Abstract
Background:
Treatment of multidrug-resistant (MDR-TB) mainly focuses on bacteriological cure. However, only limited studies have evaluated the sequelae left after the completion of treatment among MDR-TB patients.
Objective:
To assess the persistent symptoms, radiological sequelae, pulmonary function impairment and quality of life at the completion of treatment among MDR-TB patients.
Methods:
Forty six MDR-TB patients were enrolled, who completed two years of treatment under programmatic management of Drug Resistant tuberculosis at a tertiary referral institute in Delhi, India. Detailed clinical history was taken. X-ray chest, 6 Minute Walk Test and pulmonary function tests were attempted in all patients. Quality of life was evaluated using Seattle obstructive lung disease questionnaire.
Results:
At the completion of MDR-TB treatment 95.7% patients had residual symptoms; 100% patients had residual bilateral chest x-ray abnormality with 82.6% patients showing far advanced disease. PFT was abnormal in 97.6% patients with mixed pattern being the commonest abnormality. Quality of Life was impaired with mean physical function of 46%.
Conclusion:
At the completion of MDR-TB treatment, significant numbers of patients are left with post treatment sequelae. The medical management and social support for these patients should be incorporated in the national programs.
KEY WORDS: Multidrug-resistant, sequelae, tuberculosis
INTRODUCTION
According to the World Health Organization, multidrug-resistant tuberculosis (MDR-TB) is defined as resistance to isoniazid and rifampicin, with or without resistance to other drugs. An estimated 3.3% of new cases and 20% of previously treated cases have MDR-TB.[1] Although at the end of treatment, TB patients are cured bacteriologically, they are left with significant symptomatic, radiological, and physiological sequelae.[2] Anatomical changes result in radiographic and functional alterations.[3] In the literature, many studies have described the sequelae after treatment of TB. However, very few reports are available in the literature who have studied the sequelae in MDR-TB patients at the end of the treatment. Pulmonary impairment after TB is associated with disability worldwide and calls for more aggressive case prevention strategies and posttreatment evaluation. This study was planned to assess post-TB sequelae in MDR-TB patients at the end of treatment.
PATIENTS AND METHODS
The study was conducted at National Institute of Tuberculosis and Respiratory Diseases (NITRD), New Delhi India. It was a cross-sectional study of patients who completed MDR-TB treatment at NITRD under the programmatic management of drug-resistant tuberculosis (PMDT) under the national program. The patients were diagnosed as per PMDT guidelines through mycobacterial cultures and sensitivity testing.[4] They were treated with the second-line drugs as per national protocol with an intensive phase of 6–9 months and continuation phase of 18 months. The patients meeting the criteria for cured were included. The patients seropositive for human immunodeficiency virus were not included. The study was approved by the Institute Research and Ethical Committee and all participants gave written informed consent.
A detailed history was elicited from each patient. Socioeconomic status was assessed by modified Kuppuswamy socioeconomic scale.[5] Dyspnea evaluation was done by the British Medical Research Council (MRC) scale and using the Borg scale.[6,7]
Plain chest posterior-anterior radiographs were taken in all the patients. The radiographs were evaluated by two observers independently to detect the extent of involvement. The help of the third observer was taken in case of discrepancy. The chest X-rays (CXRs) were classified using the criteria used by the National Tuberculosis Association of USA.[8] The criteria defined minimal lesions as the ones which have slight to moderate density but do not contain demonstrable cavitations. They may involve a small part of one or both the lungs, but the total extent, regardless of distribution, should not exceed the volume of lung on one side that occupies the space above the second chondrosternal junction and the spine of the fourth or body of the fifth vertebra. Moderately advanced lesions are defined as the lesions which may be present in one or both lungs, but the total extent should not exceed the following limits: Disseminated lesions of slight to moderate density that may extend throughout the total volume of one lung or the equivalent in both lungs; dense and confluent lesions limited in extent to one-third the volume of one lung; total diameter of cavitations, if present, should be <4 cm. Far advanced lesions are defined as the lesions which are more extensive than moderately advanced. The other features on CXR such as unilateral/bilateral and cavitatory/noncavitatory were also recorded.
