Abstract
A reversed phase high performance liquid chromatography (HPLC) method was established for the simultaneous determination of 12, 13-dihydroxyeuparin and glycyrrhizic acid in Yanyanfang mixture. A Grace Apollo C18 column (250 mm×4.6 mm, 5 μm) was used as the stationary phase and the mobile phase was composed of acetonitrile and aqueous phosphoric acid (0.2%, v/v). Gradient elution was carried out at the flow rate of 1.0 mL/min and the column temperature was 30 °C. An ultraviolet (UV) detector was used with a selected wavelength of 240 nm. Calibration curves were linear within the concentration range of 4.6–45.75 μg/mL for 12, 13-dihydroxyeuparin (r>0.9999) and 106.9–1068.9 μg/mL for glycyrrhizic acid (r>0.9999), respectively. Recoveries were 102.18% for 12, 13-dihydroxyeuparin and 101.17% for glycyrrhizic acid. The method developed could be applied to the simultaneous determination of 12, 13-dihydroxyeuparin and glycyrrhizic acid in Yanyanfang mixture.
Keywords: 12, 13-Dihydroxyeuparin; Glycyrrhizic acid; Yanyanfang mixture; High performance liquid chromatography
1. Introduction
Yanyanfang mixture is a traditional Chinese medicine preparation consisted of Radix Eupatorii Chinensis, Radix et Caulis Ilicis Asprellae and Radix et Rhizoma Glycyrrhizae. It is commonly used for the treatment of acute and chronic faucitis and amygdalitis.
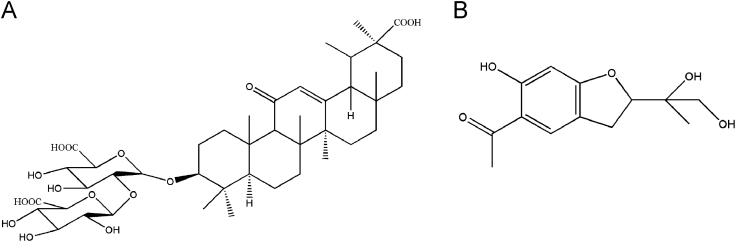
Among the three components, Radix et Rhizoma Glycyrrhizae is recorded in Chinese Pharmacopoeia (Ch. P.) (2010) [1], and according to which the assay is carried out by determining glycyrrhizic acid (Fig. 1A) and glycyrrhizin using high performance liquid chromatography. Glycyrrhizic acid exerts excellent antitumor, antivirus, antiserum and immunoregulation effects [2]. Radix Eupatorii Chinensis is recorded in Standard of Traditional Chinese Medicinal Materials for Guangdong Province (vol. 1) [3] but there is no official assay until now. As the effective constituents of Radix Eupatorii Chinensis remain unknown and the reported determination of adenosine is of low specificity [4], seeking other constituents for the quality control of Radix Eupatorii Chinensis is absolutely essential. Xie et al. [5] first isolated and prepared 12, 13-dihydroxyeuparin (Fig. 1B) from Radix Eupatorii Chinensis. Even though its pharmacological activity is still unclear, but to a great degree it could be a candidate marker of Radix Eupatorii Chinensis because of good specificity in not only the herb but also some preparations of Radix Eupatorii Chinensis.
Figure 1.
Chemical structures of glycyrrhizic acid (A) and 12, 13-dihydroxyeuparin (B).
In this paper, an accurate, simple and convenient method for the simultaneous determination of 12, 13-dihydroxyeuparin and glycyrrhizic acid has been established, which can be used for the quality control of Yanyanfang mixture.
2. Experimental
2.1. Materials and reagents
Reference standard of ammonium glycyrrhetate was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Batch number: 110,731–200,615). Reference standard of 12, 13-dihydroxyeuparin was provided by Guangdong Natural Product Reference Material Research and Development Central Lab with purity of 98% (Batch number: 100,518). Yanyanfang mixtures were provided by the First Affiliate Hospital of Sun Yat-Sen University (Batch numbers: 100,901, 100,902, 100,903; 120 mL per bottle). The negative sample without Radix Eupatorii Chinensis and negative sample without Radix et Rhizoma Glycyrrhizae were homemade according to the pharmaceutical formulation of Yanyanfang mixture. Acetonitrile (HPLC grade) was purchased from Anhui Fulltime Specialized Solvents and Reagents Co., Ltd. (Anhui, China). Ultrapure water with a resistivity more than 18 mΩ was collected from a certified Option system (ELGA LabWater, UK). Other analytical reagents were purchased from Damao Chemical Reagents Works (Tianjin, China).
2.2. Apparatus and chromatographic conditions
HPLC analysis was carried out on a Shimadzu HPLC system (Shimadzu, Japan) equipped with an SIL-20A automatic sampler and an SPD-20A double-wavelength UV detector. The system was controlled by LC solution chromatography workstation. The analytical column was a Grace Apollo C18 (250 mm×4.6 mm, 5 μm) column kept at 30 ° during the analysis. The mobile phase consisted of acetonitrile (A) and aqueous phosphoric acid (0.2%, v/v) (B) pumped at 1.0 mL/min. Gradient elution began with a composition of 10% A lasting for 10 min and gradually changed to 22% A in the following 42 min and then kept for 10 min. For the next 25 min, it gradually changed to 63% A. The injection volume was 20 μL and the wavelength was set at 240 nm.
