Abstract
The present study was aimed at the comparison of the pharmacokinetics of pure chlorogenic acid and extract of Solanum lyratum Thunb. The animals were allocated to two groups, and were administered chlorogenic acid or extract of S. lyratum Thunb. at a dose of 50.0 mg/kg orally. Blood samples were collected up to 8 h post-dosing. Plasma chlorogenic acid analyses were performed using an HPLC method with UV detector. The pharmacokinetic parameters were evaluated using non-compartmental assessment. Significant differences existed in the two groups for AUC0−t, AUC0−∞ and CLz/F. The reliable HPLC method was successfully applied to the determination of chlorogenic acid in rat plasma at dosage of 50.0 mg/kg.
Keywords: Solanum lyratum Thunb, Chlorogenic acid, Pharmacokinetic, HPLC
1. Introduction
Solanum lyratum Thunb (Solanaceae) is one of the most valued Chinese traditional medicines. It is well known as “Hedrba Solani Lyrati” in mainland of China, which has been used for regulating body immune function and ability [1], [2], [3], [4]. It was also reported to have anticancer activity [5]. The plant is known to contain steroidal glucuronides, steroidal alkaloid glucosides, coumarin and phenolic acid, etc. [6], [7], [8], [9], [10].
Chlorogenic acid (3-O-caffeoylquinic acid) is composed of quinic acid and caffeic acid. It is the major active component of S. lyratum, and its amount could reach up to 3.0 mg/g [11]. The compound has a variety of biological activities such as anti-microbes, anti-virus, oxidation prevention, anti-tumor and anti-hypertension [12].
Several HPLC methods were developed for the pharmacokinetic studies of chlorogenic acid [13], [14], [15], [16], [17], [18]. Ren et al. [19] reported that the pharmacokinetic behavior of chlorogenic acid after oral administration has obvious difference among different dosages (200, 400 and 600 mg/kg). It is well known that the contents of active ingredients in traditional Chinese medicine are usually low, so the studies of their pharmacokinetic behavior at small dose (50.0 mg/kg) are important and necessary. In addition, other components in S. lyratum may change the pharmacokinetics of chlorogenic acid, and there was no report related to this issue.
In this study, a reliable HPLC method was established to determine the concentration of chlorogenic acid in rat plasma. The pharmacokinetic behaviors between chlorogenic acid and extract of S. lyratum after oral administration were compared. It is important for understanding of the synergism of components among S. lyratum and designing rational dosage regimens.
2. Materials and methods
2.1. Chemicals and reagents
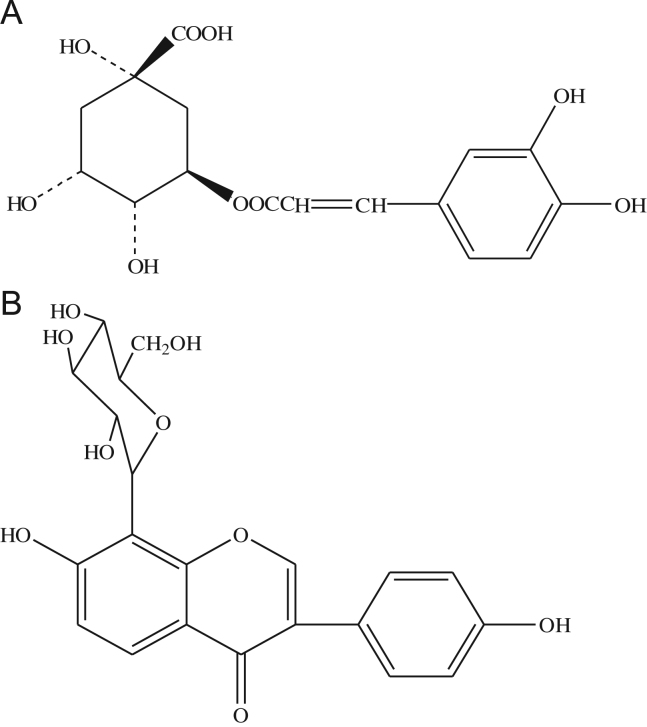
Chlorogenic acid (Fig. 1A) and the internal standard (IS), puerarin (Fig. 1B), were provided by the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). S. lyratum was purchased from Xuhui Pharmaceutical Co. Ltd. (Shanghai, China) and was genuinely identified by Prof. Qi-Shi Sun (Shenyang Pharmaceutical University, China). Methanol (HPLC grade) and other analytical grade reagents were obtained from Yuwang Reagent Company (Shenyang, China).
