Abstract
Background:
The need for more effective management of acute asthma has led to research on drugs which are otherwise approved for use in chronic asthma.
Objective:
To study and compare the effects of oral montelukast with oral ozagrel in acute asthma.
Materials and Methods:
One hundred and twenty patients with acute asthma were recruited for the study. Out of 120 study patients, forty each were randomized into placebo, montelukast, and ozagrel groups. After the first dose of the drug or placebo was administered, peak expiratory flow rate (PEFR), number of rescue medications and also vital signs were noted at 6 h, 12 h, 24 h, 48 h, and at discharge. In addition, same recordings were done on the morning (8 a.m. – 10 a.m.) following admission. The difference in mean PEFR of each group at above-mentioned time points was the primary endpoint whereas need for rescue medications the secondary end-point.
Results:
The respective mean PEFR recordings of the placebo, montelukast, and ozagrel groups at various time points were as follows: at 6 h (235.19 ± 3.18, 242.86 ± 3.26, 228.18 ± 3.25); at 12 h (254.37 ± 5.23, 265.62 ± 5.38, 242.99 ± 5.36); at 24 h (267.46 ± 7.41, 291.39 ± 7.61, 268.14 ± 7.58); and at 48 h (277.99 ± 7.35, 303.22 ± 7.56, 285.27 ± 7.53); and discharge (301.94 ± 7.07, 317.32 ± 7.27, 298.99 ± 7.23). The mean PEFR between the treatment groups were not statistically significant (P = 0.102). The mean PEFR in the three groups at 8–10 a.m. following admission was 257.60 ± 5.52, 264.23 ± 5.98, and 249.94 ± 5.96; P = 0.266. Total number of rescue doses needed were 7, 4, and 13, respectively (P = 0.67).
Conclusion:
Montelukast or ozagrel when added to the standard treatment of acute asthma does not result in any additional benefit.
KEY WORDS: Acute asthma, montelukast, ozagrel
INTRODUCTION
The need for effective medication, in addition to bronchodilators and corticosteroids, in the management of acute asthma, continues to kindle research on other categories of drugs. The delayed onset of action of corticosteroids; and their inability to inhibit the leukotriene- and thromboxane-induced inflammation leaves a lacuna in the armamentarium of acute asthma management that some of these newer drugs may have the potential to plug.[1,2,3,4] The current role of leukotriene-modifiers (e.g., montelukast) in the management of asthma is limited to their use in chronic cases.[5,6] In recent times, there has been some interest in probing their role in acute asthma – the results ranging for favorable to outright negative.[1,7,8] Thromboxane A2 has role in bronchoconstriction; and the use of thromboxane A2 synthase inhibitors (e.g., ozagrel) in chronic asthma have shown varied results in different population groups – Asians showing favorable results. Studies on their use in acute asthma hardly exist. Hence, our endeavor in this study was to address the issues of any usefulness of montelukast and ozagrel in acute asthma.[9]
Aims and objective
This study aims to study and compare the effects of oral montelukast with oral ozagrel in acute asthma.
MATERIALS AND METHODS
This randomized, prospective, placebo-controlled, double-blinded, single-center, comparative study was initiated after obtaining permission from the Institutional Ethics Committee. The study was conducted in the Department of Respiratory Medicine of a tertiary care teaching hospital attached to a medical college. Patients presenting to outpatient unit or emergency triage from October 2014 to March 2016 with a primary diagnosis of acute exacerbation of asthma requiring hospitalization, were included in the study after taking written informed consent in the language understandable to them. The inclusion criteria were age between 18 and 65, previously diagnosed case of bronchial asthma, a primary diagnosis of acute exacerbation of asthma on presentation, peak expiratory flow rate (PEFR) less than or equal to 75% of personal best within the last 12 months or of predicted value, no other acute pathology complicating the present condition such as cardiac, metabolic, or other respiratory causes. The exclusion criteria were smoking history more than 10 pack years, pregnant or breastfeeding females, patient on regular leukotriene receptor antagonists or thromboxane A2 synthase inhibitors within 2 weeks of presentation, intake of oral or parenteral steroids for >5 days within 1 month of presentation, intake of theophylline within 1 week of presentation, need of intubation before presentation, patients on regular Rifampicin, Phenytoin, or Phenobarbitone, history of allergy to montelukast or ozagrel, baseline renal function test, and liver function test derangement.
