Abstract
An efficient method is provided to detect simultaneously some important veterinary drugs from different classes in highly complex animal tissue matrix. This method using matrix solid-phase dispersion (MSPD) and high performance liquid chromatography (HPLC) with diode array detection (DAD) is developed to effectively determine two fluoroquinolones (enoxacin and lomefloxacin), two sulfonamides (sulfanilamide and sulfamethoxazole) and one tetracycline (tetracycline) simultaneously in porcine tissues. In the process, MSPD methodology was used to treat samples, washed by n-hexane to remove lipid, eluted the analytes with acetonitrile–dichloromethane (1:1, v/v). Solvent acetonitrile and solvent acetic acid (0.1%) were combined in a gradient. HPLC–DAD analysis of the tissue samples was performed within 15 min at a flow rate of 1.0 mL/min. The results showed that a recovery at 0.1, 0.5 and 1.0 μg/g fortification levels ranged from 80.6% to 99.2% with satisfactory relative standard deviations (RSDs) (below 6.1%, n=3) and the limits of quantitation (LOQ) ranged from 7 μg/kg to 34 μg/kg in porcine tissues. Utilization of the method in successfully simultaneous analysis of porcine tissue incurred with veterinary drug multiresidues is described.
Keywords: Matrix solid-phase dispersion, High performance liquid chromatography, Fluoroquinolones, Sulfonamides, Tetracyclines, Multiresidues
1. Introduction
Veterinary drugs are used inevitably in animal breeding for therapeutic or disease-preventive reasons in parallel with promoting growth of livestock [1]. Such compounds have become an integral part of the livestock-producing industry. However, when withdrawal periods are not obeyed, unsafe antibiotic residues, or their metabolites, may be present in edible products such as milk, eggs and meat. It is reported that the traces of antibiotics in food can be dangerous for consumers because of their direct toxicity and the emergence of antibiotic-resistant bacteria [1], [2]. Currently, the fluoroquinolones (FQs), sulfonamides (SAs) and tetracyclines (TCs) are types of broad spectrum antibiotics, which are used commonly in human and animal medicine. Some of FQs have many effects, such as carcinogenicity, mutation and so on, and several of them may cause photosensitization and allergic reaction, etc. [3], [4], [5]. SAs can cause side effects that make micturition and hematopoietic disorder [6], and TCs can damage liver and kidney and influence the growth of skeleton and other side effects [7]. And furthermore, all of the above-mentioned veterinary drugs can enhance the drug-resistance of bacteria [2]. Human health will be threatened by excess residues of FQs, SAs and TCs in the animal products.
To prevent consumers from suffering with the possible health problems, the authorities have regulated the use of veterinary drugs by setting the maximum residue limits (MRLs) or by prohibiting the use of many substances. According to the US Food and Drug Administration (FDA), none of the (FQs are allowed to be present in the food supply. China and the European Union (EU) have established MRLs for FQs in foods of animal origin at 10–1900 μg/kg [8], [9]. MRLs in animal food products, established by EU, Japan, America and China, equals 100 μg/kg for SAs and tetracycline (TC) [10].
This study focuses on these groups of antibiotics in developing a sensitive method that can be utilized to investigate their fate in complicated animal tissues matrix. Multiresidue methods, which enhance the efficiency of analysis, are available for determination of FQs, SAs and TCs in wastewater [11], [12]. Multiresidue methods, which will simultaneously determine more than one class of veterinary drugs in any matrix, are still limited and are largely confined to liquid chromatography–mass spectrometry (LC–MS) methods [13], [14]. LC–MS methods are capable of identifying individual antibiotics within a class but involve relatively expensive and complex instrumentation, which may not always be available for routine monitoring. LC–MS methods can be valuable when confirmation is required, but are not always necessary for quantitation. LC with fluorescence detection (FLD) has been reported to have a low detection limit. However, the technique certainly requires derivatization to improve the fluorescence properties for detection [15]. The use of diode array detector (DAD) as a detector for high performance liquid chromatographic (HPLC) has proved to be a powerful tool in the determination and identification of compounds as it makes possible the on-line acquisition of their UV spectra. In addition, most of the above mentioned methods are for one class of antibiotics only [16], [17], [18], [19], [20], [21]. A challenge is presented in the simultaneous extraction and analysis of multiple classes of compounds.