Figure 1.
Chemical structure of chlorogenic acid (A) and puerarin (B).
2.2. Instruments and chromatographic conditions
The HPLC system consisted of a Shimadzu LC-10A pump, an SPD-10AV detector and a column oven. Data were processed using Anastar software (Autoscience Instrument Co. Ltd., China).
The separation was performed on a Diamonsil-C18 column (250 mm×4.6 mm, 5 μm). The mobile phase was methanol–0.05% phosphoric acid (23:77, v/v) at a flow rate of 1 mL/min. UV detector was set at 327 nm and the column temperature was maintained at 30 °C. The sample injection volume was 20 μL.
2.3. Preparation of standards
The working solution of chlorogenic acid was prepared at concentrations over 0.250–75.0 mg/L in methanol. Quality control (QC) solutions were prepared at 0.50, 2.50, 60.0 mg/L in the same way. Internal standard working solution of 5.00 mg/L was prepared by dissolving the accurately weighed puerarin in methanol. All the solutions were stored at 4 °C away from light.
The calibration standards were prepared by spiking 100 μL of blank plasma with 20 μL chlorogenic acid standard solution to obtain final serial concentrations of 0.0500, 0.100, 0.200, 0.500, 1.00, 2.00, 5.00 and 15.0 mg/L. QC samples were prepared at concentrations of 0.100, 0.500 and 12.0 mg/L in the same manner.
2.4. S. lyratum extracts preparations
The dried and crushed herbs of S. lyratum (400 g) were extracted with 90% ethanol under refluxing three times for an hour per time. The crude extract obtained by vacuum concentration was subjected to absorbent resin (AB-8) column chromatography with a gradient of ethanol in water. The ratios of ethanol used during the procedure of column chromatography were 0, 20%, 40%, 60% and 90%, and initial eluant was water absolutely. LC with UV detector was used to monitor the elution process of chlorogenic acid. Chlorogenic acid (7.8 g) was separated from fraction 1 (using water as elute) and fraction 2 (using 20% ethanol as elute). The content of chlorogenic acid in the extractive was 15.1 mg/g detected by LC.
2.5. Plasma samples preparations
Plasma samples (100 μL) were spiked with 20 μL puerarin (IS, 5.00 mg/L) and were acidified by adding 10 μL hydrochloric acid (0.8 mol/L). The mixture was extracted with 2 mL isopropyl alcohol for 7 min. After centrifuged at 12,000 rpm for 5 min, the supernatant was transferred to another tube and evaporated to dryness under a stream of nitrogen at 40 °C. The residue obtained was reconstituted in 50 μL mobile phase, 20 μL of which was injected into the HPLC system.
2.6. Method validation
Blank rat plasma obtained from six different sources was processed and analyzed for the assessment of potential interferences with endogenous substances.
Calibration curves were constructed over the concentration range of 0.0500–15.0 mg/L by plotting the observed peak area ratios of chlorogenic acid and IS against known concentration of chlorogenic acid. The results were fitted to linear regression analysis using 1/x2 as weighting factor. The lower limit of quantification (LLOQ) was estimated using signal to noise ratio of 10.
The precision and accuracy were assessed by determining QC samples at three concentration levels on three different days. The accuracy was expressed by relative error (RE) and the precision was defined as the relative standard deviation (RSD).