History was noted, findings of physical examination were recorded, and laboratory investigations were noted. Forty subjects were included in each arm of the study as we anticipated a minimum difference of 40 L/min in PEFR with a standard deviation of 100 L/min for 80% power at 95% confidence level. Baseline PEFR was measured for all enrolled patients using Mini Wright's PEF meter. The patients were randomized into three groups after 5 min of measuring the baseline PEFR. Patients in all the three groups received standard treatment for asthma exacerbation, i.e., nebulized salbutamol 2.5 mg 6th hourly, nebulized ipratropium bromide 500 mcg 6th hourly, and intravenous methyl prednisolone 40 mg 8th hourly. The investigational drugs required for the study were kept in 3 different packets, labeled as 1 (oral montelukast 10 mg tablet), 2 (oral ozagrel 200 mg tablet), and 3 (placebo) before enrolling the first patient into the study. Patients randomized to Group 1 in addition to standard treatment received a tablet from packet “1” at enrollment and a placebo tablet after 12 h. Patients randomized to Group 2 received tablets from packet “2” − one tablet at enrollment and one tablet after 12 h. Patients randomized to Group 3 received placebo tablets from packet “3” – one at enrollment and one after 12 h. The investigational medications were continued till the patient got discharged from the hospital or developed any adverse effects to these drugs. Rescue medication (nebulized salbutamol) was given when indicated. Additional oxygen and methylxanthines were given when required.
PEFR values, rescue medication details, and vitals (pulse rate, blood pressure, respiratory rate, and SpO2 by pulse oximetry) were recorded at 6 h, 12 h, 24 h, and 48 h of drug or placebo administration and at discharge. These parameters were recorded on the morning after admission (8 a.m. – 10 a.m.) as well.
The primary end-point was the mean PEFR at each measured time point following treatment. Secondary end-point was the need for rescue medications.
Statistical analysis
The measured mean PEFR in treatment groups at different time point after starting treatment was analyzed by repeated measures analysis of variance (ANOVA) (with baseline PEFR set as covariate). The mean PEFR measured at morning after admission between 8 a.m. and 10 a.m. was analyzed by univariate ANOVA (with baseline PEFR as covariate). The need for rescue medication was analyzed by Kruskal–Wallis test. The need for methylxanthine and oxygen was analyzed by Chi-square test. The number of hospital stay was analyzed with Mann–Whitney test adjusted for Type 1 error for pairwise comparison and expressed as median and interquartile ranges. Analysis was performed using SPSS 22.0 (IBM Co., Armonk, NY, USA), and the means and percentages were expressed in graphs and tables.
RESULTS
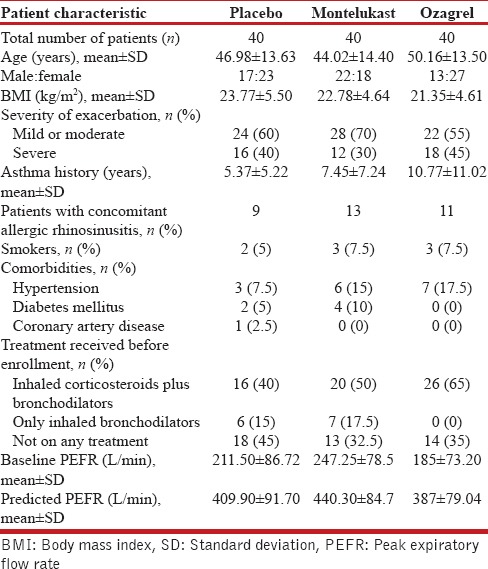
A total 142 patients with acute exacerbation symptoms of asthma were screened, out of which 22 were excluded. Forty patients each were randomized to placebo, montelukast, and ozagrel groups. There were no dropouts in the study. All patients completed the study [Figure 1]. Baseline characteristics of each study group are shown in the Table 1.
Figure 1.
