Abstract
A fast screening protocol was developed for the simultaneous determination of nine anti-estrogenic agents (aminoglutethimide, anastrozole, clomiphene, drostanolone, formestane, letrozole, mesterolone, tamoxifen, testolactone) plus five of their metabolites in human urine. After an enzymatic hydrolysis, these compounds can be extracted simultaneously from urine with a simple liquid–liquid extraction at alkaline conditions. The analytes were subsequently analyzed by fast-gas chromatography/mass spectrometry (fast-GC/MS) after derivatization. The use of a short column, high-flow carrier gas velocity and fast temperature ramping produced an efficient separation of all analytes in about 4 min, allowing a processing rate of 10 samples/h. The present analytical method was validated according to UNI EN ISO/IEC 17025 guidelines for qualitative methods. The range of investigated parameters included the limit of detection, selectivity, linearity, repeatability, robustness and extraction efficiency. High MS-sampling rate, using a benchtop quadrupole mass analyzer, resulted in accurate peak shape definition under both scan and selected ion monitoring modes, and high sensitivity in the latter mode. Therefore, the performances of the method are comparable to the ones obtainable from traditional GC/MS analysis. The method was successfully tested on real samples arising from clinical treatments of hospitalized patients and could profitably be used for clinical studies on anti-estrogenic drug administration.
Keywords: Anti-estrogens, Fast-GC/MS, Urine screening, Validation, Breast cancer
1. Introduction
According to their pharmacological properties, substances with anti-estrogenic activity can be classified in aromatase inhibitors (aminoglutethimide, anastrozole, exemestane, formestane, letrozole, testolactone), selective estrogen receptor modulators (raloxifene, tamoxifen, toremifene), substances with secondary anti-estrogenic activity (drostanolone, mesterolone) and other anti-estrogenic substances (clomiphene, cyclofenil, fulvestrant).
The therapeutic prescription for aromatase inhibitors is the treatment of metastatic breast cancer in post-menopausal women, because the inhibition of estrogens biosynthesis is supposed to prevent the growth of tumor tissue [1], [2], [3]. Selective estrogen receptor modulators (SERMs) exhibit a mixed pharmacologic profile, characterized by estrogen agonist activity in some tissues and estrogen antagonist activity in others. SERMs have many potential pharmacological applications, and are currently prescribed for women with breast cancer (tamoxifen, toremifene) or at risk for breast cancer (tamoxifen), and lastly for post-menopausal women with a risk of osteoporosis (raloxifene) [4], [5]. Clomiphene and cyclofenil have stimulating effects on the secretion of hypophysical gonadotropic hormones and are mainly used in the treatment of infertility [6], [7].
Drostanolone and mesterolone are mainly considered as anabolic agents, but they also show some secondary anti-estrogenic effects [8].
Moreover, anti-estrogenic agents can be illicitly used in sport as they are able to treat and prevent the side effects produced from anabolic androgenic steroids misuse [9]. Accordingly, the use of anti-estrogenic agents is prohibited by World Anti-Doping Agency (WADA) regulations for both male and female athletes in- and out-competitions [10].
Different conventional approaches, carried out on biological fluids (blood and urine), were reported for the detection of anti-estrogens, including immuno-analytical techniques [11], and chromatographic methods [12], [13]. Since the consumption of these compounds by the athletes is prohibited in sport events, several screening procedures were developed for the detection of anti-estrogens in human urine for anti-doping purposes. Several GC/MS screening procedures applied to anti-estrogenic agents were developed [14], [15], [16], [17], even if an increasing number of procedures based on LC/MS have been published [18], [19], [20], [21], [22]. Anti-estrogenic agents are often screened together with other class of substances, mainly using LC-MS/MS procedures, usually more sensitive than GC/MS ones.
Fast GC/MS is gaining importance in routine analytical laboratories, since it improves sample throughput and global productivity, as is necessary in laboratories where a considerable number of daily samples have to be processed [23], [24]. The time needed for instrumental determination can be drastically shortened using short and narrow columns (5–10 m, i.d. 0.05–0.1 mm), fast temperature ramping and high carrier gas flow rate as recently demonstrated [25], [26], [27], [28], [29].
Aim of the present work was to develop an analytical procedure addressed to the fast screening of fourteen anti-estrogenic drugs and metabolites (Fig. 1), using fast-GC instrumentation hyphenated to electron ionization mass spectrometry. Further “in vitro” metabolic experiments using the microsomal fraction from rat liver were carried out in order to supply us the reference material for a larger panel of metabolites. Lastly, urinary samples from patients under treatment with anti-estrogenic drugs were collected and successfully analyzed to test the actual efficiency of the proposed method.
Figure 1.
Chemical structures of the anti-estrogenic agents considered in this study.
2. Experimental
2.1. Chemicals and reagent
Methanol, t-butyl methyl ether (TBME) and various inorganic salts were supplied by Riedel-de Haën (Seelze, Germany); β-glucuronidase (from Escherichia coli) and dithioerythritol were from Sigma–Aldrich (Milan, Italy). N-Methyl–N-trimethylsilyltrifluoroacetamide (MSTFA) was obtained from Macherey–Nagel (Düren, Germany).
Aminoglutethimide, clomiphene citrate (57% cis form and 43% trans form), formestane and 17α-methyltestosterone were purchased from Sigma–Aldrich (Milan, Italy); drostanolone (17β-hydroxy-2α-methyl-5α-androstan-3-one), mesterolone (17β-hydroxy-1α-methyl-5α-androstan-3-one) were from Steraloids (Newport, RI, USA); drostanolone metabolite (3α-hydroxy-2α-methyl-5α-androstan-17-one), mesterolone metabolites (1α-methyl-5α-androstan-3α,17β-diol, 3α-hydroxy-1α-methyl-5α-androstan-17-one) were from LGC Promochem (Milan, Italy), testolactone was from US Pharmacopeia (Rockville, MD, USA). Tamoxifen citrate was kindly supplied by SOLMAG (Garbagnate, Italy); letrozole was kindly provided from Novartis Pharma (Basel, Switzerland) and anastrozole was kindly supplied by AstraZeneca (London, UK).