The aim of this study is to develop a method for simultaneous determination of selected antibiotics drugs: FQs [Enoxacin (ENO) and lomefloxacin (LOM)], SAs [sulfanilamide (SN) and sulfamethoxazole (SMZ)] and TCs [tetracycline (TC)] (Fig. 1) in high complex porcine tissues matrix. The method involves sample pre-treatment with matrix solid-phase dispersion (MSPD) and analytical determination with high performance liquid chromatography (HPLC) coupled with diode array detector (DAD).
Figure 1.
Chemical structures of the selected drugs in this study.
2. Experimental
2.1. Materials
Enoxacin (ENO, 91.1%), lomefloxacin (LOM, 90.0%), sulfanilamide (SN), sulfamethoxazole (SMZ) and tetracycline (TC) were obtained from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). HPLC-grade methanol (MeOH) and acetonitrile (ACN) were obtained from Burdick & Jackson (Muskegon, MI, USA) and HPLC-grade acetic acid (HAc) from Kermel Chemical Reagents Development Centre (Tianjin, China). C18 (50 μm) was obtained from Baseline Chrom. Tech. (Tianjin, China). Triple distilled water (18.3 MΩ cm resistivity) was prepared by a Molelement water purification system (Molecular, Shanghai, China). All solutions prepared for HPLC were filtered through a 0.45 μm filter before used. Porcine tissues, which were purchased from a local food market, were served as samples and were deep-frozen prior to the analysis.
2.2. Standard solutions
Individual standard stock solutions such as ENO, LOM, SN, SMZ and TC with concentration of 500 μg/mL were diluted with 10% methanol and stored protected from light at 4 °C. A fortification mixture of ENO, LOM, SN, SMZ and TC (10 μg/mL) in 10% methanol was prepared from these stock solutions on the day of the analysis. When the lower level of fortification solution was required, additional dilution with 10% methanol was conducted. To ensure an accurate analysis, the preparation of fortification solutions was performed on the day of the analysis.
2.3. The procedure of matrix solid-phase dispersion (MSPD)
The porcine tissue samples were cut into pieces and blended. 0.50 g sample was placed in a glass mortar with the external diameter of 90 mm, and the standard mixture solution was added. The sample was placed in the dark chamber sitting for 20 min. Two grams C18, 0.05 g EDTA–Na2 and 0.05 g oxalic acid were then added in the mortar and gently ground with the sample with a pestle to obtain a homogeneous material. One gram anhydrous sodium sulfate, 0.25 g C18, the C18/tissue matrix blend and 0.5 g anhydrous sodium sulfate were introduced in order into a 10 mL syringe barrel pre-plugged with a filter disk, and the barrel was then placed on a vacuum manifold. Flow was controlled at 1.0 mL/min. The C18/tissue matrix blend was washed with 6 mL n-hexane to remove lipids, and eluted with 8 mL ACN–dichloromethane (1:1, v/v). The eluate was evaporated to dryness with a gentle stream of air, and then the residue was dissolved in 1 mL 10% methanol. The final solution was filtered through a 0.45 μm disposable syringe filter unit and 20 μL volume of the filtrate was injected into the HPLC system.
2.4. HPLC–DAD analysis
The LC analyses were accomplished with an LC-10Avp (Shimadzu, Japan) HPLC system consisting of an LC-10ATvp secondary pump system, DGU-12A on-line degasser, CTO-10ASvp thermostatted column compartment and SPD-M10Avp diode array detector. CLASS-VP software controlled the LC components and processed ultraviolet data and a Kromasil C18 chromatography column (150 mm×4.6 mm, 5 μm) was used.