The extraction recovery of chlorogenic acid at three QC levels was determined by comparing peak areas of chlorogenic acid with those obtained from direct injection of the corresponding standards dissolved in the supernatant of the processed blank plasma.
The stability was evaluated by determining QC samples (0.100, 0.500 and 12.0 mg/L) after storage at room temperature (25 °C) away from light for 24 h.
2.7. Pharmacokinetic study in rats
Male Wistar rats (240–260 g) were supplied by the Experimental Animal Center of Shenyang Pharmaceutical University. They were kept under controlled conditions (room temperature 22±2 °C, relative humidity 50±20%) for three days before the experiment start. Before drug administration, they were fasted overnight and allowed free access to water. Animal experiments were carried out in accordance with the Guidelines for Animal Experimentation of Shenyang Pharmaceutical University (Shenyang, China) and the procedure was approved by the Animal Ethics Committee of this institution.
Male Wistar rats were divided into two groups (six rats each group). The first group was given oral administration of chlorogenic acid at a dose of 50.0 mg/kg while the second group was given 3.31 g/kg extract of S. lyratum (equal to 50.0 mg/kg chlorogenic acid) orally. Blood samples (0.3 mL) were collected from the eye vein before (0 h) and 0.17, 0.33, 0.50, 0.75, 1.0, 2.0, 4.0, 6.0 and 8.0 h after administration into heparinized tubes. These samples were immediately centrifuged at 3000 rpm for 10 min, and the plasma obtained was stored at −20 °C until analysis.
2.8. Data analysis
Pharmacokinetic parameters were evaluated using non-compartmental assessment using the drug and statistics software (DAS, version 2.0, Pharmacology Institute of China). The area under the plasma concentration–time curve from zero to the time of the last measurable sample (AUC0−t) was calculated by the linear–trapezoidal method. The area under the plasma concentration–time curve from zero to infinity (AUC0−∞) was estimated by means of the trapezoidal rule with extrapolation to infinity with apparent elimination rate constant (Ke). The Ke was estimated by log–linear regression of concentrations observed during the terminal phase of elimination, and the corresponding elimination half-life (t1/2) was then calculated