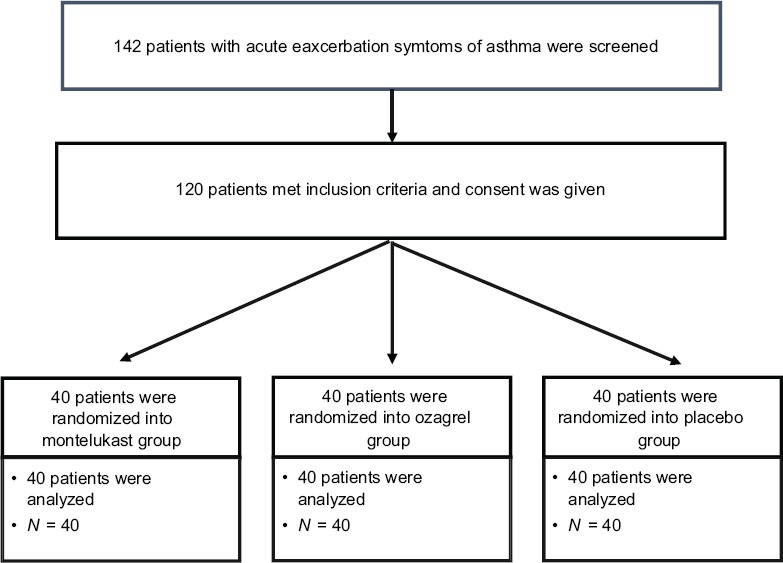
Consort diagram of the study
Table 1.
Baseline patient characteristics
Primary outcome measures
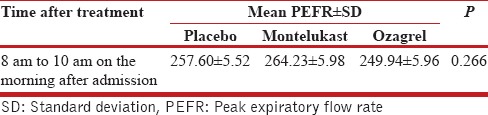
The mean PEFR for the three study arms were calculated from admission to discharge using repeated measures ANOVA with baseline PEFR as covariate. The measured values are shown in the Table 2 and Figure 2. The differences in mean PEFR between the treatment groups were not statistically significant (P = 0.102). The difference in mean PEFR of montelukast was not statistically significant when compared to ozagrel (P = 0.37) and placebo group (P = 0.51) at discharge, with baseline PEFR set as covariate. The difference in mean PEFR of ozagrel was not statistically significant when compared with placebo (P = 1.0) at discharge, with baseline PEFR set as covariate. The mean PEFR for the three study arms at morning after admission (8–10 a.m.) was analyzed using univariate ANOVA with baseline PEFR set as covariate. The difference in PEFR at morning after admission was not statistically significant (P = 0.266) [Table 3].
Table 2.
Mean peak expiratory flow rate at different points of time after starting treatment. (baseline peak expiratory flow rate [214.58 L/min] set as covariate)
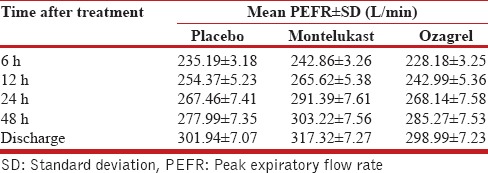
Figure 2.
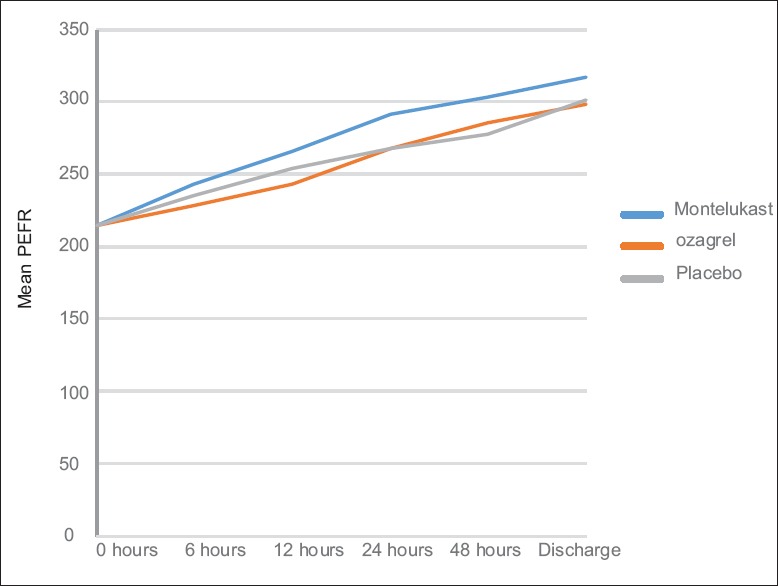
Mean peak expiratory flow rate at different points of time after starting treatment (baseline peak expiratory flow rate (214.58 L/min) set as covariate)
Table 3.