2.3. Preparation of standard solutions
2.3.1. 12, 13-Dihydroxyeuparin standard solution
An accurately weighed 12, 13-dihydroxyeuparin reference standard was dissolved in methanol-water (80:20, v/v) and diluted quantitatively to obtain a 4.57 μg/mL standard solution.
2.3.2. Ammonium glycyrrhetate standard solution
An accurately weighed ammonium glycyrrhetate reference standard was dissolved in methanol-water (80:20, v/v) and diluted quantitatively to obtain a 87.3 μg/mL standard solution (equal to 85.5 μg/mL for glycyrrhizic acid).
2.3.3. Mixed standard solution
Accurately weighed 12, 13-dihydroxyeuparin and ammonium glycyrrhetate reference standards were dissolved in methanol-water (80:20, v/v) and diluted stepwise to obtain a mixed standard solution of 3.66 μg/mL for 12, 13-dihydroxyeuparin and 87.3 μg/mL for ammonium glycyrrhetate (equal to 85.5 μg/mL for glycyrrhizic acid).
2.4. Sample solutions
An accurate volume of 5 mL Yanyanfang mixture was diluted to 25 mL with methanol-water (50:50, v/v) in a volumetric flask. The supernatant was filtered through a 0.45-μm membrane filter and prepared in duplicate. Each preparation was injected twice.
2.5. Negative solutions
Preparations of negative solutions were operated the same as sample solutions with negative sample without Radix Eupatorii Chinensis and negative sample without Radix et Rhizoma Glycyrrhizae.
3. Results and discussion
3.1. Selection of wavelength
The UV scanning spectrum of 12, 13-dihydroxyeuparin ranging from 200 to 400 nm revealed that its maximum absorption wavelength was at 240 nm while glycyrrhizic acid at 252 nm. Considering the content of 12, 13-dihydroxyeuparin in Yanyanfang mixture was much less than glycyrrhizic acid and the latter still had strong ultraviolet absorption at 240 nm, 240 nm was selected as detection wavelength in this method.
3.2. Specificity
The chromatographic behaviors of 12, 13-dihydroxyeuparin (Peak 1) and glycyrrhizic acid (Peak 2) were out of interference by other components, which indicated a good specificity for the method. The chromatograms are shown in Fig. 2.
Figure 2.
HPLC chromatograms of Yanyanfang mixture sample solution, mixed standard solution and negative solutions. (A) Yanyanfang mixture; (B) mixed standard solution; (C) negative solution without Radix Eupatorii Chinensis; (D) negative solution without Radix et Rhizoma Glycyrrhizae. (1) 12, 13-Dihydroxyeuparin; (2) glycyrrhizic acid.
3.3. Linearity
Calibration curves were constructed by plotting the peak area (Y) versus injection volume (X), with the result shown in Table 1.
Table 1.
Linearity of mixed standard solution.
| Compound | Regression equation | r | Range of concentration (μg/mL) |
|---|---|---|---|
| 12, 13-Dihydroxyeuparin | Y=36597X−93.52 | 1.0000 | 4.6–45.75 |
| Glycyrrhizic acid | Y=55743X−1092.5 | 1.0000 | 106.9–1068.9 |
3.4. Repeatability
Six sample solutions (Batch number: 100,903) were prepared for repeatability test. The result is listed in Table 2, indicating that the method is of great repeatability.
Table 2.
Repeatability of samples.
| No. | Concentration(μg/mL) |
|
|---|---|---|
| 12, 13-Dihydroxyeuparin | Glycyrrhizic acid | |
| 1 | 19.71 | 612.26 |
| 2 | 19.87 | 618.92 |
| 3 | 20.18 | 627.37 |
| 4 | 19.08 | 604.03 |
| 5 | 20.07 | 619.80 |
| 6 | 19.63 | 600.73 |
| Average content | 19.76 | 613.85 |
| RSD (%) | 1.98 | 1.65 |
3.5. Stability
A sample solution (Batch number: 100,903) stored at room temperature was analyzed at regular time within 24 h and was found to be stable within 24 h (Table 3).
Table 3.
Stability test of samples.
| Time (h) | Peak area |
|
|---|---|---|
| 12, 13-Dihydroxyeuparin | Glycyrrhizic acid | |
| 0 | 780,420 | 1,624,719 |
| 2 | 780,923 | 1,620,854 |
| 4 | 780,740 | 1,607,828 |
| 8 | 771,305 | 1,622,726 |
| 12 | 778,234 | 1,629,868 |
| 24 | 788,156 | 1,572,591 |
| Average peak area | 779,963 | 1,613,098 |
| RSD (%) | 0.69 | 1.31 |
3.6. Recovery
Recovery test was carried out by adding the standard solutions to known amounts of sample solutions. The results are summarized in Table 4, Table 5. These results indicated a good accuracy for this method.