as 0.693/Ke. The maximum plasma concentration (Cmax) and their time of occurrence (Tmax) were obtained directly from the measured data. Pharmacokinetic parameters after oral administration of chlorogenic acid and extract of S. lyratum were expressed as mean±SD and were compared by a two one-sided t test using the Statistical Package for the Social Science (SPSS, version 16.0). The significant difference was defined as P<0.05.
3. Results
3.1. Method validation
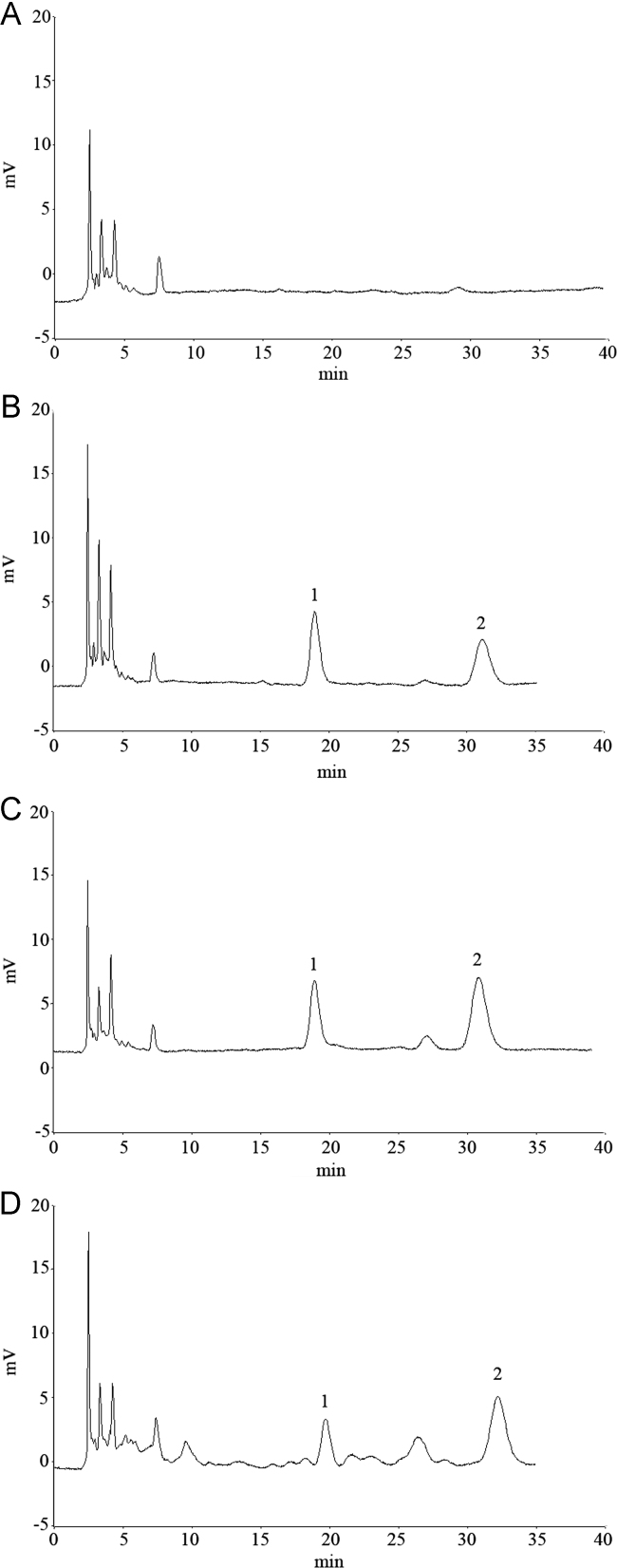
Typical chromatograms of blank plasma, blank plasma spiked with chlorogenic acid and IS, rat plasma sample 1 h after oral administration of chlorogenic acid and rat plasma sample 1 h after oral administration of extract of S. lyratum are presented in Fig. 2. The retention times for chlorogenic acid and IS were 18.5 and 31.7 min, respectively. The biological matrix did not interfere with the determination of chlorogenic acid and IS.
Figure 2.
Chromatograms of chlorogenic acid in rat plasma. A: blank plasma; B: plasma spiked with chlorogenic acid (0.500 mg/L) and puerarin (IS, 2.50 mg/L); C: plasma sample 1 h after oral administration of 50.0 mg/kg chlorogenic acid; D: plasma sample 1 h after oral administration of extract of S. lyratum. Peak 1, chlorogenic acid (18.5 min). Peak 2, puerarin (31.7 min).
The calibration curve of chlorogenic acid was linear in the range of 0.0500–15.0 mg/L with a mean correlation coefficient (r) (n=3) of 0.9982, a slope of 2.025 and an intercept of 4.498×10−2. The LLOQ obtained for the analyte was 0.0500 mg/L, with the RSD and RE of 7.6% and 9.1%, respectively.
The intra-day and inter-day precisions of chlorogenic acid at low to high concentrations were better than 1.8% and 8.6%, respectively. The accuracy (RE) of chlorogenic acid ranged from −6.8% to −1.8%. The results indicated that the reproducibility of the method was acceptable.
The mean extraction recovery (n=5) at three concentration levels was 68.6%, 71.8% and 72.9%, and RSD was 7.2%, 4.2% and 5.1%. Mean recovery of the IS was 90.5% with the RSD of 4.6% (n=15).
Stability of chlorogenic acid in prepared samples at room temperature (25°C) away from light for 24 h was determined by QC samples (n=5) at three concentration levels. RE of the three concentration levels was −3.6%, 5.3% and −8.5%, respectively.
