Abstract
Objective: To develop the representative fingerprint for the quality control of placenta polypeptide injection.
Methods
The chromatographic separation was performed using a Phenomenex Gemini C18 column (250 mm×4.6 mm, 5 μm) maintained at 30 °C. 0.1% aqueous trifluoroacetic acid (Solvent A) and acetonitrile contained 0.1% TFA (Solvent B) were used as mobile phase with a gradient elution. Detection wavelength was 280 nm with the sample injection volume of 50 μL; the flow rate was 1.0 mL/min. The fingerprints of different samples were investigated by similarity analysis.
Results
Nine peaks were identified as the characteristic common peaks. The similarities of the fingerprints of the 10 batches of samples were above 0.992.
Conclusion
This method showed high precision and good repeatability, and provided the basis for the improvement of the quality control of placenta polypeptide injection.
Keywords: Placenta polypeptide injection, Fingerprint, Similarity analysis, High performance liquid chromatography
1. Introduction
Placenta has been used as a nourishing medicine since ancient times [1]. With further research over the past several centuries, the function of the placenta was continued to be explored in depth. Placenta polypeptide injection, one of most widely used traditional Chinese preparations, is made from healthy human placenta, and mainly composed of amino acids, peptides, proteins, lipid fatty acids and nucleic acids. With the biological activity of enhancing cellular immune function and inhibiting peroxidation reaction, placenta polypeptide injection can be used for the treatment of reduction or disorder of cellular immune function, diseases caused by surgery healing, cell infection and leucopenia. It was also reported that placenta polypeptide injection has very good actions in inhibiting tumors and prolonging life [2].
However, the authentic standard for the quality control of placenta polypeptide injection was too simple to reflect the real and comprehensive internal quality of placenta polypeptide injection, for example, only total nitrogen item was assigned for the assay of preparation, and furthermore, no adequate method was used for total control of the quality of placenta polypeptide injection.
Fingerprint technique is a powerful tool for the quality control of multi-component herbal medicines and has been widely accepted as a useful means for the evaluation and quality control of herbal materials and their finished products. Fingerprint analysis has been introduced and accepted by World Health Organization (WHO) as a strategy for the assessment of herbal medicines [3]. Recently, fingerprint is also required by the Drug Administration Bureau of China to standardize injections made from traditional Chinese medicines and their raw materials [4]. Cheng and Yang [5] established a fingerprint method for analyzing the quality consistence of human placenta tissue hydrolysate of different lots, while fingerprint analysis of placenta polypeptide injection has not been reported.
The present study aimed at developing the HPLC fingerprint of placenta polypeptide injection. Then the fingerprint model could accurately reflect the quality and guarantee clinical efficacy of placenta polypeptide injection.
2. Experimental
2.1. Materials and reagents
Acetonitrile of HPLC grade was from Fisher Scientific (NJ, USA). Trifluoroacetic acid of HPLC grade was from J&K Chemical Ltd. (Utah, USA). Other reagents were of analytical grade. High purity water was obtained by Millipore Milli-Q water purification system (MA, USA). Placenta polypeptide injection was provided by Qianfeng Biological Products Co. (Guizhou Province, China), the specification is 4 mL of each, the batch numbers were 20100532, 20100953, 20100954, 20100955, 20100533, 20100578, 20100627, 20100783, 20100784 and 20100848, and are represented by 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 below, respectively.
2.2. Instrumentation and conditions
HPLC analysis was carried out on a Shimadzu UFLC XR HPLC equipped with binary solvent delivery pump, an auto sampler and photodiode array detector (PAD) connected to an LC Solution Software. The chromatographic separation was performed using a Phenomenex Gemini C18 column (250 mm×4.6 mm, 5 μm) maintained at 30 °C. Detection wavelength was 280 nm with the sample injection volume of 50 μL; the flow rate was 1.0 mL/min. 0.1% aqueous trifluoroacetic acid (TFA) (Solvent A) and acetonitrile contained 0.1% TFA (Solvent B) were used as mobile phase with a gradient elution starting with 100% A, then to reach 99.5%A and 0.5% B at 15 min and 50% A and 50% B at 35 min, finally to reach 100% A at 36 min, and maintain 100% A at 50 min.