Bis-4-cyanophenylmethanol (a letrozole metabolite), 4-hydroxy-clomiphene (a clomiphene metabolite) and 4-hydroxy-tamoxifen (a tamoxifen metabolite) were obtained from “in vitro” phase I metabolism from rat liver microsomal fraction [30], [31], [32]. All reagents were supplied by Sigma–Aldrich (Milan, Italy).
Stock standard solutions were prepared in methanol at a concentration of 1000 μg/mL and were stored at −20 °C until used. All solution and buffers were prepared using deionized water obtained from Milli-Q System (Millipore Corporate Headquarters, Billerica, USA).
2.2. In vitro synthesis of phase I letrozole, clomiphene and tamoxifen metabolites
2.2.1. In vitro metabolic assays
Liver microsomal fraction was prepared as described elsewhere [31]. Stock solutions (500 μM) of each substrate tested for in-vitro metabolic activity (letrozole, clomiphene and tamoxifen) were prepared in DMSO. Reaction mixtures were prepared in a test tube by adding 225 μL of 0.1 M Tris HCl buffer (pH 7.4), 225 μL of a microsomal suspension (approximately 2 mg of proteins), 2000 μL of NADPH generating system (consisting of 0.32 mM NADPH, 6.4 mM glucose-6-phosphate, 0.5 U/mL glucose-6-phosphate dehydrogenase, 5 mM MgCl2 solution and 0.8 mM of EDTA solution) and 50 μL of 500 μM stock solution containing the tested substrate. The reaction mixtures were incubated at 37 °C under gentle agitation for 30 min and lastly the reaction was stopped by cooling the mixture on ice. Metabolized substrate suspensions were stored at −80 °C until used. The extraction of metabolism products from the resulting suspensions was carried out following the same procedure used for the urine samples and described as follows.
2.3. Characterization of “in-vitro” metabolites
The substrates obtained by “in vitro” metabolic assays were characterized by GC/MS after solvent extraction and trimethylsilylation, as described in the subsequent paragraph. GC/MS analyses were performed using an Agilent 6890N/5975 instrument (Agilent Technologies, Milan, Italy) with a 17 m HP-1 fused silica capillary column (J&W Scientific), with i.d. 0.20 mm and film thickness 0.11 μm. The carrier gas was helium (flow rate 1 mL/min, split ratio 1:10). The oven temperature program was: isothermal at 180 °C for 3 min, heating rate of 5 °C/min up to 240 °C, then 10 °C/min to 270 °C, and lastly 40 °C/min to 320 °C (isothermal for 3 min). The temperatures of transfer line, injection port and MS detector were all set at 280 °C. Acquisition was carried out in full scan mode from m/z 50 to m/z 550. The correct identity of metabolites was determined by both mass spectral interpretation and comparison with published spectra.
2.4. Sample preparation
The sample preparation introduced minor modifications from the standard operating procedure described by Donike et al. [33] for the detection of anabolic steroids. Urine samples (3 mL) were added with 15 μL of a 10 μg/mL methyltestosterone solution, used as the internal standard (IS), and then were buffered to pH 7.4 with 2 mL of a 0.1 M phosphate buffer (prepared by dissolving 4.63 g of KH2PO4 and 11.75 g of Na2HPO4·2 H2O in 1 L of water). 30 μL of β-glucuronidase from E. coli were subsequently added and the mixture was incubated at 55 °C for 1 h. Once the hydrolysis was completed, the mixtures were cooled at room temperature and 2 mL of 0.1 M carbonate buffer (prepared by dissolving 2.12 g of Na2CO3 and 6.72 g of NaHCO3 in 1 L of water) were added to raise the pH to 9.6. Liquid–liquid extraction (LLE) was performed by adding 10 mL of TBME and shaking in a multimixer for 10 min. After centrifugation at 2200 rpm for 3 min, the organic layer was transferred into a test tube and dried under nitrogen at 70 °C. The dry residue was derivatized with 50 μL of a MSTFA/NH4I/dithioerythritol (1000:2:4, v/w/w) solution for 30 min at 70 °C. An aliquot of 1 μL was injected into the fast-GC/MS system.
2.5. Fast-GC/MS analysis
Fast-GC/MS determinations were performed using a 6890N GC, combined with a 5975 inert Mass Selective Detector (Agilent Technologies, Milan, Italy), with electron ionization at 70 eV. A 5 m fused-silica capillary column (Supelco “Equity 1”), with i.d. 0.1 mm and film thickness 0.11 μm was utilized for GC separation. The final analytical solution (1 μL) was injected into the fast-GC system operating in the split mode (5:1). Helium was employed as carrier gas at constant pressure of 33.21 psi. The GC oven temperature was isothermally set at 180 °C for 0.5 min, then initially raised to 220 °C with a 35 °C/min heating rate and subsequently to 310 °C with a rate of 60 °C/min, followed by a isothermal period of 1 min at 310 °C. The total run time was 4.14 min, while the overall cycle time, including oven cooling and thermal equilibration was about 6 min. The GC injector and transfer line were maintained at 280 °C. Data were acquired in the selected-ion monitoring (SIM) mode at dwell time of 15 ms, using for each compound detection the diagnostic ions listed in Table 1.
Table 1.