3.2. Pharmacokinetics study
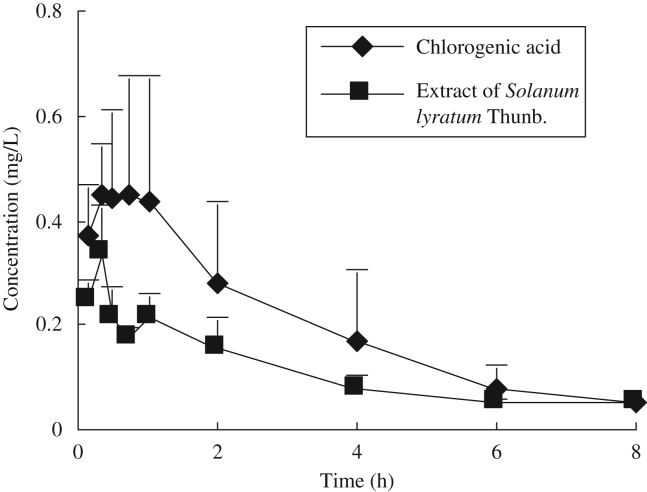
Comparative pharmacokinetics of chlorogenic acid was performed after oral administration of 50.0 mg/kg of chlorogenic acid or extract of S. lyratum (equal to 50.0 mg/kg chlorogenic acid) to rats. Pharmacokinetic parameters were obtained using non-compartmental methods. The mean plasma concentration–time curve profiles are illustrated in Fig. 3 and their pharmacokinetic parameters are listed in Table 1. Pharmacokinetic parameters after oral administration of chlorogenic acid and extract of S. lyratum were compared by two one-sided t test using SPSS program (version 16.0). The AUC0−t and AUC0−∞ for extract of S. lyratum were 0.76±0.26 and 0.83±0.27 mg h/L, and were significantly lower than those obtained after administration of chlorogenic acid. The clearance (CLz/F) for extract of S. lyratum was 65±21 L/(kg h), which was significantly higher than that after administration of pure chlorogenic acid.
Figure 3.
Plasma concentration versus time profiles of chlorogenic acid in rats (n=6) after oral administration.
Table 1.
Pharmacokinetic parameters after oral administration of chlorogenic acid (50.0 mg/kg) and extract of S. lyratum (equal to 50.0 mg/kg chlorogenic acid) (n=6/group).
| Parameters | Chlorogenic acid (P.O.) | Extract of S. lyratum (P.O.) |
|---|---|---|
| AUC0−t (mg h/L) | 1.50±0.91 | 0.76±0.26* |
| AUC0−∞ (mg h/L) | 1.61±0.92 | 0.83±0.27* |
| t1/2 (h) | 1.70±0.24 | 1.7±0.2 |
| Tmax (h) | 0.48±0.29 | 0.36±0.07 |
| CLz/F [L/(h kg)] | 39±17 | 65±21* |
| Vz/F (L/kg) | 97.5±47 | 154±33 |
| Cmax (mg/L) | 0.55±0.22 | 0.37±0.12 |
| Ke (1/h) | 0.41±0.06 | 0.41±0.19 |
Represent significant difference or P<0.05.
4. Discussion
4.1. Optimization of plasma samples preparations
Reagents like methanol, acetonitrile and perchloric acid were tested for protein precipitation; however, the recovery of chlorogenic acid was low. Chlorogenic acid was a biprotic acid with pKa1=3.59, pKa2=8.59, and was difficult to extract by liquid–liquid extraction in nearly neutral environment. So variety of concentrations (0.4, 0.6, 0.8, 1.0, 1.2 mol/L) of hydrochloric acid (10 μL) were tried to adjust pH of plasma samples. The results indicated that recovery of chlorogenic acid obviously increased with concentrations of hydrochloric acid until 0.8 mol/L, and endogenous interference increased significantly when concentration of hydrochloric acid was higher than 0.8 mol/L. Therefore, 10 μL of hydrochloric acid (0.8 mol/L) was added to plasma samples during sample preparation. Ethyl acetate, ethyl acetate–isopropanol (10:90, v/v) and isopropanol were tested as extract solvent for chlorogenic acid extraction from plasma samples. Isopropanol gave the highest recovery of chlorogenic acid, and was finally chosen as the extraction solvent.