Mean peak expiratory flow rate at morning after admission between 8 a.m. and 10 a.m. (baseline peak expiratory flow rate [214.58 L/min] set as covariate)
Secondary outcome measures
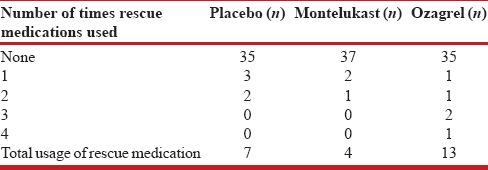
The need for rescue medications was analyzed by Kruskal–Wallis test. Three patients required rescue medications in montelukast group - two patients receiving rescue medication once each, and the other received rescue medications twice. In ozagrel group, five patients received rescue medications: one patient received it four times; two patients received three times each, one patient twice and another one once. In placebo group, five patients received rescue medication: two patients received it twice and three patients received once [Table 4]. The difference in the frequency of rescue medication use in three groups was not statistically significant (P = 0.67).
Table 4.
Need for rescue medication in each group
Need for methylxanthines and oxygen
Twenty-five patients out of 120 patients had received methylxanthines during study for relieving symptoms. Five patients required oxygen supplementation for maintaining oxygen saturation. Distribution of these in different groups is summarized in Table 5. The difference in the requirement for methylxanthines, and oxygen in different groups was not statistically significant (P = 0.518).
Table 5.
Need for methylxanthines and supplemental oxygen in study population

Number of days of hospital stay and condition at discharge
There were no specified criteria for discharge of the patients. The duration of hospital stay (in days) was expressed as median (interquartile range): placebo 5.5 (4–7), montelukast 5 (4–6.75), and ozagrel 7 (6–8). The difference in number of days of hospital stay between the three groups was significant (P < 0.001). The difference between montelukast and ozagrel group showed statistical significance (P < 0.001). However, there was no statistical significance between montelukast and placebo group (P = 0.232). The difference in number of days of hospital stay between ozagrel and placebo was statistically significant (P = 0.002). All patients improved symptomatically at discharge. All patients showed either improvement or no change in vital signs compared to admission, except seven patients. However, all the seven patients were discharged based on symptomatic improvement.
Adverse events
Imminent respiratory arrest and need for intubation and mechanical ventilation were not reported in any of the study arm. No deaths were reported in any of the three study arms. There were no reported serious adverse effects of drugs during the study.
DISCUSSION
Montelukast or ozagrel when added to the standard treatment of acute asthma does not result in any additional beneficial effect: Our data show that a study, done by Ramsay et al., in the United Kingdom demonstrated a significant improvement in PEFR in patients with acute asthma when they were given montelukast in addition to standard treatment.[1] In a randomized, placebo-controlled study, done in acute asthma setting, better forced expiratory volume in 1 s (FEV1) values were obtained after giving oral montelukast than after placebo. Moreover, intravenous formulation of montelukast performed even better.[10] Significant improvement in FEV1 in acute asthma, after administering intravenous montelukast, has been reported by various other studies as well.[11,12,13,14] Zhang et al. in a systematic review and meta-analysis found that montelukast administration led to significant improvement in peak expiratory flow percent in acute asthma (P = 0.008) and, in addition, there was reduction in systemic corticosteroid requirement (P = 0.005).[15] Very few studies from India have assessed the role of leukotriene modifiers in acute asthma. One such randomized, placebo-controlled, three-arm study from a tertiary care teaching hospital in Karnataka has reported that addition of zileuton to the standard treatment of acute asthma was associated with significant improvement in PEFR compared to placebo. Montelukast, however, failed to demonstrate such an effect. The montelukast arm had lowest baseline values of PEFR at the start of this study and, hence, this could have affected the results pertaining to this arm of the study. It is noteworthy that montelukast and zileuton both significantly reduced the need for rescue medication.