Molecular weights of the 14 analytes and their TMS-derivatives, GC retention times, characteristic ions (EI mode) and corresponding retention time windows used in SIM experiments.
| Analytes | Analyte molecular weight | No. of TMS groups | TMS derivatives molecular weight | RT (min) |
Ions (m/z) EI |
RT time window (min) | |
|---|---|---|---|---|---|---|---|
| Target | Others | ||||||
| Anastrozole | 294 | 0 | 294 | 1.90 | 209 | 293, 266 | 1.80–2.40 |
| Letrozole metabolitea | 234 | 1 | 306 | 1.94 | 217 | 291, 190 | 1.80–2.40 |
| Aminoglutethimide | 232 | 3 | 448 | 2.14 | 291 | 276, 433 | 1.80–2.40 |
| Aminoglutethimide | 232 | 4 | 520 | 2.14 | 491 | 520, 505 | 1.80–2.40 |
| Drostanolone met (3α-ol-17-one) | 304 | 2 | 448 | 2.44 | 433 | 448, 343 | 2.40–2.54 |
| Mesterolone met (3α-ol-17-one) | 304 | 2 | 448 | 2.52 | 433 | 448, 343 | 2.40–2.54 |
| Mesterolone met (3α,17β-diol) | 306 | 2 | 450 | 2.56 | 345 | 450, 435 | 2.54–2.66 |
| Tamoxifen | 371 | 0 | 371 | 2.59 | 58 | 72, 371 | 2.54–2.66 |
| Mesterolone | 304 | 2 | 448 | 2.59 | 141 | 157, 433 | 2.54–2.66 |
| Drostanolone | 304 | 2 | 448 | 2.63 | 448 | 405, 141 | 2.54–2.66 |
| ISTD | 302 | 2 | 446 | 2.74 | 446 | 301, 431 | 2.66–2.80 |
| Tamoxifen metabolitea | 387 | 1 | 459 | 2.74 | 58 | 72, 459 | 2.66–2.80 |
| Testolactone | 300 | 2 | 444 | 2.75 | 444 | 429, 206 | 2.66–2.80 |
| Formestane | 302 | 3 | 518 | 2.85 | 518 | 503, 147 | 2.80–2.88 |
| Clomiphene (two isomers) | 405 | 0 | 405 | 2.92 | 86 | 100, 405 | 2.88–4.13 |
| 405 | 0 | 405 | 2.99 | 86 | 100, 405 | 2.88–4.13 | |
| Clomiphene metabolitea | 421 | 1 | 493 | 3.33 | 86 | 100, 493 | 2.88–4.13 |
Reference material obtained by in vitro metabolism experiments.
2.6. Method validation
2.6.1. Selectivity
Ten different blank urine samples were deconjugated, extracted, derivatized and analyzed as described above. The occurrence of possible interferences from endogenous substances or derivatization byproducts was tested by monitoring the selected-ion chromatograms, characteristic for each investigated compound, at the retention time interval expected for their elution.
2.6.2. Linearity
The linear calibration model was checked by analyzing (three replicates) negative urine samples spiked with standard solutions at six concentration levels for each analyte. The linear calibration parameters were obtained using the least squares regression method, while the correlation coefficients (R2) were utilized to estimate linearity. Quantitative results from area counts were corrected using the IS signal.
2.6.3. Limit of detection (LOD)
LOD values were estimated as the analyte concentrations whose response provided a S/N value equal to 3, as determined from the least abundant among qualifier ions. LOD numerical values were extrapolated from S/N values at the lowest concentration level (LCL) using the corresponding calibration curve. The noise was measured from −0.05 min before the peak onset till the beginning of the GC peak for each analyte. Extrapolated LOD values were experimentally confirmed by studying urine samples spiked with all analytes at LODs concentrations.
2.6.4. Extraction recovery
Extraction recoveries were calculated by comparing two experimental sets of data. In the first set, ten blank urine samples were spiked before the extraction step with all analytes at 50, 250 and 500 ng/mL final concentration, except for testolactone added at 250, 500 and 1000 ng/mL concentration. In the second set, ten blank urine samples were spiked after the extraction step, with standard working solutions, at the same final concentration of 50, 250 and 500 (or 1000) ng/mL. Recovery (%) was calculated as the ratio between the response (peak area) obtained from the two separate series of samples.
2.6.5. Repeatability (intra-assay precision)
Intra-assay precision (repeatability), expressed as percent variation coefficient (CV%), was assessed by extracting and analyzing ten replicates of negative urine samples, spiked with the standard solutions at two concentration levels (final concentrations of 50 ng/mL and 500 ng/mL for each analyte), performed by the same operator. Standard criteria taken from the literature [34] designate satisfactory intra-assay precision for qualitative screening methods when CV% values are below 15% at 500 ng/mL concentrations and below 25% at 50 ng/mL concentrations.
2.6.6. Robustness
The method robustness was evaluated by changing the operators involved in both preparative and instrumental phases of analysis, as well as the batch of chemicals and reagents employed in the sample treatment and the spiking level of several urine samples. For each analyte, compliance of RT, relative ion abundances and approximate concentrations were verified.
2.7. Real urine samples
Three urine samples from women suffering from breast metastatic cancer and under treatment with Arimidex® (anastrozole), Femara® (letrozole) and Nolvadex® (tamoxifen citrate) were collected and analyzed. Aliquots of 30 mL of urine were collected and stored at−20 °C until analysis. All selected patients provided a fully informed permission for the scientific use of these analyses.
3. Results and discussion
3.1. GC/MS characterization of phase I letrozole, clomiphene and tamoxifen metabolites
The derivatized extracts obtained from “in-vitro” metabolic assays yielded relatively clean chromatographic profiles, from which the identification of the starting drugs and their phase I metabolic products was straightforward. However, the experimental mass spectra obtained from these metabolites were compared with literature spectra, for a correct structural identity assignment [15], [35].
