-elemene and curcumol (purity>99%) were from the National Institute for the Control of Pharmaceutical and Biological Products (Shanghai, China), and germacrone and curdione (purity>99%) were obtained from Ding Rui Chemical Co., Ltd. (Shanghai, China). Pure water was purchased from Wahaha Co., Ltd. (Shanghai, China).
2.2. Optimization of ultrasound extraction parameters
Ultrasound extraction (CQF-I-6, Shanghai, China) was performed by using equipment constructed in our laboratory. Sample powder (1 g) and n-hexane (10 mL), which was used as extraction solvent, were placed in a 10 mL flask, and ultrasound (200 V, 50 Hz) was applied for 30 min. Then the extract was centrifuged (TGL-16; Anting, Shanghai, China) for 5 min at 5000 rpm and 1 μL of the supernatant (analytical sample) was injected into the GC–MS for analysis.
2.3. GC–MS analysis
GC–MS was performed with a Thermo Trace GC Ultra instrument coupled to a Thermo DSQ II mass spectrometer with Xcalibur software (Thermo Fisher Scientific, USA). Compounds were separated on a 30 m×0.25 mm×0.25 μm HP-5 MS capillary column. Helium was used as carrier gas at a flow rate of 1 mL/min. The temperature of the split injector was 250 °C and the split ratio was 1:10. The injection volume was 1 μL. The column temperature was set at 50 °C and then programmed at 20 °C/min to 150 °C, followed by 2 °C/min to 180 °C, then at 20 °C/min to 200 °C, and this temperature was then maintained for 3 min. The GC–MS interface and ion source temperatures were 260 and 200 °C, respectively. The selected ion monitoring (SIM) method was used for quantification of five sesquiterpenes. Fragment ions were used for quantification; m/z 93 for α-pinene, m/z 81 for β-elemene and the internal standard, m/z 105 for curcumol, m/z 107 for germacrone and m/z 180 for curdione. The electron energy was 70 eV.
2.4. Preparation of solutions
n-hexane stock solutions containing the five analytes were prepared and diluted to appropriate concentrations for the construction of calibration curves. Six concentrations of each of the five analytes were injected in triplicate. Linearity test solutions were prepared at five concentration levels from the limit of quantitation (LOQ) to 150% of the working level. The correlation coefficient, regressive equation and linear range are listed in Table 1.
Table 1.
Regression equations, correlation coefficients (R2) and linear range for α-pinene, β-elemene, curcumol, germacrone and curdione.
| Reference substances | Regression equation | R2 | Linear range (μg/mL) |
|---|---|---|---|
| α-pinene | y=0.3387x+0.0036 | 0.9976 | 0.0113–0.5631 |
| β-elemene | y=0.0636x−0.0104 | 0.9996 | 0.2501–12.5061 |
| Curcumol | y=0.0285x−0.0148 | 0.9988 | 0.5155–25.7339 |
| Germacrone | y=0.1138x−0.0932 | 0.9993 | 3.3326–166.6294 |
| Curdione | y=0.1161x−0.073 | 0.9993 | 1.9348–96.7398 |
2.5. Method validation
Method validation was designed according to the Pharmacopoeia of the People's Republic of China [2]. The limit of detection (LOD) and LOQ for α-pinene, β-elemene, curcumol, germacrone and curdione were estimated at a signal-to-noise ratio of 3:1 and 10:1, respectively, by injecting a series of dilute solutions with known concentration. A precision study was also carried out by injecting six replicates (n=6). The accuracy and stability were evaluated at the working level. The percentage recovery was calculated. Precision was checked by injecting six standard solutions (n=6). The RSDs of their areas were less than 2%.
3. Results and discussion
3.1. Selection of extraction conditions
For UE, the effects of the experiment conditions on the extraction and enrichment efficiency for the five components were evaluated. n-hexane, diethyl ether and ethanol were tested, and finally n-hexane was adopted because of its high extraction efficiency and limited interference. The effects of the extraction time on the yields of the volatile compounds recovered were studied from 10 to 60 min with other conditions fixed. Finally, an extraction time of 30 min was chosen for further study due to its high extraction efficiency.
