Abstract
Recurrent Guillain-Barré syndrome (GBS) is a rare, immune-mediated disease of the peripheral nervous system. It has been reported to occur at intervals ranging from four months to 10 years; published case studies suggest that 1%–6% of patients who have had GBS will experience recurrent attacks. The most commonly identified infections coinciding with GBS are Campylobacter jejuni, Haemophilus influenzae, Mycoplasma pneumonia, and cytomegalovirus, while an antecedent infection with Escherichia coli is very uncommon. In this case report, we present a rare episode of recurrent GBS, which followed a urinary tract infection (UTI) by E. coli, and an accompanying literature review. A 75-year-old woman with a prior history of acute motor axonal neuropathy (AMAN), a subtype of GBS, presented with subsequent weakness of limbs and areflexia following 10 days of fever, frequency, and dysuria. Base on nerve conduction studies, cerebrospinal fluid analysis and other clinical investigation, we diagnosed the patient with recurrent GBS caused by E. coli. The patient recovered with mild subjective weakness following treatment of intravenous immunoglobulin with ceftriaxone. We suggest that E. coli causes UTI could be one of the diverse trigger factors involved in recurrent GBS.
Keywords: Guillain-Barré Syndrome, Polyneuropathies, Urinary Tract Infection, Uropathogenic Escherichia coli
Graphical Abstract
INTRODUCTION
Guillain-Barré syndrome (GBS) is an acute inflammatory polyradiculoneuropathy; an immune-mediated disease involving the peripheral nervous system that is considered a neurological emergency warranting prompt diagnosis and treatment.1 GBS is generally considered to be monophasic, but recurrent Guillain-Barré syndrome (RGBS), defined as two or more episodes of acute monophasic neuropathy with near or complete recovery between episodes,2 can occur in 1%–10% of patients, depending on the length of the asymptomatic period.3 Although the cause of GBS is still unclear, antecedent infections are recognized as triggering agents and about two-thirds of GBS patients report infections with Campylobacter jejuni, cytomegalovirus (CMV), Epstein-Barr virus (EBV), or Mycoplasma pneumoniae. Conversely, cases of GBS in which Escherichia coli was an antecedent infectious agent are very rare.4 To our knowledge, this is the first documented case in which RGBS follows a urinary tract infection (UTI) by E. coli.
CASE DESCRIPTION
A 75-year-old woman with a prior history of acute motor axonal neuropathy (AMAN), a subtype of GBS, presented to the emergency department following 10 days of fever, frequency, and dysuria on February 20, 2015. Additionally, she had experienced four days of bilateral upper and lower limbs weakness with areflexia, and one day of worsening dyspnea. Vital signs revealed a temperature of 38.5°C and an oxygen saturation of 92% on room air.
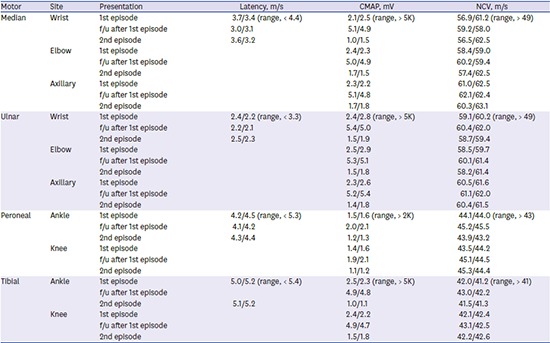
The patient's first episode manifested weakness of upper and lower limbs with areflexia accompanied by dyspnea, fever, and urinary frequency and dysuria on October 15, 2015. Nerve conduction studies (NCSs) were performed on her fourth day of first admission. NCSs revealed axonal motor type polyneuropathy (Table 1). No organisms grew in a culture of the patient's stool and sputum. The urine analysis showed pyuria, and a cultured urine sample revealed the presence of E. coli. The patient was diagnosed with AMAN and the cause was determined to be a UTI, following an E. coli infection. She was treated with intravenous immunoglobulins (IVIGs) and third generation cephalosporin (ceftriaxone). When re-examined three months after treatment, the patient had a nearly complete recovery and showed significant improvement in follow-up NCSs (Table 1).