4.2. Pharmacokinetic study
The current HPLC method was developed and validated for the determination of chlorogenic acid in rat plasma. The pharmacokinetics of chlorogenic acid after oral administration of pure chlorogenic acid and extract of S. lyratum was compared. The main differences between the pharmacokinetic data of the two groups reflected in the AUC0−t, AUC0−∞ and CLz/F, suggesting that other compounds in S. lyratum may prohibit the absorption of chlorogenic acid and accelerate the elimination of chlorogenic acid in rats. This phenomenon was reported for the first time, and further investigation is already in progress.
In addition, a second absorption peak appeared about 1 h after oral administration in both of the two plasma concentration–time curves, it may be caused by the reabsorption of hepatoenteral circulation. This phenomenon has not been reported before.
The major part of chlorogenic acid was not absorbed in the proximal part of the gut and reached the large intestine where it was hydrolyzed by the microflora [20], [21]. Most of chlorogenic acid was metabolized easily in intestine. The main metabolites were benzoic acid, benzene propanoic acid and cinnamic acid [22]. The information about the absorption and metabolism of chlorogenic acid may relate to the reabsorption of hepatoenteral circulation. Moreover, some other components of S. lyratum in intestine could restrain the absorption of chlorogenic acid.
5. Conclusion
In conclusion, pharmacokinetic behaviors of chlorogenic acid after oral dosing of chlorogenic acid and extract of S. lyratum had significant differences. The absorption and excretion of chlorogenic acid can be inhibited by the interaction between other components of S. lyratum. These conclusions could provide references for further studies on developing new dosage forms of S. lyratum. The results also confirmed the theory that monomeric compound was unable to represent the whole herb on the pharmacokinetic study of traditional Chinese medicine. To get reliable and accurate results, traditional Chinese medicine should be investigated as a whole.
Acknowledgments
The study was supported by the Science and Technology Bureau of Liaoning Province (No. 2007226011) and the Shenyang Technology Bureau (No. 1071164-9-00).
References
- 1.Kim H.M., Kim M.J., Li E. The nitric oxide-producing properties of Solanum Lyratum. J. Ethnopharmacol. 1999;67:163–169. doi: 10.1016/s0378-8741(99)00011-2. [DOI] [PubMed] [Google Scholar]
- 2.Kang B., Lee E., Hong I. Abolition of anaphylactic shock by Solanum lyratum Thunb. Int. J. Immunopharmacol. 1997;19(11/12):729–734. doi: 10.1016/s0192-0561(97)00035-0. [DOI] [PubMed] [Google Scholar]
- 3.Kim H.M., Lee E.J. Solanum lyratum inhibits anaphylactic reaction and suppresses the expression of l-histidine decarboxylase mRNA. Immunopharm. Immunol. 1998;20(1):135–146. doi: 10.3109/08923979809034813. [DOI] [PubMed] [Google Scholar]
- 4.Kang S.Y., Sang H., Park J.H. Hepatoprotective activity of scopoletin, a constituent of Solanum lyratum. Arch. Pharm. Res. 1998;21(6):7l8–722. doi: 10.1007/BF02976764. [DOI] [PubMed] [Google Scholar]
- 5.Kuo K.W., Hsu S.H., Li Y.P. Anticancer activity evaluation of the Solanum glycoalkaloid solamargine. Biochem. Pharmacol. 2000;60:1865–1873. doi: 10.1016/s0006-2952(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 6.Murakami K., Ezima H. Studies on the constituents of Solanum plants. V. The constituents of S. lyratum Thunb. II. Chem. Pharm. Bull. 1985;33(1):67–73. [Google Scholar]