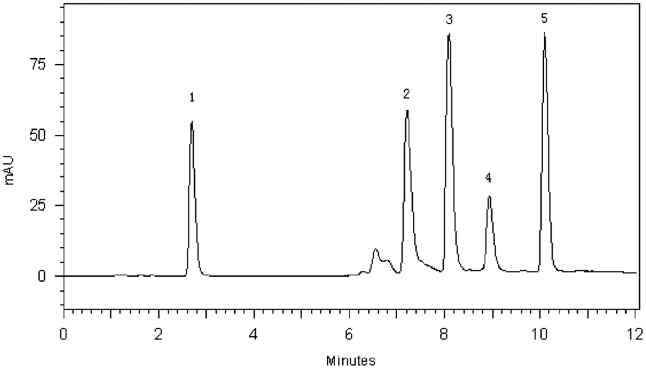
Solvent A (0.1% HAc) and solvent B (MeOH) were combined in a gradient as follows: 15–25% B (3 min), 25–45% B (3 min), 45% B (5 min), 45–15% B (4 min). The flow rate was 1.0 mL/min, and the column heater was set at 25 °C. The investigated drugs were eluted for 15 min and a 15 min post time allowed re-equilibration of the column. ENO, LOM, SN, SMZ and TC were monitored at absorbance wave-length of 280 nm (Fig. 2). The retention time for the drugs is shown in Table 1.
Figure 2.
HPLC–DAD chromatograms of a 5 μg/mL standard solution, λ=280 nm. 1, SN; 2, ENO; 3, LOM; 4, TC; 5, SMZ.
Table 1.
The maximum UV-detection wavelengths and retention time of the five drugs.
| Drugs | λ (nm) | Typical retention time (min) |
|---|---|---|
| SN | 259 | 2.69 |
| ENO | 269 | 7.21 |
| LOM | 288 | 8.08 |
| TC | 264 | 8.93 |
| SMZ | 270 | 10.09 |
3. Results and discussion
3.1. The procedure for MSPD
The traditional extraction–purification of the antibiotics involves numerous and varying analytical steps, which are labor intensive and time consuming. In this study the matrix solid-phase dispersion extraction (MSPD) first developed by Staren Barker et al. [22] was chosen, for its easy use, possible automation, and multiresidue potential. MSPD involves homogenizing and dispersing of a small amount of matrix with adsorbent (usually C18 or C8), washing with a small amount of solvent and elution to extract a wide range of compounds. The MSPD mechanism appears to encompass sample homogenization, cellular disruption, extraction, fractionation and purification in one single process.
3.1.1. Optimization of the rinsing and eluting conditions
Based on 2.0 g C18 and 0.50 g porcine tissue, the rinsing and eluting conditions were investigated (the spiking level was 0.5 μg/g). n-Hexane, the different mixture of ACN and CH2Cl2, and the mixture of MeOH and CH2Cl2 were used to optimize the rinsing and eluting conditions. The average recoveries of the drugs in different rinsing and eluting conditions are shown in Table 2.
Table 2.
Recoveries (%) of the drugs in different ringing and eluting conditions.
| Rinsing and eluting solvents | SN | ENO | LOM | TC | SMZ |
|---|---|---|---|---|---|
| 6 mL C6H14 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 8 mL CH3CN/CH2Cl2 (v/v=1:3) | 93.5 | 80.2 | 85.1 | 78.6 | 90.7 |
| 8 mL CH3CN/CH2Cl2 (v/v=1:1) | 94.3 | 93.1 | 95.3 | 79.6 | 86.4 |
| 8 mL CH3CN/CH2Cl2 (v/v=3:1) | 68.6 | 90.2 | 94.3 | 80.8 | 62.0 |
| 8 mL CH3OH/CH2Cl2 (v/v=1:1) | 84.3 | 75.3 | 77.5 | 75.0 | 80.9 |
n.d.: not detected; the relative standard deviations (RSDs) were between 2.5% and 9.6% (n=5).