Lung function tests included spirometry, lung volume study, and diffusion study. Spirometry was done in all patients by a dry rolling seal spirometer (Morgan Transfer Test Model C). The calibration of the equipment and the procedure adopted during the test was in accordance with the equipment and procedure requirements of the American Thoracic Society (ATS) Snowbird Workshop on the standardization of pulmonary function testing.[9] Obstructive disease was defined as forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio <0.8, with a FVC ≥80% of the predicted value. Restrictive disease was diagnosed if the FVC was <80% of the predicted values and FEV1/FVC ratio >0.8. Combined disease was defined as an FVC <80% of the predicted value and an FEV1/FVC ratio <0.8.[10]
The results were expressed at body temperature, ambient pressure, saturated. They were reported in absolute volumes as well as percent predicted based on regression equations of the European Community of Coal and Steel.[11] Lung volume study was conducted using the helium dilution method. The diffusion study was done using the single-breath carbon monoxide diffusion method. The severity of lung function impairment based on FEV1 and severity of diffusion capacity were defined as per the European Respiratory Society/ATS guidelines 2005.[12]
Six-min walk test (6MWT) was done as per the ATS guidelines.[13] The predicted distance walked was calculated using standard reference equations given by Enright and Sherrill, and results were expressed as percentage of the predicted values.[14]
The Seattle Obstructive Lung Disease Questionnaire (SOLDQ) was used for assessing the quality of life for all enrolled patients. The questionnaire had 29 items measuring four health dimensions; physical function, emotional function, coping skills, and treatment satisfaction were evaluated. The higher score denoted a good quality of life for all the components of the questionnaire.[15] We used a Hindi translation of this questionnaire which had been pretested.
RESULTS
Forty-six patients meeting the eligibility criteria were enrolled with mean ± standard deviation (SD) age of 27.6 ± 10.5 years. Table 1 shows the demographic profile of the patients under study, and there were 25 males and 21 females. It was observed that 54.3% of the patients were unemployed and 54.3% of patients belonged to upper and lower class on modified Kuppuswamy scale.
Table 1.
Characteristics of the patients under study (total patients=46)
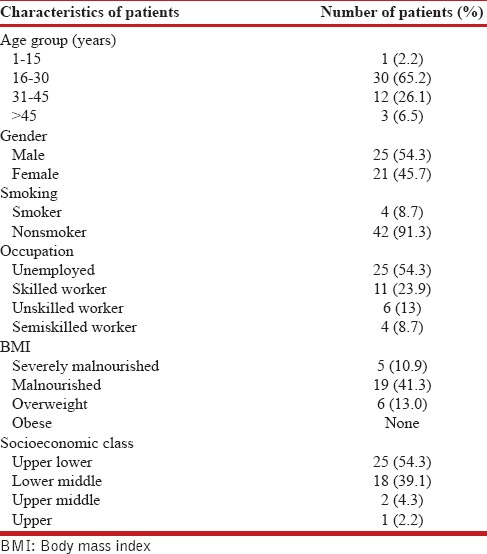
Among the 46 study patients, 44 (95.7%) were symptomatic, and the most common residual symptom after 2 years of MDR-TB treatment was breathlessness, present in 40 (87%) of the patients. The evaluation of level of breathlessness demonstrated Grade II dyspnea in 30 (65.2%) patients on MRC scale and 23 (50%) patients belonged to scale 4 on Borg scale.
On radiological evaluation, all the patients had bilateral disease. The extent of CXR involvement showed far advanced disease in 82.6% of the patients, moderately advanced disease in 17.4%, and none of the patients had minimal disease. Multiple cavities were reported in 24 (52.2%) patients.
Pulmonary function tests are shown in Table 2. The patients had a mean ± SD FEV1 of 56.1 ± 21.0% of predicted normal, and the most common pattern of lung function abnormality was mixed type, presents in 27 (64.3%) patients, followed by restrictive abnormality present in 23.8%. Out 46 patients under study, 32 patients could perform diffusion studies. Table 3 shows the diffusion capacity abnormality among these 42 patients. The diffusion capacity was found to be moderately decreased in 15 (46.9%) patients.
Table 2.
Pulmonary function abnormalities among 42 patients*

Table 3.