2.3. Sample preparation
Placenta polypeptide injection was filtrated through 0.45 μm filter before HPLC analysis.
2.4. Method validation
The method validation was performed mainly on precision, reproducibility and stability.
2.5. Obtain of HPLC fingerprint and similarity analysis
To establish the representative chromatographic fingerprint, 10 batches of placenta polypeptide injection were analyzed under the established HPLC method. The obtained fingerprints of 10 batches of placenta polypeptide injection were analyzed automatically by professional software named Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine composed by Chinese Pharmacopoeia Commission (Version 2004A) (Beijing, China).
3. Results and discussion
3.1. Optimization of HPLC condition
To give the most chemical information and best separation in the chromatograms, the mobile phase and its flow rate, conditions for elution, column temperature and detection wavelength were investigated in this study.
In the selection of mobile phase, three elution systems were investigated. Solvent A was chosen among pure water, aqueous TFA and aqueous formic acid; solvent B was acetonitrile contained TFA. The aqueous TFA-acetonitrile contained TFA system showed a better separation, chromatographic peaks had moderate retention time and good resolution, so this system was chosen to be the mobile phase. The concentration of TFA in water and acetonitrile was further investigated. TFA concentrations of 0.05% and 0.1% were selected for gradient elution, the results showed that 0.1% of TFA both in water and acetonitrile was better. The elution gradient was also optimized and the best condition was described in Section 2.2.
From the results of comparative study of column temperature of 25, 30 and 35 °C and flow rate of 0.8, 1.0 and 1.2 mL/min, the flow rate was set at 1.0 mL/min when column temperature was kept at 30 °C considering resolution and running time. As we know, peptide bond has strong UV absorption around 215 nm, and aromatic rings have strong absorption at 280 nm, so 215 nm and 280 nm were monitored and compared. In gradient elution, the baseline was stable at 280 nm while fluctuated with the change in the proportion of mobile phase at 215 nm, so the detection wavelength was finally set at 280 nm.
3.2. Method validation
In order to obtain stable and repeatable chromatographic fingerprint of placenta polypeptide injection for quality control, the method validation of HPLC fingerprint analysis was performed on the basis of the relative retention time (RRT) and the relative peak area (RPA). Method precision was based on replicated analysis of samples, which were injected into HPLC system for six times. The RSDs of retention time and peak areas of all peaks were not exceeding 1.0% and 3.0%. The method reproducibility was studied through six-replicated sample solutions from the same batch. The RSDs of RRT and RPA were below 1.0% and 3.0%. The stability test was performed with a sample solution for 24 h. The RSDs of the RRT and RPA were found less than 1.0% and 3.0%, respectively. The result indicated that the developed method was validated and applicable for sample analysis.
3.3. HPLC fingerprints and similarity analysis
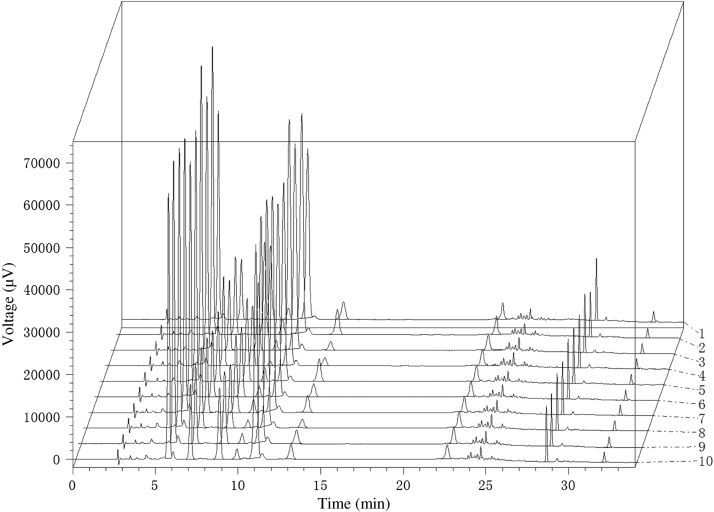
Under the optimal conditions, the fingerprints of 10 batches of placenta polypeptide injection were obtained (Fig. 1). It was found that these samples had similar HPLC profiles.