[7] Similar effect of montelukast on requirement of inhaled short-acting beta-agonist has been reported in a three-arm study − oral montelukast plus intravenous steroid, intravenous steroid alone and placebo – which, in addition, noted a trend toward better improvement in PEFR in the montelukast arm, though not statistically significant.[16] However, in the present study, montelukast arm did not show any significant effect on PEFR. Although the requirement for rescue medication was the lowest in the montelukast arm, it did not reach statistical significance. Similar results were obtained by Zubairi et al. in a randomized, double-blind, placebo-controlled study in which the investigators evaluated the efficacy of oral montelukast in hospitalized patients with acute asthma. In this study from the Indian subcontinent, 100 patients were randomized into montelukast and placebo group, and the standard treatment for acute asthma was given to all the patients in both groups. The investigators found no statistically significant difference in PEFR over the course of stay in the hospital (P = 0.20 at day 2 and P = 0.47 at day 3) and at discharge (P = 0.15) when compared to placebo group.[8] Ferreira et al. have reported in a study, done on twenty patients from Portugal, that there was no significant differences between montelukast and placebo, so far as the improvement in PEFR and length of stay in emergency room was considered. However, montelukast group did have lesser need for aminophylline or steroids (P = 0.03). In our study, the need for additional methylxanthines, even though lesser in the montelukast group, failed to show any statistical significance (P = 0.518).[17,18] Even the utility of intravenous montelukast − which has offered some promise as shown by the studies mentioned above − is mired in this maze of conflicting results; and this is highlighted by a study done on asthmatic children in the United States. In this study, it was observed than intravenous montelukast was not better that placebo in terms of improvement in symptoms, FEV1 values, or clinical course during hospitalization.[18] Thus, the question mark on the utility of montelukast in acute asthma persists in view of these contradictory results thrown up by various studies. Whether these contrasting results are a reflection of the differences in study populations, study designs or drug characteristics itself needs to be looked into. At present, oral leukotriene modifiers are not recommended for use in acute asthma, but to ascertain the role of intravenous formulations of montelukast, in such a clinical setting, more research is needed.[5]
Thromboxane A2, metabolite of arachidonic acid along the cyclooxygenase pathway, causes acute bronchoconstriction in response to allergen exposure among asthma patients; and the drugs that inhibit its production, i.e., thromboxane A2 synthetase inhibitors (e.g., ozagrel), have demonstrated beneficial effects, such as reduction in bronchial hyperresponsiveness, in Asian population.[19,20] Studies from Japan have reported that thromboxane modulators may have role as an add-on therapy to inhaled corticosteroids in the event of poor response to corticosteroids, in the setting of chronic asthma.[4,9] However, to the best of our knowledge, this group of drugs does not appear to have been tested in acute asthma setting. In our study, ozagrel did not demonstrate any benefit over and above the standard treatment of acute asthma. On the contrary, there was trend toward increased need for the rescue medication in ozagrel arm, though it was not statistically significant. Notwithstanding these results, the search for more therapeutic options for the management of acute asthma must continue as this clinical situation is associated with a high morbidity.
The limitation of the present study was the use of PEFR and not FEV1 as indicator of airflow obstruction. FEV1 is a better indicator and has been used in various similar studies. Assessment of subjective measures of clinical improvement such as the BORG dyspnea score could have improved the study design. The absence of well laid out criteria for discharge of patients may have affected the results so far as the duration of hospital stay is concerned. Moreover, our study was only a single-center study in a geographically localized population in south India.