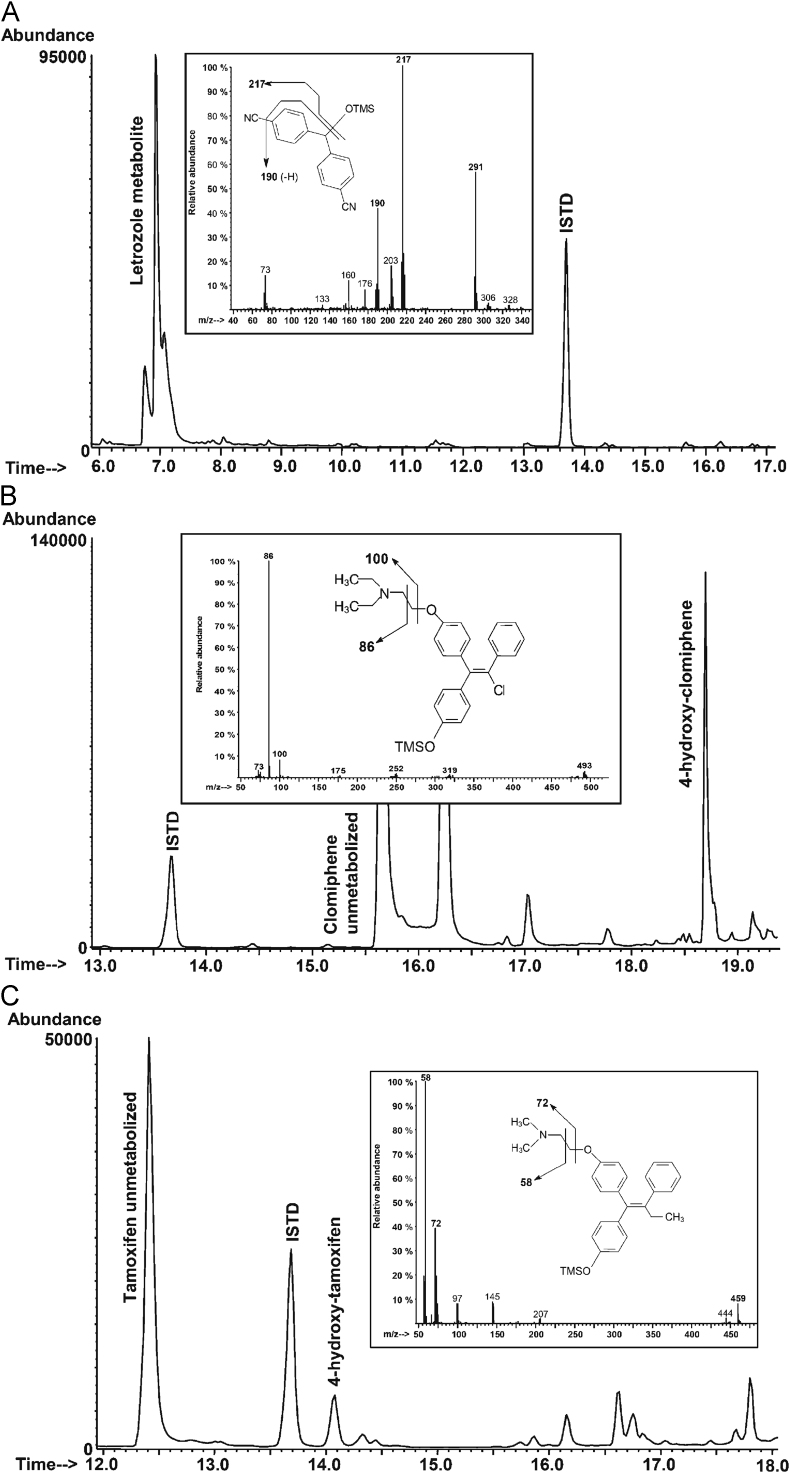
Fig. 2 shows the total-ion chromatograms, mass spectra and fragmentation patterns for the derivatized metabolites of letrozole (bis-4-cyanophenyl-methanol), clomiphene (4-hydroxy-clomiphene) and tamoxifen (4-hydroxy-tamoxifen), obtained from “in vitro” metabolic experiments.
Figure 2.
GC/MS-SIM chromatograms of the derivatized extracts obtained from “in-vitro” metabolic assays, mass spectra and proposed fragmentations pattern of letrozole (A), clomiphene (B) and tamoxifen (C) metabolites.
The bis-4-cyanophenyl-methanol is the main inactive metabolite of letrozole [15]. The mono-TMS derivative shows the molecular ion at m/z 306 (relative abundance of 2%) and a high-mass fragment m/z 291 (56%), due to the loss of a methyl radical from the TMS residue. The fragment at m/z 217 (base peak) is generated by the trimethylsiloxy-radical elimination, while a subsequent rearrangement leads to the m/z 190 ion (44%) by HCN loss.
Hydroxylation of the aromatic ring represents the main phase I metabolic route for clomiphene [31]. The molecular ion of main clomiphene metabolite, namely 4-hydroxy-clomiphene, is detected from its mono-TMS derivative at m/z 493 (2%); cleavage of the C–C bond in α-position to the amino group leads to the fragment at m/z 86 (base peak), while cleavage of the C–C bond in β-position to the amino group yields the fragment at m/z 100 (8%).
Hydroxylation of the aromatic ring also occurs in the tamoxifen metabolism, yielding 4-hydroxy-tamoxifen [36], [37]. The major fragmentation of its mono-TMS derivative is determined by the localization of the positive charge on the amino-group, yielding both the base peak at m/z 58 (base peak) and the major fragment at m/z 72 (39%) resulting from the cleavage in α- and β-position with respect to the amino group. The presence of the hydroxyl group on the tamoxifen structure is testified by the metabolite molecular ion at m/z 459 (8%).
3.2. Derivatization products
Most anti-estrogenic agents and their metabolites listed in Fig. 1 contain polar groups in their structures, that make them non-volatile and unsuitable for gas chromatographic separation. Therefore, the development of an efficient GC/MS protocol, for identifying anti-estrogen in biological matrices, requires preliminary derivatization of these polar groups. Trimethylsilylation makes the analytes more volatile and produces a molecular weight increase of 72 u, for each reactive hydrogen. For steroids and anti-estrogenic agents such as drostanolone and mesterolone containing more than one polar group, MSTFA is proved to be the most efficient agent to give TMS ethers, since it produces single fully substituted derivatives nearly without side-products, whose significant mass spectra contain diagnostic target ions, ideal for screening purposes [15], [16], [25], [35].
MSTFA reacts with all hydroxyl and keto groups of anti-estrogenic agents, and amine and amide hydrogens of aminoglutethimide, to give their tris–TMS and tetrakis–TMS derivatives, respectively. The absence of exchangeable hydrogens on anastrozole, clomiphene and tamoxifen does not prevent their detection, as unmodified molecules. The molecular weights of the original anti-estrogenic agents and their derivatives, together with the number of TMS-groups introduced by the derivatization, are reported in Table 1.