The data in Table 2 indicates that 6 mL n-hexane cannot elute the drugs. When eluting the drugs with 8 mL ACN/CH2Cl2 (1:3, v/v), the recoveries of two SAs were >90%. When eluting the drugs with 8 mL ACN/CH2Cl2 (3:1, v/v), the recoveries of two FQs were >90%. However, the best recoveries of all the drugs were obtained with 8 mL ACN/CH2Cl2 (1:1, v/v). Therefore, the proper rinsing and eluting solvents were 6 mL n-hexane and 8 mL ACN/CH2Cl2 (1:1, v/v), respectively. Based on the above procedures, the rinsing and eluting conditions for porcine tissue were tested, and similar results were obtained. Under the optimum conditions, the recoveries of the drugs were all above 80%. The results demonstrate that the MSPD-based method can reduce analysis time, solvent waste, and cost without affecting the quality of residue detection and measurement.
3.1.2. Optimization of the eluting solvent volume
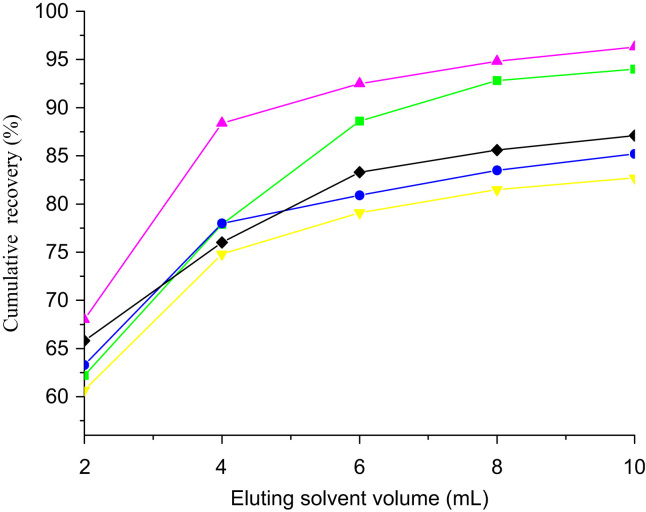
The effect of the eluting solvent volume was studied by collecting each drug in every 0.50 g porcine tissue during eluting with a sample fortified at 0.5 μg/g. The cumulative recoveries on eluting with 10 mL of ACN are shown in Fig. 3. The recovery for each drug increased rapidly to 70% after elution with 4 mL of eluent, followed by a slow increase in the eluent volume and finally reaching an equilibrium value with 8 mL of eluent. The eluent volume used in the subsequent studies was accordingly set at 8 mL.
Figure 3.
Cumulative recoveries of the five drugs in porcine tissue at 0.5 μg/g as a function of eluent volume.  =SN;
=SN;  =ENO;
=ENO;  =LOM;
=LOM;  =TC; =SMZ.
=TC; =SMZ.
3.2. HPLC–DAD
3.2.1. HPLC conditions
The isolation of FQs, SAs and TCs, and their separation from matrices are complicated, since their groups have a propensity to form chelate complexes with metal ions and sample matrix proteins, and interact strongly with silanol groups of siliceous sorbents. In this paper, EDTA–Na2 and oxalic acid were added in the MSPD-process to chelate with metal ions. Improved resolution of these different components was achieved by manipulating the solvent and additive composition (HAc, ACN, MeOH were used), the volume proportion of the solvents in the mobile phase and the concentration of HAc. MeOH (B) and 0.1% HAc (A) were selected. Further improvement in separation was obtained by mobile phase gradient. The gradient condition is as follows: 15–25% B (3 min), 25–45% B (3 min), 45% B (5 min), 45–15% B (4 min). Experimental conditions selected enable separation in 15 min.
3.2.2. Identification
The HPLC–DAD method chosen allows the separation of the drugs and identification of them by their retention time and their spectra (see Table 1 and Fig. 2). With a photodiode array detector, the absorption spectra of ENO, LOM, SN, SMZ and TC standards in the mobile phase were measured for the selection of the HPLC monitoring wavelength. The measurement was conducted at 280 nm, which gave an average maximum absorbance for all of the drugs.