Table 4.
Recovery test of 12, 13-dihydroxyeuparin.
| No. | Sampling volume (mL) | Initial amount (μg) | Added amount (μg) | Found amount (μg) | Recovery (%) | Average recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 39.52 | 18.30 | 58.17 | 101.91 | 102.18 | 1.43 |
| 2 | 39.52 | 18.30 | 58.10 | 101.53 | |||
| 3 | 39.52 | 18.30 | 57.84 | 100.11 | |||
| 4 | 39.52 | 36.60 | 76.57 | 101.23 | |||
| 5 | 39.52 | 36.60 | 76.32 | 100.55 | |||
| 6 | 39.52 | 36.60 | 77.73 | 104.40 | |||
| 7 | 39.52 | 54.90 | 96.25 | 103.33 | |||
| 8 | 39.52 | 54.90 | 96.23 | 103.30 | |||
| 9 | 39.52 | 54.90 | 96.23 | 103.30 |
Table 5.
Recovery test of glycyrrhizic acid.
| No. | Sampling volume (mL) | Initial amount (μg) | Added amount (μg) | Found amount (μg) | Recovery (%) | Average recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1228.16 | 516.59 | 1750.40 | 101.09 | 101.17 | 0.65 |
| 2 | 1228.16 | 516.59 | 1747.39 | 100.51 | |||
| 3 | 1228.16 | 516.59 | 1750.37 | 101.09 | |||
| 4 | 1228.16 | 1033.18 | 2272.01 | 101.03 | |||
| 5 | 1228.16 | 1033.18 | 2289.33 | 102.71 | |||
| 6 | 1228.16 | 1033.18 | 2274.81 | 101.30 | |||
| 7 | 1228.16 | 1549.77 | 2799.71 | 101.41 | |||
| 8 | 1228.16 | 1549.77 | 2790.89 | 100.84 | |||
| 9 | 1228.16 | 1549.77 | 2786.20 | 100.53 |
3.7. Application to assay of Yanyanfang mixtures
Three batches of Yanyanfang mixtures were analyzed and the results are shown in Table 6. There was no great variation in the content of 12, 13-dihydroxyeuparin and glycyrrhizic acid among different batches, indicating the stable manufacturing technique for Yanyanfang mixture.
Table 6.
Content of 12, 13-dihydroxyeuparin and glycyrrhizic acid in Yanyanfang mixtures.
| Batch number | 12, 13-Dihydroxyeuparin |
Glycyrrhizic acid |
||
|---|---|---|---|---|
| Content (μg/mL) | RSD (%) | Content (μg/mL) | RSD (%) | |
| 100,901 | 19.6 | 1.0 | 624.2 | 1.1 |
| 100,902 | 20.4 | 1.5 | 635.4 | 1.2 |
| 100,903 | 19.8 | 0.6 | 614.1 | 0.6 |
4. Conclusion
There were few reports on the determination of 12, 13-dihydroxyeuparin and glycyrrhizic acid in Yanyanfang mixture. The present study demonstrated that this method manifests good reliability and accuracy, which could be applied to the quality control of Yanyanfang mixture.
Acknowledgments
This work was supported by Guangdong Natural Product Reference Material Research & Development Central Lab, the First Affiliate Hospital of Sun Yat-sen University and the Industry-University-Research Cooperation Program from Science and Technology Department of Guangdong Province (No: 2010B090400533).
Contributor Information
Zhi-Sheng Xie, Email: cleverxzs@163.com.
Xin-Jun Xu, Email: xxj2702@sina.com.
References
- 1.Chinese Pharmacopeia Commission . vol. 1. China Medical Science Press; Beijing: 2010. (Pharmacopeia of the People's Republic of China (Version 2010)). pp. 80–81. (in Chinese) [Google Scholar]
- 2.Qi Min. The research progress on pharmacological action and function of glycyrrhizic acid. J. Mudanjiang Med. Univ. 2009;30(3):79–81. (in Chinese) [Google Scholar]
- 3.Guangdong Food and Drug Administration . vol. 1. Guangdong Science and Technology Press; Guangzhou, China: 2004. (Standard of Traditional Chinese Medicinal Materials for Guangdong Province (Version 2004)). pp. 21–22. (in Chinese) [Google Scholar]
- 4.Lu Q.W., Li J., Xiao P. Determination of adenosine in Radix Eupalorri Chinense Linn by RP-HPLC. J. Guangdong Coll. Pharm. 2007;23(2):131–133. (in Chinese) [Google Scholar]
- 5.Xie X.L., Xu X.J., Chen X. Preparation of 12, 13-dihydroxyeuparin reference substance from Radix Eupatorii Chinensis and Yanyanfang mixture. Lishizhen Med. Mater. Med. Res. 2010;21(7):1730–1732. (in Chinese) [Google Scholar]