Figure 1.
The fingerprints of 10 batches of placenta polypeptide injection. 1–10 represent the batches of 20100532, 20100953, 20100954, 20100955, 20100533, 20100578, 20100627, 20100783, 20100784, 20100848, respectively.
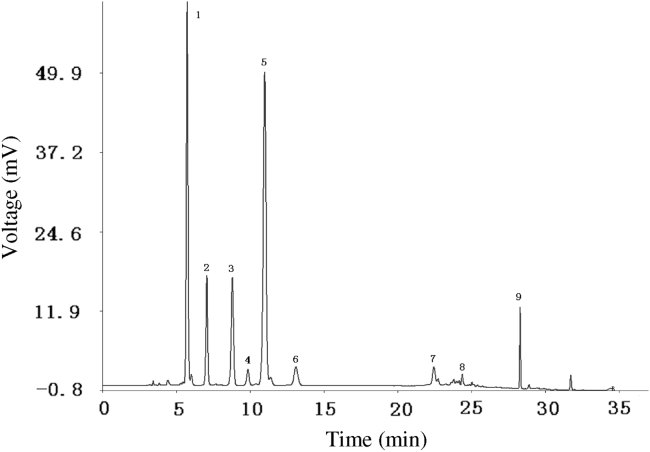
The similarity degrees of these samples should be evaluated by similarity analysis (SA), which has been compulsorily carried by State Food and Drug Administration (SFDA) of China. Using the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine, the representative standard fingerprint was generated by the median method (Fig. 2).
Figure 2.
Standard fingerprint of placenta polypeptide injection. Peaks 1–9 were assigned as “characteristic common peaks”.
Peaks existed in the standard fingerprint with reasonable heights and good resolutions were assigned as “characteristic common peaks”. There were 9 characteristic peaks (from peak 1 to peak 9) in the chromatogram, which cover more than 90% of the total area. To calculate the RRT and RPA, a reference peak should be chosen. Peak 5 had a considerably high content of more than 30% of the total area, and it also had moderate retention time, stable peak area and good shape. Therefore, it was chosen as the reference peak (S). Then the retention time and peak area of the 9 common peaks were measured (Table 1, Table 2) and RRT and RPA of all characteristic common peaks with respect to this reference peak were calculated (Table 3, Table 4).
Table 1.
Retention time of 10 batches of placenta polypeptide injection.
| Peak number | Retention time (s) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1 | 5.83 | 5.82 | 5.80 | 5.81 | 5.81 | 5.80 | 5.80 | 5.79 | 5.79 | 5.83 |
| 2 | 7.21 | 7.18 | 7.16 | 7.16 | 7.15 | 7.14 | 7.14 | 7.12 | 7.12 | 7.21 |
| 3 | 8.98 | 8.93 | 8.91 | 8.91 | 8.90 | 8.90 | 8.89 | 8.87 | 8.87 | 8.98 |
| 4 | 10.07 | 10.00 | 9.98 | 9.98 | 9.97 | 9.96 | 9.95 | 9.94 | 9.93 | 10.07 |
| 5 (S) | 11.23 | 11.15 | 11.13 | 11.13 | 11.12 | 11.11 | 11.10 | 11.08 | 11.07 | 11.23 |
| 6 | 13.38 | 13.30 | 13.29 | 13.27 | 13.27 | 13.24 | 13.25 | 13.23 | 13.20 | 13.38 |
| 7 | 23.01 | 22.85 | 22.81 | 22.78 | 22.78 | 22.71 | 22.73 | 22.73 | 22.65 | 23.01 |
| 8 | 28.68 | 28.68 | 28.68 | 28.67 | 28.67 | 28.66 | 28.65 | 28.64 | 28.65 | 28.68 |
| 9 | 32.16 | 32.16 | 32.15 | 32.15 | 32.15 | 32.14 | 32.14 | 32.13 | 32.14 | 32.16 |
Table 2.