CONCLUSION
Montelukast or ozagrel when added to the standard treatment of acute asthma does not result in any additional beneficial effect: improvement in lung functions, decrease in requirement of rescue medication, or decrease in the need for methylxanthine and oxygen.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ramsay CF, Pearson D, Mildenhall S, Wilson AM. Oral montelukast in acute asthma exacerbations: A randomised, double-blind, placebo-controlled trial. Thorax. 2011;66:7–11. doi: 10.1136/thx.2010.135038. [DOI] [PubMed] [Google Scholar]
- 2.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2000:CD002178. doi: 10.1002/14651858.CD002178. [DOI] [PubMed] [Google Scholar]
- 3.Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. 2001;119:1533–46. doi: 10.1378/chest.119.5.1533. [DOI] [PubMed] [Google Scholar]
- 4.Myou S, Fujimura M, Leff AR. Additive effect of cysteinyl leukotriene or thromboxane modifiers to inhaled corticosteroids in asthmatic patients. Allergol Int. 2004;53:211–7. [Google Scholar]
- 5.British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2014;69(Suppl 1):1–192. [PubMed] [Google Scholar]
- 6.Watts K, Chavasse RJ. Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children. Cochrane Database Syst Rev. 2012:CD006100. doi: 10.1002/14651858.CD006100.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magazine R, Shahul HA, Chogtu B, Kamath A. Comparison of oral montelukast with oral zileuton in acute asthma: A randomized, double-blind, placebo-controlled study. Lung India. 2016;33:281–6. doi: 10.4103/0970-2113.180805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zubairi AB, Salahuddin N, Khawaja A, Awan S, Shah AA, Haque AS, et al. A randomized, double-blind, placebo-controlled trial of oral montelukast in acute asthma exacerbation. BMC Pulm Med. 2013;13:20. doi: 10.1186/1471-2466-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez JM, Janssen LJ. Revisiting the usefulness of thromboxane-A2 modulation in the treatment of bronchoconstriction in asthma. Can J Physiol Pharmacol. 2015;93:111–7. doi: 10.1139/cjpp-2014-0364. [DOI] [PubMed] [Google Scholar]
- 10.Dockhorn RJ, Baumgartner RA, Leff JA, Noonan M, Vandormael K, Stricker W, et al. Comparison of the effects of intravenous and oral montelukast on airway function: A double blind, placebo controlled, three period, crossover study in asthmatic patients. Thorax. 2000;55:260–5. doi: 10.1136/thorax.55.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo CA, Jr, Smithline HA, Malice MP, Green SA, Reiss TF. A randomized controlled trial of intravenous montelukast in acute asthma. Am J Respir Crit Care Med. 2003;167:528–33. doi: 10.1164/rccm.200208-802OC. [DOI] [PubMed] [Google Scholar]
- 12.Camargo CA, Jr, Gurner DM, Smithline HA, Chapela R, Fabbri LM, Green SA, et al. A randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthma. J Allergy Clin Immunol. 2010;125:374–80. doi: 10.1016/j.jaci.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Adachi M, Taniguchi H, Tohda Y, Sano Y, Ishine T, Smugar SS, et al. The efficacy and tolerability of intravenous montelukast in acute asthma exacerbations in Japanese patients. J Asthma. 2012;49:649–56. doi: 10.3109/02770903.2012.690479. [DOI] [PubMed] [Google Scholar]
- 14.James JM. A randomized, controlled trial of intravenous montelukast in acute asthma. Pediatrics. 2004;114:547. [Google Scholar]
- 15.Zhang HP, Jia CE, Lv Y, Gibson PG, Wang G. Montelukast for prevention and treatment of asthma exacerbations in adults: Systematic review and meta-analysis. Allergy Asthma Proc. 2014;35:278–87. doi: 10.2500/aap.2014.35.3745. [DOI] [PubMed] [Google Scholar]
- 16.Cýllý A, Kara A, Ozdemir T, Oǧüş C, Gülkesen KH. Effects of oral montelukast on airway function in acute asthma. Respir Med. 2003;97:533–6. doi: 10.1053/rmed.2003.1479. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira MB, Santos AS, Pregal AL, Michelena T, Alonso E, de Sousa AV, et al. Leukotriene receptor antagonists (Montelukast) in the treatment of asthma crisis: Preliminary results of a double-blind placebo controlled randomized study. Allerg Immunol (Paris) 2001;33:315–8. [PubMed] [Google Scholar]
- 18.Morris CR, Becker AB, Piñieiro A, Massaad R, Green SA, Smugar SS, et al. A randomized, placebo-controlled study of intravenous montelukast in children with acute asthma. Ann Allergy Asthma Immunol. 2010;104:161–71. doi: 10.1016/j.anai.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 19.Dogné JM, de Leval X, Benoit P, Rolin S, Pirotte B, Masereel B, et al. Therapeutic potential of thromboxane inhibitors in asthma. Expert Opin Investig Drugs. 2002;11:275–81. doi: 10.1517/13543784.11.2.275. [DOI] [PubMed] [Google Scholar]
- 20.Rolin S, Masereel B, Dogné JM. Prostanoids as pharmacological targets in COPD and asthma. Eur J Pharmacol. 2006;533:89–100. doi: 10.1016/j.ejphar.2005.12.058. [DOI] [PubMed] [Google Scholar]