3.3. Fast GC/MS characterization
Full scan electron ionization mass spectra of all parent drugs were recorded from reference standards, in order to determine the most appropriate SIM protocol for accurate and sensitive detection of these target analytes. Likewise, full scan mass spectra for target metabolites were recorded from in vitro metabolic mixtures.
A previous study [15] including GC/MS experiments on letrozole reference standards found that the detection of this compound is difficult, due to its poor chromatographic properties using a standard polysiloxane column. We encountered the same problem and decided to detect letrozole from its main metabolite, namely bis-4-cyanophenylmethanol, whose determination does not involve difficulties.
The relative abundances of three characteristic ions (qualifier ions vs. target ion) along the GC peak were used for the positive identification of each target compound together with the coincidence of its retention time with the expected one. All target analytes were easily characterized by different fragments. The SIM protocol developed proved efficient in discriminating the interferences.
Table 1 reports retention times and selected ions, used for the identification of anti-estrogenic agents or their trimethylsilyl derivatives in the EI mode.
Fig. 3 shows the fast-GC/MS chromatogram of a mixture of analytes at the calibration level concentration of 250 ng/mL obtained in the SIM mode. It is noteworthy that the entire GC/MS run was completed in about 3 min. Two more minutes were needed for oven cooling, temperature equilibration and injection. Satisfactory chromatographic separation of all target analytes was obtained including clomiphene isomers. Even if a fast gradient was applied for the chromatographic separation, the high MS-sampling rate, using a modern benchtop quadrupole mass analyzer, guaranteed a minimum of 10 points across the chromatographic peak without significant loss of information. The peaks were adequately described allowing a good integration reproducibility, as demonstrated by satisfactory CV% values reported in Table 2.
Figure 3.
Fast GC/MS-SIM chromatogram of a blank urine sample fortified with all target compounds at the concentration of 250 ng/mL: (1) anastrozole, (2) aminoglutethimide, (3) drostanolone metabolite (3a-ol-17-one), (4) mesterolone metabolite (3a-ol-17-one), (5) mesterolone metabolite (3a, 17bdiol), (6) mesterolone, (7) tamoxifen, (8) drostanolone, (9) testolactone, (10) formestane, (11) clomiphene (two isomers).
Table 2.
Percentage recovery (%) and repeatability (intra-assay precision) (CV%) for each analyte tested. The metabolites from in-vitro assays are not included.
| Detected analytes |
Extraction efficiency |
Repeatability |
|||
|---|---|---|---|---|---|
| Conc. (ng/mL) | Recovery (%) | CV% | CV% at 50 ng/mL | CV% at 500 ng/mL | |
| Anastrozole | 50 | 89.2 | 13.3 | n/a | 7.6 |
| Aminoglutethimide (3TMS) | 50 | 88.9 | 11.9 | 20.2 | 11.1 |
| Aminoglutethimide (4TMS) | 50 | 92.8 | 17.7 | 10.2 | 8.5 |
| Drostanolone met (3α-ol-17-one) | 50 | 35.3 | 17.8 | 13.8 | 12.0 |
| Mesterolone met (3α-ol-17-one) | 50 | 85.8 | 17.7 | 13.0 | 9.4 |
| Mesterolone met (3α,17β-diol) | 50 | 83.7 | 12.3 | 9.4 | 9.0 |
| Tamoxifen | 50 | 94.8 | 14.2 | 7.6 | 7.3 |
| Mesterolone | 50 | 102.7 | 17.0 | 12.2 | 9.1 |
| Drostanolone | 50 | 97.7 | 12.2 | 9.6 | 8.5 |
| Testolactone | 250 | 40.6 | 10.2 | n/a | 9.1 |
| Formestane | 50 | 97.9 | 11.5 | 9.2 | 8.6 |
| Clomiphene (two isomers) | 50 | 93.9 | 12.1 | 8.7 | 9.7 |
| 50 | 91.5 | 12.2 | 8.1 | 9.5 | |
n/a: not available.
The SIM protocol described in Table 1 was used to build the calibration graphs for all analytes.
3.4. Method validation
According to ISO 17025 requirements and ICH guidelines [38], all validation parameters useful to evaluate the overall performance of a qualitative analytical method were investigated, including selectivity, linearity, limit of detection, recovery, repeatability and robustness.
3.4.1. Selectivity
Ion-chromatograms from 10 negative urine samples showed no interfering signals (i.e. S/N ratio minor than 3) at the retention time where each analyte is expected to elute. This demonstrated that the method is selective for all 14 anti-estrogenic agents and free from positive interference from urine components and column bleeding.
3.4.2. Linearity and limit of detection
The range of concentration studied was planned according to the approximate response factors obtained from the preliminary experiments with standard solutions. All calibration curves proved linear as demonstrated by correlation values comprised between 0.990 and 0.999. Calibration curves are not available for clomiphene, letrozole and tamoxifen metabolites because their purity and exact concentration are not known with certainty. The experimental data relative to calibration curves, limits of detection and S/N values for all target analytes are reported in Table 3. LOD values obtained for most target compound were found at 10 ng/mL, including the ones for formestane, tamoxifen, drostanolone, mesterolone (and their metabolites), while few LOD values (15–45 ng/mL) slightly exceeded this limit except the one for testolactone that was 200 ng/mL.
Table 3.