3.3. Method validation
3.3.1. Linearity
Eight point calibration curves were prepared for each analysis day. Quantitation utilized the UV peak area for each drug. The calibration curves were found to be linear over the range of 0.001–10 μg/mL studied (0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10 μg/mL levels were used). The linear equations, correlation coefficients and linear range of the drugs are presented in Table 3. The results indicate that the correlation coefficient is equal to 0.9987–0.9997.
Table 3.
Linear equations, correlation coefficients and linear range of the five drugs.
| Compound | Regression equation | Correlation coefficient (r) | Linear range (μg/mL) |
|---|---|---|---|
| SN | y=4.4×104x+1.8×103 | 0.9997 | 0.005–10 |
| ENO | y=3.8×104x−9.7×102 | 0.9997 | 0.005–10 |
| LOM | y=7.2×104x+1.7×104 | 0.9992 | 0.005–10 |
| TC | y=2.0×104x−7.5×102 | 0.9987 | 0.01–10 |
| SMZ | y=7.5×104x+2.4×103 | 0.9997 | 0.001–10 |
y: peak area; x: concentration (μg/mL).
3.3.2. Intra-day and inter-day repeatability
Analysis of the calibration standards was applied in determining the intra-day (three repetitions of each concentration) and inter-day repeatability (three repetitions of each concentration, three days). The results (for three levels) are shown in Table 4. The intra-day RSDs were lower than 9.3% and lower than 10.2% for inter-day assays. These results indicate that the method developed had acceptable precision.
Table 4.
Intra-day and inter-day repeatability.
| Analytes | Amount injected (ng) | Intra-day repeatability | Inter-day repeatability |
|---|---|---|---|
| RSD (n=3) (%) | RSD (n=9) (%) | ||
| SN | 1.0 | 3.6 | 4.2 |
| 0.5 | 4.0 | 5.7 | |
| 0.1 | 4.8 | 6.0 | |
| ENO | 1.0 | 1.5 | 2.3 |
| 0.5 | 2.8 | 3.1 | |
| 0.1 | 3.5 | 4.0 | |
| LOM | 1.0 | 4.6 | 4.8 |
| 0.5 | 4.2 | 4.9 | |
| 0.1 | 5.4 | 5.9 | |
| TC | 1.0 | 2.1 | 2.7 |
| 0.5 | 5.2 | 5.8 | |
| 0.1 | 9.3 | 10.2 | |
| SMZ | 1.0 | 2.0 | 2.6 |
| 0.5 | 3.3 | 5.2 | |
| 1.0 | 3.9 | 5.5 | |
3.3.3. Accuracy
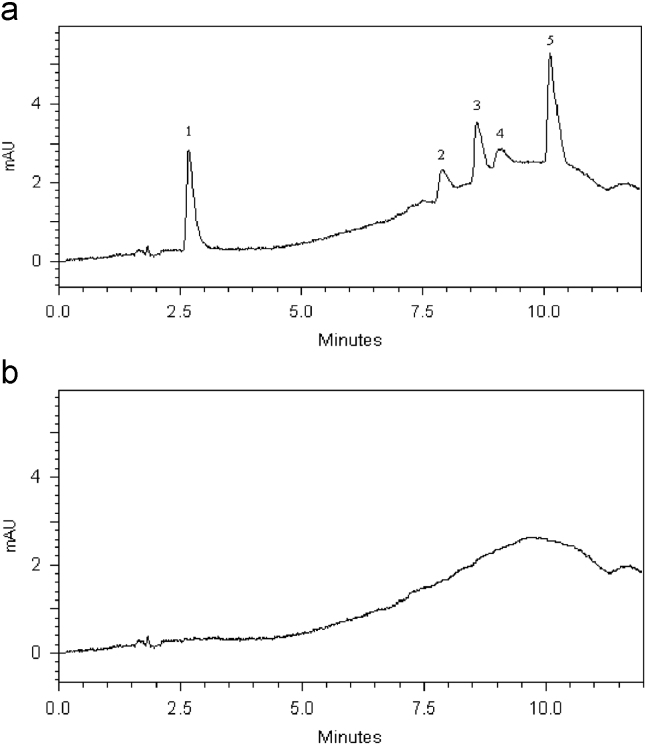
Accuracy of the method was tested by fortification of porcine samples at three known levels of 0.1, 0.5 and 1.0 μg/g, pre-processing, analysis and determination of the recovery for each drug, respectively. Fig. 4 shows chromatograms of a representative blank and spiked porcine tissue at 0.1 mg/kg level and Table 5 summarizes the recoveries and the RSD obtained for each analyte. The results indicate that the average recoveries range from 80.6% to 103.1% and RSD of the peak areas change from 0.3% to 6.1%.