Peak area of 10 batches of placenta polypeptide injection.
| Peak number | Peak area |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1 | 524,487 | 438,464 | 525,247 | 436,020 | 409,142 | 476,088 | 486,085 | 497,303 | 463,991 | 524,487 |
| 2 | 160,226 | 141,303 | 181,250 | 140,673 | 133,299 | 150,633 | 162,197 | 161,293 | 151,417 | 160,226 |
| 3 | 251,021 | 179,741 | 158,479 | 219,473 | 184,876 | 206,502 | 166,073 | 189,241 | 187,931 | 251,021 |
| 4 | 47,769 | 30,809 | 13,591 | 39,250 | 31,704 | 37,589 | 21,946 | 29,267 | 29,342 | 47,769 |
| 5 (S) | 697,044 | 640,986 | 771,392 | 622,568 | 605,187 | 659,920 | 699,245 | 701,027 | 662,799 | 697,044 |
| 6 | 98,719 | 31,415 | 28,749 | 80,223 | 47,892 | 59,598 | 31,274 | 50,383 | 53,125 | 98,719 |
| 7 | 52,573 | 49,512 | 51,761 | 45,396 | 43,510 | 47,148 | 47,211 | 48,139 | 45,130 | 52,573 |
| 8 | 47,785 | 61,492 | 56,256 | 58,104 | 63,767 | 55,827 | 58,925 | 55,691 | 58,870 | 47,785 |
| 9 | 14,745 | 15,013 | 14,164 | 12,932 | 14,668 | 15,190 | 14,751 | 14,886 | 14,781 | 14,745 |
Table 3.
Relative retention time (RRT) of 10 batches of placenta polypeptide injection.
| Peak number | RRT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | RSD (%) | |
| 1 | 0.520 | 0.519 | 0.522 | 0.521 | 0.522 | 0.522 | 0.522 | 0.522 | 0.522 | 0.523 | 0.223 |
| 2 | 0.643 | 0.642 | 0.644 | 0.643 | 0.643 | 0.643 | 0.643 | 0.643 | 0.643 | 0.643 | 0.054 |
| 3 | 0.800 | 0.800 | 0.800 | 0.800 | 0.801 | 0.801 | 0.801 | 0.801 | 0.801 | 0.801 | 0.039 |
| 4 | 0.896 | 0.897 | 0.897 | 0.897 | 0.897 | 0.897 | 0.897 | 0.897 | 0.897 | 0.897 | 0.014 |
| 5 (S) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0 |
| 6 | 1.193 | 1.192 | 1.193 | 1.194 | 1.193 | 1.194 | 1.192 | 1.194 | 1.194 | 1.193 | 0.063 |
| 7 | 2.048 | 2.049 | 2.049 | 2.049 | 2.047 | 2.049 | 2.045 | 2.048 | 2.052 | 2.047 | 0.098 |
| 8 | 2.552 | 2.555 | 2.572 | 2.576 | 2.576 | 2.579 | 2.580 | 2.582 | 2.585 | 2.589 | 0.381 |
| 9 | 2.860 | 2.865 | 2.884 | 2.888 | 2.889 | 2.892 | 2.894 | 2.896 | 2.900 | 2.904 | 0.398 |
Table 4.