Calibration intervals; gradients, intercepts and R2 values obtained for calibration curves; LOD and S/N at Lowest Calibration Level (LCL) values for the TMS derivatives of the investigated 11 anti-estrogenic agents. The metabolites obtained from in-vitro assays are not included.
| Analytes | Calibration level concentration (ng/mL) | Slope | Intercept | R2 | LOD (ng/mL) | S/Nat LCL |
|---|---|---|---|---|---|---|
| Anastrozole | 100–1000 | 0.595 | −0.586 | 0.996 | 45 | 7.0 |
| Aminoglutethimide (3TMS) | 50–1000 | 0.173 | −0.0401 | 0.997 | 25 | 6.8 |
| Aminoglutethimide (4TMS) | 50–1000 | 0.0413 | −0.0179 | 0.990 | 35 | 4.7 |
| Drostanolone met (3α-ol-17-one) | 25–1000 | 0.344 | −0.123 | 0.994 | 10 | 8.7 |
| Mesterolone met (3α-ol-17-one) | 25–1000 | 0.485 | −0.122 | 0.997 | 10 | 7.4 |
| Mesterolone met (3α,17β-diol) | 25–1000 | 0.103 | 0.0725 | 0.997 | 10 | 9.0 |
| Tamoxifen | 25–1000 | 1.22 | −0.0466 | 0.999 | 10 | 7.5 |
| Mesterolone | 25–1000 | 1.64 | 0.246 | 0.999 | 10 | 7.5 |
| Drostanolone | 25–1000 | 0.622 | −0.221 | 0.998 | 10 | 8.2 |
| Testolactone | 250–1000 | 0.0046 | 0.0106 | 0.990 | 200 | 4.4 |
| Formestane | 25–1000 | 1.08 | −0.102 | 0.999 | 10 | 9.6 |
| Clomiphene (two isomers) | 25–1000 | 0.730 | −0.122 | 0.999 | 15 | 5.3 |
| 25–1000 | 0.123 | −0.112 | 0.999 | 15 | 5.2 |
A blank urine sample, spiked with the target analytes at the LOD concentrations indicated in Table 3, was analyzed in triplicate. All experimental S/N values observed exceeded the critical value of 3, as expected.
3.4.3. Extraction recovery
Extraction recoveries were calculated by comparing the results from blank urine samples spiked before the extraction step with the ones from negative urine samples, which were first extracted and then spiked, before the analysis. The results obtained at 50 ng/mL concentration are reported in Table 2. Most recovery values ranged from 102.7% (mesterolone) to 83.7% (mesterolone metabolite—3α,17β-diol), with the exception of testolactone and drostanolone metabolite (3α-ol-17-one) showing average recovery efficiencies of 40.6% and 35.3%, respectively. For the latter, the limited extraction recovery that we observed is reflected in literature data [34], while no previous data are available for liquid–liquid testolactone extraction efficiency. It should be noted that the extraction recoveries were quite stable, even for the two compounds with lower efficiency, as is demonstrated by CV% values, all ranging between 10% and 18%. Taking into account that a screening method for multiple target analytes is not likely to provide homogeneously high extraction recoveries and that a 35% recovery is acceptable for a qualitative screening, provided good extraction repeatability is obtained, it can be concluded that the observed extraction efficiencies are perfectly compatible with the screening purpose of the present method. Extraction efficiency data obtained at higher concentrations (250 and 500 ng/mL) confirmed the results presented in Table 3, with a slight decrease of most recovery values. For example, at the concentration of 500 ng/mL, recovery efficiency values were comprised between 101.4% of formestane and 72.9% of aminoglutethimide as tris-TMS derivatives. Recovery value for drostanolone metabolite (3α-ol-17-one) spiked at the highest concentration dropped to 20.3%, while for testolactone no important difference with the result obtained at lower concentration was observed (40.0%).
3.4.4. Repeatability (intra-assay precision) and robustness
The identification criteria, including the coincidence of retention times and the relative abundances of characteristic ions, were respected all over the repeated tests. The repeatability proved satisfactory for all the target analytes, as the coefficient of variation percentage (CV%) was always abundantly lower than 25% for the samples spiked at 50 ng/mL, and lower than 12% for the samples spiked at 500 ng/mL. The repeatability for testolactone was obviously tested only at 500 ng/mL.
No significant variation of retention times, relative ion abundance or limits of detection were observed by changing the operator, the instrument (same model), the chemical batches, the stock standard solutions and the sample volume. In all experiments, no statistically significant variations of detected concentrations were obtained (data not shown).
3.5. Real urine samples
Real urine samples were collected during outpatient medical examination, not allowing a strict control of the time elapsed from the last drug administration. The patients were being treated with tamoxifen, anastrozole and letrozole. All the samples examined turned out positive for the administered drug. Chromatograms obtained by fast-GC/MS analysis of three real urine samples from patients under treatment with different anti-estrogenic agents are reported in Fig. 4. In the first urine sample (Fig. 4A) the estimated concentration of anastrozole was 98 ng/mL. GC plots in the first column show the superimposed profiles of the selected ions used for the different drugs identification. Comparison with the GC graphs represented in the third column, and relative to the real samples, provide clear evidence that the relative ion abundances are perfectly reproduced throughout each GC peak profile. The selected-ion chromatograms reported in the central column demonstrate that the method developed is selective and no interfering signals are present in the GC profiles.
Figure 4.
Selected-ion chromatograms of extracted urine samples obtained from patients treated with (A) anastrozole, (B) letrozole and (C) tamoxifen. For letrozole and tamoxifen the metabolites, not the original drug, are detected. Left: urine spiked reference standard; center: blank urine; right: real urine samples.
Thus, all target analytes examined (and/or their metabolites) were positively detected in real urine samples from patients under pharmacological treatment, showing that the presented method can find practical application for clinical purposes.
4. Conclusions
The continuous effort to increase the analytical throughput in biomedical determinations and reduce the time needed for the instrumental analysis received new impulse from the introduction of fast-gas chromatography. Although the analytical methods based on liquid chromatography combined with multiple stage mass spectrometry are gaining increasing popularity with respect to GC/MS, just because they allow more rapid determinations, the extensive application of fast-GC/MS procedures is likely to hold back this trend, by considering the large difference in price, the diffuse experience existing in the GC-based analytical methods and the absence of signal suppression/enhancement phenomena, occasionally occurring in electrospray ionization when complex biological matrices are analyzed by LC/MS.
Analytical methods have to respond to the requirements established by the specific inquiry under way. While in anti-doping screening analysis a common goal is to identify the largest number of prohibited substances with the lowest number of analytical methods, independently from their pharmacological effects, epidemiology studies are frequently addressed to the detection of a certain class of drugs. Such inquiries may take advantage of analytical methods specifically designed for the class of substances under investigation. For anti-estrogenic agents, an epidemiologic study is possibly of interest, as these compounds are daily employed for clinical purposes (mainly in the treatment of breast cancer or masculine and feminine infertility) or illicitly used by athletes in order to treat or prevent the side effects associated with anabolic androgenic steroids abuse.