- 7.Sun L.X., Fu W.W., Li W. Diosgenin glucuronides from Solanum lyratum and their cytotoxicity against tumor cell lines. Z. Naturforsch. C: Biosci. 2006;61c:171–176. doi: 10.1515/znc-2006-3-403. [DOI] [PubMed] [Google Scholar]
- 8.Sun L.X., Fu W.W., Ren J. Cytotoxic constituents from Solanum Lyratum. Arch. Pharm. Res. 2006;29(2):135–139. doi: 10.1007/BF02974274. [DOI] [PubMed] [Google Scholar]
- 9.Murakami K., Saijo R., Nohara T. Studies on the constituents of Solanum plants. I. On the constituents of the stem parts of Solanum lyratum THUNB. Yakugaku Zasshi. 1981;101(3):275–279. doi: 10.1248/yakushi1947.101.3_275. [DOI] [PubMed] [Google Scholar]
- 10.Yahara S., Morooka M., Ikeda M. Two new steroidal glucuronides from Solanum lyratum II. Planta. Med. 1986;6(12):496–498. doi: 10.1055/s-2007-969267. [DOI] [PubMed] [Google Scholar]
- 11.Qi W., Sun L.X., Zhao H.X. Simultaneous determination of chlorogenic acid and caffeic acid in Solanum lyratum Thunb. J. Shenyang Pharm. Univ. 2009;26(6):451–455. (in Chinese) [Google Scholar]
- 12.Wu W.H., Kang Z., Ouyang D.S. Progresses in the pharmacology of chlorogenic acid. Nat. Prod. Res. Dev. 2006;18(4):691–694. (in Chinese) [Google Scholar]
- 13.Li X.P., Yu J., Luo J.Y. Determination and pharmacokinetic study of chlorogenic acid in rat dosed with Yin-Huang granules by RP-HPLC. Biomed. Chromatogr. 2006;20(2):206–210. doi: 10.1002/bmc.555. [DOI] [PubMed] [Google Scholar]
- 14.Liao Q.F., Jia Y., Gao Q.T. High-performance liquid chromatographic method for determination and pharmacokinetic study of chlorogenic acid in the plasma of rats after administration of the Chinese medicinal preparation Luying decoction. Chromatographia. 2005;62(1–2):103–107. [Google Scholar]
- 15.Tsai T.H., Chen Y.F., Shum A.Y.C. Determination of chlorogenic acid in rat blood by microdialysis coupled with microbore liquid chromatography in pharmacokinetic application. J. Chromatogr. A. 2000;870(1–2):443–448. doi: 10.1016/s0021-9673(99)01153-x. [DOI] [PubMed] [Google Scholar]
- 16.He X., Zhao T.M., Jun X.D. Pharmacokinetic study on chlorogenic acid. Chin. Trad. Pat. Med. 1999;21(4):161–162. (in Chinese) [Google Scholar]
- 17.Rong G., Qiang Z., Tao G. Gradient high-performance liquid chromatography for the simultaneous determination of chlorogenic acid and baicalin in plasma and its application in the study of pharmacokinetics in rats. J. Pharm. Biomed. Anal. 2007;43(1):335–340. doi: 10.1016/j.jpba.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Ren X. Study on determination method and pharmaeokineties of chlorogenic acid in animals after mainline. Sichuan Univ., Chengdu, China. 2005:28–31. (in Chinese) [Google Scholar]
- 19.Ren J., Jiang X.H., Li C.R. Investigation on the absorption kinetics of chlorogenic acid in rats by HPLC. Arch. Pharm. Res. 2007;30(7):911–916. doi: 10.1007/BF02978845. [DOI] [PubMed] [Google Scholar]
- 20.Plumb G.W., Garcia-Conesa M.T., Kroon P.A. Metabolism of chlorogenic acid by human plasma, liver, intestine and gut microflora. J. Sci. Food Agric. 1999;79(3):390–392. [Google Scholar]
- 21.Couteau D., McCartney A.L., Gibson G.R. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001;90:873–881. doi: 10.1046/j.1365-2672.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonthier M.P., Verny M.A., Besson C. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003;133(6):1853–1859. doi: 10.1093/jn/133.6.1853. [DOI] [PubMed] [Google Scholar]