3.2. Validation of the method
3.2.1. Linearity
The calibration graphs obtained by plotting concentration against the average peak area (each sample injected in duplicate) were linear over the range. The regression equations and correlation coefficients (R2) for the compounds are listed in Table 1.
3.2.2. Repeatability
The repeatability of α-pinene, β-elemene, curcumol, germacrone and curdione was calculated based on five runs. The peak areas of selected ions were relatively stable. The RSD was 1.86%, 1.05%, 2.21%, 1.48% and 1.46%, respectively.
3.2.3. Stability
The stability of α-pinene, β-elemene, curcumol, germacrone and curdione was also determined by injecting a freshly prepared standard solution 0, 2, 4, 6, 10 and 12 h after preparation, respectively. The RSD was 3.00%, 3.92%, 1.55%, 2.62% and 3.46%, respectively. Thus, the quantitation of sesquiterpenes such as α-pinene, β-elemene, curcumol, germacrone and curdione in Ezhu and Yujin could be reliably performed within 12 h of sample extraction.
3.2.4. Sensitivity
The LOQ of this method, defined as the lowest concentration of compound in a sample that can be quantitatively determined with suitable precision and accuracy, was 0.0452, 1.0004, 0.205, 51.3 and 29.8 mg/mL, respectively.
The LOD determined as the concentration for which peak heights were three times the baseline noise (S/N=3:1) was 0.0807, 3.9698, 0.8182, 52.90 and 30.71 mg/mL, respectively.
3.2.5. Accuracy and reproducibility
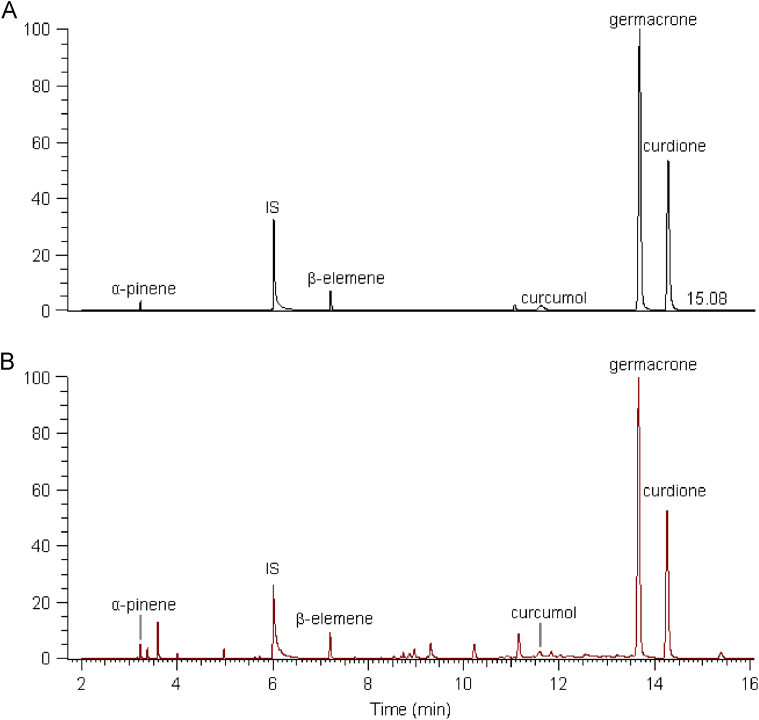
To validate the method, mixed solutions of known amounts of the standards were added into the sample. The recovery of the five tested compounds was between 95.39% and 103.09% with an RSD in the range of 1.97—4.60% (n=6). All the main compounds were well resolved, and the results are shown in Fig. 1. As it may not be possible to identify peaks without the standards when using GC alone, GC–MS was used to allow peak identification. The contents of five identified sesquiterpenes in Ezhu and Yujin were determined using this method (Table 2). The results showed that the contents of the five identified compounds varied greatly in different species and regions.
Figure 1.
GC–MS total ion chromatograms. (A) Mixture of standards, α-pinene, β-elemene, curcumol, germacrone and curdione. (B) Ezhu from Guangxi Province.
Table 2.