Fast-GC/MS analysis, performed on narrow bore columns at high heating rates, provides significant reduction of the time required for the analysis of these anti-estrogenic agents, while maintaining chromatographic separation, limits of detection, and repeatability comparable with those obtained by conventional GC. This makes the method particularly suited for routine analysis, especially when a considerable number of samples have to be analyzed, as about 10 injections/h could be performed.
The method proposed in the present study is selective for the detection of anti-estrogenic agents in human urine. The method appears to be sufficiently flexible to include new substances (and metabolites) of the same class that may appear on the market in the future. Moreover, since fast-GC/MS technique allows to lower the time needed for method validation, a future extension of the present method on blood samples could be rapidly performed.
Acknowledgments
We thank AstraZeneca (London, UK) for providing anastrozole, Novartis Pharma AG (Basel, Switzerland) for supplying letrozole and SOLMAG (Garbagnate, Italy) for providing tamoxifen.
We also thank Dr. Alfredo Berruti (Polo Oncologico “Torino Ovest”—Ospedale S. Luigi Gonzaga, Orbassano, Turin) for supplying us with urine samples from patients.
References
- 1.Lønning P.E. Aromatase inhibitors in breast cancer. Endocr. Relat. Cancer. 2004;11:179–189. doi: 10.1677/erc.0.0110179. [DOI] [PubMed] [Google Scholar]
- 2.Smith I.E., Dowsett M. Aromatase inhibitors in breast cancer. N. Engl. J. Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 3.Santen R.J., Harvey H.A. Use of aromatase inhibitors in breast carcinoma. Endocr. Relat. Cancer. 1999;6:75–92. doi: 10.1677/erc.0.0060075. [DOI] [PubMed] [Google Scholar]
- 4.MacGregor J.I., Jordan V.C. Basic guide to the mechanisms of antiestrogen action. Pharmacol. Rev. 1998;50:151–196. [PubMed] [Google Scholar]
- 5.Osborne C.K., Zhao H., Fuqua S.A.W. Selective estrogen receptor modulators: structure, function, and clinical use. J. Clin. Oncol. 2000;18:3172–3186. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- 6.Goto S., Takakura K., Nakanishi K. Efficacy of clomiphene citrate and cyclofenil for infertile women with normal ovulatory cycles. Fertil. Steril. 2001;76:409–411. doi: 10.1016/s0015-0282(01)01906-9. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H.R., Seki M., Seki K. Effects of clomiphene, cyclofenil, epimestrol and Ro 4-8347 on pituitary and serum prolactin in mature female rats. Endocrinol. Jpn. 1972;19:525–532. doi: 10.1507/endocrj1954.19.525. [DOI] [PubMed] [Google Scholar]
- 8.Marinov L., Tsekova V., Koinov K. Drostanolone propionate (masteril) in disseminated breast cancer in women. Immediate results. Khirurgiia (Sofiia) 1987;40:80–86. [PubMed] [Google Scholar]
- 9.Handelsman D.J. Clinical review: the rationale for banning human chorionic gonadotropin and estrogen blockers in sport. J. Clin. Endocrinol. Metab. 2006;91:1646–1653. doi: 10.1210/jc.2005-2569. [DOI] [PubMed] [Google Scholar]
- 10.World Anti-Doping Agency, The 2010 Prohibited List, Available at 〈http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/WADA_Prohibited_List_2010_EN.pdf〉 (accessed 8 February 2010).
- 11.Pfister C.U., Duval M., Godbillon J. Development, application and comparison of an enzyme immunoassay and a high-performance liquid chromatography method for the determination of the aromatase inhibitor CGS 20,267 in biological fluids. J. Pharm. Sci. 1994;83:520–524. doi: 10.1002/jps.2600830415. [DOI] [PubMed] [Google Scholar]
- 12.Marfil F., Pineau V., Sioufi A. High-performance liquid chromatography of the aromatase inhibitor, letrozole, and its metabolite in biological fluids with automated liquid–solid extraction and fluorescence detection. J. Chromatogr. B: Biomed. Sci. Appl. 1996;683:2581–2588. doi: 10.1016/0378-4347(96)00118-1. [DOI] [PubMed] [Google Scholar]
- 13.Beere B., Schubert B., Oberguggenberger A. Development and validation of a liquid chromatography–tandem mass spectrometry method for the simultaneous quantification of tamoxifen, anastrozole, and letrozole in human plasma and its application to a clinical study. Anal. Bioanal. Chem. 2010;398:1791–1800. doi: 10.1007/s00216-010-4075-z. [DOI] [PubMed] [Google Scholar]
- 14.Bàez H., Camargo C., Osorio H. Detection of tamoxifen metabolites by GC–MSD. J. Chromatogr. Sci. 2004;42:551–553. doi: 10.1093/chromsci/42.10.551. [DOI] [PubMed] [Google Scholar]
- 15.Mareck U., Sigmund G., Opfermann G. Identification of the aromatase inhibitor letrozole in urine by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:3689–3693. doi: 10.1002/rcm.2239. [DOI] [PubMed] [Google Scholar]
- 16.Mareck U., Sigmund G., Opfermann G. Identification of the aromatase inhibitor aminoglutethimide in urine by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:2209–2214. doi: 10.1002/rcm.838. [DOI] [PubMed] [Google Scholar]
- 17.Gärtner P., Hofbauer K., Reichel C. Synthesis and identification of hydroxylated metabolites of the anti-estrogenic agent cyclofenil. J. Mass Spectrom. 2008;43:958–964. doi: 10.1002/jms.1462. [DOI] [PubMed] [Google Scholar]