Table 1. Nerve conduction study of the patient in 1st episode, follow-up, and 2nd episode.
| Motor | Site | Presentation | Latency, m/s | CMAP, mV | NCV, m/s |
|---|---|---|---|---|---|
| Median | Wrist | 1st episode | 3.7/3.4 (range, < 4.4) | 2.1/2.5 (range, > 5K) | 56.9/61.2 (range, > 49) |
| f/u after 1st episode | 3.0/3.1 | 5.1/4.9 | 59.2/58.0 | ||
| 2nd episode | 3.6/3.2 | 1.0/1.5 | 56.5/62.5 | ||
| Elbow | 1st episode | 2.4/2.3 | 58.4/59.0 | ||
| f/u after 1st episode | 5.0/4.9 | 60.2/59.4 | |||
| 2nd episode | 1.7/1.5 | 57.4/62.5 | |||
| Axillary | 1st episode | 2.3/2.2 | 61.0/62.5 | ||
| f/u after 1st episode | 5.1/4.8 | 62.1/62.4 | |||
| 2nd episode | 1.7/1.8 | 60.3/63.1 | |||
| Ulnar | Wrist | 1st episode | 2.4/2.2 (range, < 3.3) | 2.4/2.8 (range, > 5K) | 59.1/60.2 (range, > 49) |
| f/u after 1st episode | 2.2/2.1 | 5.4/5.0 | 60.4/62.0 | ||
| 2nd episode | 2.5/2.3 | 1.5/1.9 | 58.7/59.4 | ||
| Elbow | 1st episode | 2.5/2.9 | 58.5/59.7 | ||
| f/u after 1st episode | 5.3/5.1 | 60.1/61.4 | |||
| 2nd episode | 1.5/1.8 | 58.2/61.4 | |||
| Axillary | 1st episode | 2.3/2.6 | 60.5/61.6 | ||
| f/u after 1st episode | 5.2/5.4 | 61.1/62.0 | |||
| 2nd episode | 1.4/1.8 | 60.4/61.5 | |||
| Peroneal | Ankle | 1st episode | 4.2/4.5 (range, < 5.3) | 1.5/1.6 (range, > 2K) | 44.1/44.0 (range, > 43) |
| f/u after 1st episode | 4.1/4.2 | 2.0/2.1 | 45.2/45.5 | ||
| 2nd episode | 4.3/4.4 | 1.2/1.3 | 43.9/43.2 | ||
| Knee | 1st episode | 1.4/1.6 | 43.5/44.2 | ||
| f/u after 1st episode | 1.9/2.1 | 45.1/44.5 | |||
| 2nd episode | 1.1/1.2 | 45.3/44.4 | |||
| Tibial | Ankle | 1st episode | 5.0/5.2 (range, < 5.4) | 2.5/2.3 (range, > 5K) | 42.0/41.2 (range, > 41) |
| f/u after 1st episode | 4.9/4.8 | 43.0/42.2 | |||
| 2nd episode | 5.1/5.2 | 1.0/1.1 | 41.5/41.3 | ||
| Knee | 1st episode | 2.4/2.2 | 42.1/42.4 | ||
| f/u after 1st episode | 4.9/4.7 | 43.1/42.5 | |||
| 2nd episode | 1.5/1.8 | 42.2/42.6 |
Data shown are right/left.
CMAP = compound muscle action potential, NCV = nerve conduction velocity, f/u = follow-up.
The patient's second episode occurred eight months after her first, and manifested generalized weakness and dysuria. Areflexia and weakness were more severe in distal muscle groups than proximal muscles in both the upper and lower limbs (upper limb: distal Medical Research Council [MRC] grade 3, proximal MRC grade 4; lower limb: distal MRC grade 3, proximal MRC grade 4). Sensory abnormality and ataxia were absent. NCSs performed one week after neurological deterioration revealed that the amplitude of compound muscle action potentials (CMAPs) was reduced in the median, ulnar, tibial, and peroneal nerves. Sensory nerve action potentials of the median, ulnar, and sural nerves were within the normal range (Table 1). Analysis of the cerebrospinal fluid (CSF) revealed elevated protein levels (155 mg/dL) with albuminocytologic dissociation; however, her glucose levels were normal (64 mg/dL). The following analyses all yielded negative results: serology for C. jejuni, varicella-zoster virus, and CMV and tests for hepatitis A, B, C, and E, as well as human immunodeficiency virus, EBV, herpes virus, and adenovirus. However, urine analysis showed pyuria, and a culture of the urine revealed E. coli. An antibiotic sensitivity/susceptibility test of the urine showed the same result (Table 2), and the serum tested positive for the gangliosides GM1 and GD1a, using an immunoglobulin G (IgG) antibody. We diagnosed the patient with RGBS, caused by UTI, and treated her with IVIG and ceftriaxone. Her muscle weakness and dyspnea gradually improved over one-month period. At her follow-up, four months after discharge, NCSs of the patient demonstrated improved CMAPs in the median, ulnar, and peroneal nerves.