Contents (μg/g) of five sesquiterpenes in a variety of species of Ezhu and Yujin.
| Samples no. |
Content(μg/g) |
||||
|---|---|---|---|---|---|
| α-pinene | β-elemene | Curcumol | Germacrone | Curdione | |
| 1 | 0.28 | 1.82 | 5.68 | 12.08 | 28.72 |
| 2 | 0.62 | 4.08 | 7.28 | 15.45 | 4.56 |
| 3 | 0.03 | 1.56 | 40.79 | 10.55 | 13.25 |
| 4 | 0.33 | 5.60 | 57.02 | 18.99 | – |
| 5 | 0.20 | 2.17 | 15.26 | 14.93 | 185.18 |
| 6 | 0.60 | 1.99 | 16.13 | 5.72 | 38.63 |
| 7 | 0.05 | 6.15 | 43.17 | 30.81 | – |
| 8 | 0.07 | 8.88 | 33.70 | 21.56 | – |
| 9 | 0.01 | 1.23 | 24.61 | 7.42 | 11.89 |
| 10 | 0.27 | 2.26 | 10.16 | 17.13 | 255.12 |
| 11 | 0.09 | 1.11 | 6.35 | 6.56 | 5.68 |
| 12 | 0.02 | 0.48 | 0.97 | – | 7.74 |
| 13 | 0.01 | 0.53 | 0.83 | – | 4.27 |
| 14 | 0.03 | 0.50 | 0.97 | – | 3.51 |
| 15 | 0.07 | 0.71 | 1.17 | – | 2.20 |
| 16 | 0.45 | 9.68 | 12.14 | 48.27 | 33.90 |
Numbers 1–2 are Ezhu from Yunan Province, 3–8 from Sichuan Province, 10 from Guangxi Province and 11–12 are Ezhu purchased from Leiyunshang (Shanghai, China). Numbers 13–15 are Yujin from Sichuan Province and 16 is Yujin purchased from Leiyunshang (Shanghai, China).
3.3. Comparison of volatile components between Ezhu and Yujin
As shown in Table 2, although most of the compounds present in the essential oils of Ezhu and Yujin were the same, their contents varied significantly. The difference in content is likely to be at least partly responsible for the different pharmacological effects of Ezhu and Yujin, in particular the main bioactive compounds such as β-elemene and curcumol. Indeed, the relative amounts of bioactive compounds often play an important role in the biological effects used to cure disease with TCMs. Some components were found to be more abundant in Ezhu than Yujin. For example, β-elemene and curdione were both found in Ezhu and Yujin, but their contents in Yujin were less while the content of germacrone in Yujin was more than that in Ezhu. For Ezhu and Yujin from different areas, the contents of various components also varied. For example, the contents of curcumol and curdione in Ezhu from Sichuan Province were the highest while germacrone content in Yujin from Sichuan Province was the lowest. To our knowledge, this is the first in-depth and comparative investigation of the essential oils in the two important medicines. The significant differences in the constituents of the essential oils from Ezhu and Yujin provide compelling evidence that Ezhu and Yujin must be treated as two different TCMs with very different properties.
4. Conclusion
In this paper, the essential oils in Ezhu and Yujin were analyzed using GC–MS, allowing their quantitative characteristics to be compared. The comparative results of the essential oils in Ezhu and Yujin provide valuable information to aid appropriate use of these TCMs. Comparison of the chemical components of Ezhu and Yujin is helpful in allowing elucidation of the mechanism of their therapeutic effects. Moreover, the results showed that GC–MS offers a simple and highly sensitive way to evaluate the quality of Ezhu and Yujin, which should be of significant value to industrial and regulatory bodies.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 30873196) and the Project of Modernization of Traditional Chinese Medicine of Shanghai (no. 09dZ1975100).
Contributor Information
Yi-Feng Chai, Email: yfchai@smmu.edu.cn.
Zi-Yang Lou, Email: louziyang@126.com.