Figure 4.
Chromatograms of: (a) extracted sample from spiked porcine tissues with 0.1 mg/kg and (b) blank extract, λ=280 nm. 1, SN; 2, ENO; 3, LOM; 4, TC; 5, SMZ.
Table 5.
Average recoveries, RSD and LOQ of the drugs at three levels of spiking (n=5).
| Drugs | Added (mg/kg) | Average recovery (%) | RSD (%) | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|
| SN | 1.0 | 99.2 | 3.0 | 7 | 24 |
| 0.5 | 93.1 | 1.0 | |||
| 0.1 | 88.3 | 3.6 | |||
| ENO | 1.0 | 87.1 | 2.9 | 5 | 17 |
| 0.5 | 87.5 | 3.5 | |||
| 0.1 | 87.0 | 1.1 | |||
| LOM | 1.0 | 97.4 | 2.7 | 4 | 14 |
| 0.5 | 97.5 | 1.3 | |||
| 0.1 | 89.0 | 4.0 | |||
| TC | 1.0 | 86.4 | 1.6 | 10 | 34 |
| 0.5 | 80.6 | 1.4 | |||
| 0.1 | 84.7 | 0.3 | |||
| SMZ | 1.0 | 98.5 | 3.3 | 2 | 7 |
| 0.5 | 86.4 | 6.1 | |||
| 0.1 | 82.3 | 3.0 | |||
The limit of detection (LOD) defined as a response 3 times the average height of the blank baseline noise was in the range 2–10 μg/kg and the limit of quantification (LOQ) defined as a response 10 times the average height of the blank baseline noise was 7–34 μg/kg, for porcine tissue samples, with 0.50 g in the MSPD method.
4. Conclusions
The results show that the developed method in this study is robust and sensitive for simultaneous detection and quantification of two FQs, two SAs and one TC antibiotics in animal tissues matrix. The proposed MSPD methodology is relatively simpler, more efficient and economical compared with SPE, and is suitable for multiresidue analysis of the studied drugs in porcine tissues. With MSPD, cleanup steps or the addition of chemical agents to further separate the drugs from interfering substances before HPLC analysis of extract are not necessary. Moreover, it reduces the sample sizes and requires less solvent and reagent for efficient isolation of the compounds of interest. The procedure can be treated as a screening method, which enables detection of ENO, LOM, SN, SMZ and TC in animal-product tissues at the MRLs level and estimation of their amounts.
The improvement of sensitivity and accuracy on fully quantitative grounds, and the efficient simultaneous analysis of a wide range of veterinary drug residues in animal food products will be addressed in our future investigations.
Acknowledgments
This work was supported by the Natural Science Foundation of Shaanxi Province (No. 2009jm4002-1). We also acknowledge professor Guangde Yang for his technical assistance.