- 18.Kolmonen M., Leinonen A., Pelander A. A general screening method for doping agents in human urine by solid phase extraction and liquid chromatography/time-of-flight mass spectrometry. Anal. Chim. Acta. 2007;585:94–102. doi: 10.1016/j.aca.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Mazzarino M., de la Torre X., Botrè F. A screening method for the simultaneous detection of glucocorticoids, diuretics, stimulants, anti-oestrogens, beta-adrenergic drugs and anabolic steroids in human urine by LC-ESI-MS/MS. Anal. Bioanal. Chem. 2008;392:681–698. doi: 10.1007/s00216-008-2292-5. [DOI] [PubMed] [Google Scholar]
- 20.Mazzarino M., Botrè F. A fast liquid chromatographic/mass spectrometric screening method for the simultaneous detection of synthetic glucocorticoids, some stimulants, anti-oestrogen drugs and synthetic anabolic steroids. Rapid Commun. Mass Spectrom. 2006;20:3465–3476. doi: 10.1002/rcm.2729. [DOI] [PubMed] [Google Scholar]
- 21.Mareck U., Geyer H., Guddat S. Identification of the aromatase inhibitors anastrozole and exemestane in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:1954–1962. doi: 10.1002/rcm.2545. [DOI] [PubMed] [Google Scholar]
- 22.Kang M.J., Hwang Y.H., Lee W. Validation and application of a screening method for beta2-agonists, anti-estrogenic substances and mesocarb in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:252–264. doi: 10.1002/rcm.2834. [DOI] [PubMed] [Google Scholar]
- 23.Maštovskà K., Lehotay S.J. Approaches to fast gas chromatography/tandem mass spectrometry. J. Chromatogr. A. 2003;1000:153–180. doi: 10.1016/s0021-9673(03)00448-5. [DOI] [PubMed] [Google Scholar]
- 24.Matisovà E., Dömötörovà M. Fast gas chromatography and its use in trace analysis. J. Chromatogr. A. 2003;1000:199–221. doi: 10.1016/s0021-9673(03)00310-8. [DOI] [PubMed] [Google Scholar]
- 25.Mazzarino M., Orengia M., Botrè F. Application of fast gas chromatography/mass spectrometry for the rapid screening of synthetic anabolic steroids and other drugs in anti-doping analysis. Rapid Commun. Mass Spectrom. 2007;21:4117–4124. doi: 10.1002/rcm.3326. [DOI] [PubMed] [Google Scholar]
- 26.Marcos J., Pascual J.A., De la Torre X. Fast screening of anabolic steroids and other banned doping substances in human urine by gas chromatography/tandem mass spectrometry. J. Mass Spectrom. 2002;37:1059–1073. doi: 10.1002/jms.365. [DOI] [PubMed] [Google Scholar]
- 27.Brunelli C., Bicchi C., Di Stilo A. High-speed gas chromatography in doping control: fast-GC and fast-GC/MS determination of beta-adrenoceptor ligands and diuretics. J. Sep. Sci. 2006;29:2765–2771. doi: 10.1002/jssc.200500387. [DOI] [PubMed] [Google Scholar]
- 28.Di Corcia D., Morra V., Pazzi M. Simultaneous determination of beta2-agonists in human urine by fast-gas chromatography/mass spectrometry: method validation and clinical application. Biomed. Chromatogr. 2010;24:358–366. doi: 10.1002/bmc.1300. [DOI] [PubMed] [Google Scholar]
- 29.Morra V., Davit P., Capra P. Fast gas chromatographic/mass spectrometric determination of diuretics and masking agents in human urine: development and validation of a productive screening protocol for antidoping analysis. J. Chromatogr. A. 2006;1135:219–229. doi: 10.1016/j.chroma.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 30.Tao X., Piao H., Canney D.J. Biotransformation of letrozole in rat liver microsomes: effects of gender and tamoxifen. J. Pharm. Biomed. Anal. 2007;43:1078–1085. doi: 10.1016/j.jpba.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Ruenitz P.C., Arrendale R.F., George G.D. Biotransformation of the antiestrogen clomiphene to chemically reactive metabolites in the immature female rat. Cancer Res. 1987;47:4015–4019. [PubMed] [Google Scholar]
- 32.Notley L.M., Crewe K.H., Taylor P.J. Characterization of the human cytochrome P450 forms involved in metabolism of tamoxifen to its alpha-hydroxy and alpha,4-dihydroxy derivatives. Chem. Res. Toxicol. 2005;18:1611–1618. doi: 10.1021/tx050140s. [DOI] [PubMed] [Google Scholar]
- 33.Donike M., Geyer H., Gotzmann A. Florence. In: Bellotti P., Benzi G., Ljungquist A., editors. Official Proceedings of the International Athletic Foundation World Symposium on Doping in Sport. IAAF; 1988. p. 53. [Google Scholar]
- 34.Jiménez C., Ventura R., Segura J. Validation of qualitative chromatographic methods: strategy in antidoping control laboratories. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002;767:341–351. doi: 10.1016/s1570-0232(01)00593-1. [DOI] [PubMed] [Google Scholar]
- 35.Mareck-Engelke U., Sigmund G., Opfermann G. Screening for tamoxifen, clomiphene and cyclofenil in doping analysis. In: Schänzer W., Geyer H., Gotzmann A., Mareck-Engelke U., editors. Recent Advances in Doping Analysis (9) Sport&Buch Strauß; Cologne: 2001. p. 53. [Google Scholar]
- 36.Kisanga E.R., Mellgren G., Lien E.A. Excretion of hydroxylated metabolites of tamoxifen in human bile and urine. Anticancer Res. 2005;25:4487–4492. [PubMed] [Google Scholar]
- 37.Mazzarino M., Fiacco I., de la Torre X. A mass spectrometric approach for the study of the metabolism of clomiphene, tamoxifen and toremifene by liquid chromatography time-of-flight spectroscopy. Eur. J. Mass Spectrom. (Chichester, England) 2008;14:171–180. doi: 10.1255/ejms.921. [DOI] [PubMed] [Google Scholar]
- 38.International Conference on Harmonisation, Validation of Analytical Procedures: Methodology. 〈http://www.ich.org/LOB/media/MEDIA417.pdf〉, 2004 (accessed 20 July 2010).