Relative peak area (RPA) of 10 batches of placenta polypeptide injection.
| Peak number | RPA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | RSD (%) | |
| 1 | 0.683 | 0.752 | 0.684 | 0.681 | 0.700 | 0.676 | 0.721 | 0.695 | 0.709 | 0.700 | 3.375 |
| 2 | 0.227 | 0.230 | 0.220 | 0.235 | 0.226 | 0.220 | 0.228 | 0.232 | 0.230 | 0.228 | 2.157 |
| 3 | 0.366 | 0.360 | 0.280 | 0.205 | 0.353 | 0.305 | 0.313 | 0.238 | 0.270 | 0.284 | 16.824 |
| 4 | 0.061 | 0.069 | 0.048 | 0.018 | 0.063 | 0.052 | 0.057 | 0.031 | 0.042 | 0.044 | 32.539 |
| 5 (S) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0 |
| 6 | 0.128 | 0.142 | 0.049 | 0.037 | 0.129 | 0.079 | 0.090 | 0.045 | 0.072 | 0.080 | 42.346 |
| 7 | 0.080 | 0.075 | 0.077 | 0.067 | 0.073 | 0.072 | 0.071 | 0.068 | 0.069 | 0.068 | 5.014 |
| 8 | 0.123 | 0.069 | 0.096 | 0.073 | 0.093 | 0.105 | 0.085 | 0.084 | 0.079 | 0.089 | 12.840 |
| 9 | 0.034 | 0.021 | 0.023 | 0.018 | 0.021 | 0.024 | 0.023 | 0.021 | 0.021 | 0.022 | 7.619 |
The largest variation was found in peaks 4 and 6, with both having an RSD over 30% in RPA for the 10 batches of samples. Considering the individual difference in human placenta, which is the raw materials of placenta polypeptide injection, the concentrations of some ingredients are different from batch to batch, especially those ingredients with low concentration. The single peak area of peaks 4 and 6 is less than 5% of the total area, so the two peaks' RSD of RPA could not be monitored, but the RRT must be controlled, in order to guarantee the internal quality.
The similarities between the entire chromatographic profiles of 10 batches of placenta polypeptide injection and the standard chromatographic fingerprint were calculated, and the correlation coefficients of their fingerprints were shown as 0.992, 0.992, 0.999, 0.997, 0.997, 0.999, 0.999, 0.999, 0.999 and 1.000. These results indicated that the samples shared nearly the same correlation coefficients of similarities, showing the internal quality of these samples was excellent.
4. Conclusion
An HPLC method was developed for fingerprint of placenta polypeptide injection. The fingerprints of 10 batches of placenta polypeptide injection were obtained and analyzed by the professional software. This method showed high precision and good repeatability, and provided the basis for the improvement of the quality control of placenta polypeptide injection. It is a worthy further study to characterize the 9 common peaks.
References
- 1.Yang G.Q., Zou X.H. Research advances on chemical compositions, pharmacological effect and clinic application of placenta and its extract from human and animals. J. Shenyang Agric. Univ. 2003;34(2):150–154. [Google Scholar]
- 2.Guo D.G. Activity study of placenta polypeptide injection on transplantation tumors in mice. J. Tongren Vocat. Tech. Coll. (Nat. Sci. Ed.) 2009;7(5):31–33. [Google Scholar]
- 3.World Health Organization, Guidelines for the Assessment of Herbal Medicines, Geneva, 1991.
- 4.State Food and Drug Administration, Guidance for Experimental Research on HPLC Fingerprint of Traditional Chinese Injections (Draft), Beijing, 2000, 〈http://www.sda.gov.cn/WS01/CL0237/15768.html〉.
- 5.Cheng Y.Q., Yang P.Y. HPLC fingerprint of human placenta tissue hydrolysate. Chin. J. Biochem. Pharm. 2001;14(4):238–240. [Google Scholar]