References
- 1.Anadon A., Martinez-Larranaga M.R. Residues of antimicrobial drugs and feed additives in animal products: regulatory aspects. Liv. Produc. Sci. 1999;59:183–198. [Google Scholar]
- 2.Dong Y.C. Food safety and monitored control of veterinary drug residues. Chin. J. Vet. Drug. 2009;43(10):24–28. [Google Scholar]
- 3.Shi X.F., Lu G.L. The harm and countermeasures of veterinary quinolone drug residues. Meat. Saf. 2008;25(9):16–17. [Google Scholar]
- 4.Wang Y.S., Zhou L.M., Hong Z. Relationships between mechanism of adverse reactions and structure for fluoroquinolones. Australasi. Dispute Resolution J. 2005;6:401–407. [Google Scholar]
- 5.Lu H., Zheng Y., Lu J.F. The harm and countermeasures of veterinary drug residue. J. Taizhou Polytech. Coll. 2008;8(3):75–78. [Google Scholar]
- 6.Fang G.K. Determination of trace sulfonamides residues in animal derived food. Food. Res. Dev. 2007;28(8):101–104. [Google Scholar]
- 7.Gu X.D., Chen Y.Z., Hu B. Tetracycline residue and meat health. China. Anim. Health. 2007:62–63. [Google Scholar]
- 8.Veterinary Bureau of Ministry of Agriculture The maximum residue limits of veterinary drug in animal food products by Ministry of Agriculture. Chin. J. Vet. Drug. 2003;37(3):5–11. [Google Scholar]
- 9.Posyniak A., Zmudxki J., Semeniuk S. Effects of the matrix and sample preparation on the determination of fluoroquinolone residues in animal tissues. J. Chromatogr. A. 2001;914:89–94. doi: 10.1016/s0021-9673(00)01088-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhai Z.D., Cao C.M., Ge Y. A review of application of chromatographic techniques to determination of antibiotic residues in milk and related food products. J. Food Sci. 2010;31(1):287–291. [Google Scholar]
- 11.Renew J.E., Huang C.H. Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid chromatography–electrospray mass spectrometry. J. Chromatogr. A. 2004;1042:113–121. doi: 10.1016/j.chroma.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Babic S., Asperger D., Mutavdzic D. Solid phase extraction and HPLC determination of veterinary pharmaceuticals in wastewater. Talanta. 2006;70:732–738. doi: 10.1016/j.talanta.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Hui L., Philip K.J., Sherri B. Analysis of veterinary drug residues in shrimp: a multi-class method by liquid chromatography–quadrupole ion trap mass spectrometry. J. Chromatogr. B. 2006;836:22–38. doi: 10.1016/j.jchromb.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Romero-González R., López-Martínez J.C., Gómez-Milán E. Simultaneous determination of selected veterinary antibiotics in gilthead seabream (Sparus Aurata) by liquid chromatography–mass spectrometry. J. Chromatogr. B. 2007;857:142–148. doi: 10.1016/j.jchromb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Schneider M.J., Braden S.E., Reyes-Herrera I. Simultaneous determination of fluoroquinolones and tetracyclines in chicken muscle using HPLC with fluorescence detection. J. Chromatogr. B. 2007;846:8–13. doi: 10.1016/j.jchromb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Hou X., Dong X.C., Yang G.Y. Study on determination of seven fluoroquinolones in pork sample by rapid high performance liquid chromatography. J. Yunnan University (Nat. Sci.) 2007;16(3):225–227. [Google Scholar]
- 17.Lin L., Yang C.L., Cha Y.B. Determination of four fluoroquinolone residues in eggs by HPLC. Anal. Instrum. 2010;2:17–20. [Google Scholar]
- 18.Song Y.Q., Guo W.P., Zhao R. Determination of five different kinds of sulfonamides residues in meat products with high performance liquid chromatography. Meat. Ind. 2006;4:33–36. [Google Scholar]
- 19.Zhang P., Chen Y., Wang X.L. SPE–HPLC simultaneous determination of twelve sulfonamides residues in egg power. Chin. J. Pharm. Anal. 2010;30(12):2338–2343. [Google Scholar]
- 20.Tao D.Y., Jiao H.H., Zhang L.Y. Determination of three kinds of tetracyclines residues in pig liver by HPLC. Prog. Vet. Med. 2006;27(1):81–83. [Google Scholar]
- 21.Luo W.X., Zhao Q.R., Zhu Y. Tetracyclines and metabolites in pork by HPLC. Chin. J. Health Lab. Technol. 2010;20(5):1018–1020. [Google Scholar]
- 22.Barker S.A., Long A.R., Short C.R. Isolation of drug residues from tissues by solid phase dispersion. J. Chromatogr. 1989;475:353–361. doi: 10.1016/s0021-9673(01)89689-8. [DOI] [PubMed] [Google Scholar]