References
- 1.Deng C., Li N., Zhang X. Rapid determination of essential oil in Acorus tatarinowii Schott by pressurized hot water extraction followed by solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. A. 2004;1059(1–2):149–155. doi: 10.1016/j.chroma.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Chinese Pharmacopoeia Committee . Chemical Industry Press; Beijing, China: 2010. The Pharmacopoeia of the People's Republic of China. Part 1. [Google Scholar]
- 3.Mehla J., Reeta K.H., Gupta P. Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Life Sci. 2010;87(19–22):596–603. doi: 10.1016/j.lfs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Senft C., Polacin M., Priester M. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer. 2010;10(1):491. doi: 10.1186/1471-2407-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Z., Lin L., Liu Z. Potential therapeutic effects of curcumin: relationship to microtubule-associated proteins 2 in Aβ1-42 insult. Brain Res. 2010;1368:115–123. doi: 10.1016/j.brainres.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Nie X.H., Ao Z.H., Yin G.Y. Studies on the isolation,purification and characterization of an extra cellular polysaccharide from Grifola frondosa. Pharm. Biotechnol. 2003;10:152–154. [Google Scholar]
- 7.Xia Q., Huang Z.G., Li S.P. The experiment study of the anti-virus effects of zedoary oil on influenzavirus and respiratory syncytial virus. Chin. Pharmacoll. Bull. 2004;20(3):357–358. (in Chinese) [Google Scholar]
- 8.Li X., Sun Y.T., Zhang Z.Q. Determination of β-element, curcumol, germacrone and curdione of zedoary turmeric oil. Chin. J. Pharm. Anal. 2009;11:1832–1836. (in Chinese) [Google Scholar]
- 9.Liu X., Cheng L., Lu G.J. Simultaneous determination of curcumol contentin and β-elemene in Zedoary Turmeric Oil by GC. Chin. Tradit. Patent Med. 2010;32(3):426–429. (in Chinese) [Google Scholar]
- 10.Yang W., Zhang S.H., QM L. Determination of the contents of germacrone and β-elemene in oleum curcumae by capillary column gas chromatography. J. Shenyang Pharm. Univ. 2006;23(12):785–787. (in Chinese) [Google Scholar]
- 11.Zhang G., Li C. Analysis of volatile oils in Rhizoma Curcumae by GC–MS. Lishizhen Med. Materia Medica Res. 2007;18(5):1126–1128. (in Chinese) [Google Scholar]
- 12.Du X., Wu L.H., HG Z. GC-MS determination of β-elemene in Zedoary turmeric oil. Chin. J. Pharm. Anal. 2007;27(2):216–218. (in Chinese) [Google Scholar]
- 13.Wang D.W., Zhang L., Li Y.F. Microwave-assisted extraction and gas chromatography–mass spectrometric analysis of volatile oil from Rhizoma Curcumae. Chin. J. Anal. Chem. 2008;36(10):1454. (in Chinese) [Google Scholar]
- 14.Cheng J., Wei J.K., Yun L. Development and validation of UPLC method for quality control of Curcuma longa Linn.: fast simultaneous quantitation of three curcuminoids. J. Pharm. Biomed. Anal. 2010;53(1):43–49. doi: 10.1016/j.jpba.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 15.He H., Ma S., Tian S. HPLC determination of six components in zedoary turmeric oil and its related injections. Zhongguo Zhong Yao Za Zhi. 2010;35(5):593–597. doi: 10.4268/cjcmm20100511. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 16.Li M., Zhang N. Primary study on HPLC fingerprint of Radix curcumae. Zhong Yao Cai. 2009;32(2):194–197. (in Chinese) [PubMed] [Google Scholar]
- 17.Manzan A.C., Toniolo F.S., Bredow E. Extraction of essential oil and pigments from Curcuma longa [L] by steam distillation and extraction with volatile solvents. J. Agric. Food Chem. 2003;51(23):6802–6807. doi: 10.1021/jf030161x. [DOI] [PubMed] [Google Scholar]
- 18.Xia Q., Zhao K.J., Huang Z.G. Molecular genetic and chemical assessment of Rhizoma Curcumae in China. J. Agric. Food Chem. 2005;53(15):6019–6026. doi: 10.1021/jf0508495. [DOI] [PubMed] [Google Scholar]