Table 2. Culture and sensitivity for urine and cerebrospinal fluid of patient.
| Presentation | 1st episode | 2nd episode | |
|---|---|---|---|
| Specimen | Urine | Urine | |
| Culture | Escherichia coli | Escherichia coli | |
| Sensitivity | |||
| Extended-spectrum beta-lactamases | Negative | Negative | |
| Ampicillin/clavulanic acid | R | R | |
| Amoxicillin | S | S | |
| Piperacillin/tazobactam | S | S | |
| Cefazolin | S | S | |
| Cefoxitin | S | S | |
| Cefotaxime | S | S | |
| Ceftazidime | S | S | |
| Cefepime | S | S | |
| Aztreonam | S | S | |
| Ertapenem | S | S | |
| Imipenem | S | S | |
| Amikacin | S | S | |
| Gentamicin | S | S | |
| Ciprofloxacin | S | S | |
| Tigecycline | S | S | |
| Trimethoprim/sulfamethoxazole | R | R | |
S = sensitive, R = resistant.
DISCUSSION
In this report, we describe a novel case of RGBS, in which the patient presented with symptoms following a UTI caused by E. coli infection. Two-thirds of patients with GBS have antecedent respiratory or gastrointestinal symptoms, and a number of infectious agents have been implicated in the pathogenesis of GBS. Conversely, UTIs, which are predominantly cause by uropathogenic E. coli (UPEC), are one of the most common bacterial infections encountered in clinical practice and account for significant morbidity, yet GBS cases in which an antecedent UTI or infection with E. coli have been implicated are rare.4
E. coli has traditionally been regarded as an extracellular pathogen; however, in recent years UPEC has been shown to be an opportunistic intracellular pathogen. Upon entering the cytosol of facet cells in the bladder UPEC rapidly multiply, forming large inclusions called intracellular bacterial communities (IBCs),5 which may be associated with UTI recurrence.6 The patient in our case was an elderly woman, and postmenopausal women aged > 70 years face unique challenges with regard to recurrent UTIs, due to institutionalization, decreased functional status, and a number of other risk factors.7 In particular, reduced levels of estrogen can increase vaginal pH, decrease endogenous vaginal microflora, and increase the incidence of prolapse due to muscle weakness. Each of these factors may have predisposed our patient to UPEC colonization.
Our patient presented with two separate episodes of areflexic quadriparesis, occurring eight months apart. The second episode followed a nearly complete recovery from the first; for eight months, there was no evidence or symptoms of the preceding infection. Therefore, we believe that the case of RGBS described herein may have been caused by a UPEC, in which IBCs were formed.
The present patient developed AMAN with positive IgG antibody to GM1 and GD1a. It is known that there was a strong association between AMAN and IgG antibodies against GM1, GD1a.8 However, no data is available addressing the homology between lipopolysaccharide of E. coli and the GM1, GD1a ganglioside antibodies.
Taken together, our results indicate that E. coli may be an under-recognized and underdiagnosed pathogen in GBS cases. We further suggest patients with GBS, in which symptoms manifest following a UTI caused by an E. coli infection, may be more prone to recurrences, and clinicians should be aware of this, as well as of the diverse trigger factors involved in RGBS. Examination of the exact pathophysiological mechanisms linking RGBS and UPEC, in IBC, requires additional experimental studies.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Chung H, Na SJ. Investigation: Jo YS, Choi JY, Chung H, Na SJ. Project administration: Chung H, Na SJ. Writing - original draft: Jo YS. Writing - review & editing: Kim Y, Na SJ.
References
- 1.Tse AC, Cheung RT, Ho SL, Chan KH. Guillain-Barré syndrome associated with acute hepatitis E infection. J Clin Neurosci. 2012;19(4):607–608. doi: 10.1016/j.jocn.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Mossberg N, Nordin M, Movitz C, Nilsson S, Hellstrand K, Bergström T, et al. The recurrent Guillain-Barré syndrome: a long-term population-based study. Acta Neurol Scand. 2012;126(3):154–161. doi: 10.1111/j.1600-0404.2012.01667.x. [DOI] [PubMed] [Google Scholar]
- 3.Grand'Maison F, Feasby TE, Hahn AF, Koopman WJ. Recurrent Guillain-Barré syndrome. Clinical and laboratory features. Brain. 1992;115(4):1093–1106. doi: 10.1093/brain/115.4.1093. [DOI] [PubMed] [Google Scholar]
- 4.Kono Y, Nishitarumizu K, Higashi T, Funakoshi K, Odaka M. Rapidly progressive Guillain-Barré syndrome following Escherichia coli infection. Intern Med. 2007;46(9):589–591. doi: 10.2169/internalmedicine.46.6330. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 6.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4(12):e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raz R, Gennesin Y, Wasser J, Stoler Z, Rosenfeld S, Rottensterich E, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis. 2000;30(1):152–156. doi: 10.1086/313596. [DOI] [PubMed] [Google Scholar]
- 8.Ogawara K, Kuwabara S, Mori M, Hattori T, Koga M, Yuki N. Axonal Guillain-Barré syndrome: relation to anti-ganglioside antibodies and Campylobacter jejuni infection in Japan. Ann Neurol. 2000;48(4):624–631. [PubMed] [Google Scholar]