Distribution of severity of diffusion capacity among 32 patients*

In 6MWT, the mean ± SD distance walked in males was 493.6 ± 64.7 and in females was 468.1 ± 40.8 m. The distance walked in 6MWT was 71.3% predicted normal in males and 64.8% predicted normal in females.
Table 4 shows the quality of life impairment among 46 patients with post-MDR-TB sequelae. The most affected domain was physical domain with mean physical function of 46%.
Table 4.
Quality of life by Seattle Obstructive Lung Disease Questionnaire in mean percent of 46 patients under study

DISCUSSION
The present study was aimed at evaluating persistent symptoms, radiological sequelae, pulmonary function test, and quality of life at completion of 2 years of treatment among MDR-TB patients registered under PMDT. In our study, 95.7% of patients had residual symptoms. The most common residual symptom was breathlessness being observed in 87% of patients. In a study, in India, it was reported that 78% of the MDR-TB patients were left with some residual symptoms at the end of the treatment.[2]
After treatment of TB, the lung is left with residual fibrosis, scarring, cavitation and distortion of lung architecture leading to volume loss, and bronchiectasis.[16] Pulmonary TB also involves airways leading to the edema, hypertrophy, and hyperplasia of mucous glands, thus affecting the caliber of the airways. Through the mechanism of cicatricial fibrosis, there is also a reduction of total lung capacity. It has also been demonstrated that there is a significant increase in functional limitations in MDR-TB patients who had undergone multiple treatments compared to patients cured in the first treatment.[3]
In our study, among forty-six patients, all patients had bilateral CXR involvement. Far advanced lesions were present in 82.6% whereas 17.4% showed moderate lesions on CXR. Residual radiological lesions persist even after successful treatment of TB.[17] Other studies have also demonstrated abnormal CXRs after completion of treatment in MDR pulmonary TB. Another study among 33 cured MDR-TB patients observed radiographic abnormalities affecting an average of 36% of lung fields, and out of the 33 patients, more than half had cavities.[10] In our study, also 52.2% of the patients had multiple cavities. In another study, out of 45 patients, 98% showed residual CXR abnormalities, and multiple cavities were present in 53% of the patients.[2] These extensive residual lung lesions are predictors of permanent disability because of tissue destruction and susceptibility to opportunistic infections thus, leading to reduced quality of life.[18] Radiologic evidence suggestive of inactive TB has also been found to be associated with an increased risk of airflow obstruction.[19]
In our study, among 42 patients who could perform the pulmonary function tests, 97.6% had lung function abnormality. Functional impairments have been studied in patients following treatment of pulmonary TB.[3,20,21,22,23,24,25,26,27,28,29,30] Controversy exists regarding which pattern of abnormality is most prevalent in TB sequelae. Mixed disorder was the most prevalent as per the study done by Ramos et al.[31] Panda et al. reported restrictive defect as the most common followed by mixed disorder.[32] However, there are very limited studies on pulmonary function impairment among MDR-TB patients. de Vallière and Barker[10] studied residual lung damage after treatment completion in 33 MDR-TB patients and found that 31 out of 33 patients had abnormal lung function tests. The median FEV1 was 63% and FVC was 57% of the predictive value; restrictive and mixed patterns were the predominant abnormalities. In our study, also, the most prevalent pattern of lung function abnormality was mixed type seen in 64.3%, followed by restriction pattern seen in 23.8%.
In another study done on 18 cured MDR-TB patients, the spirometric patterns were abnormal in 78% of patients.[33] It has also been observed that patients with multiple treatments showed significantly lower levels of vital capacity and FEV1 when compared to patients with single treatment.[3]
In our study, 6MWT was done in all forty-six patients and observed to be reduced being 71.3% of predicted normal in males and 64.8% in females. 6MWT has been found to be significantly low in patients of pulmonary TB at the end of the treatment.[29,34] However, limited studies have assessed six minute walk distance in treated MDR-TB patients. In a study done, among 47 treated MDR-TB patients, the 6MWT was observed to be significantly low in males as well as female patients.[2] In another study, 6MWT was done in adult patients diagnosed with pulmonary TB treated as outpatients over 6 months (Group I) and adult patients with multiple treatments of longer duration and MDR-TB patients (Group II). It was found that Group II patients showed a greater functional impairment as the average distance walked in 6MWT was over 100 m lower.[3]
The studies among TB patients have shown that TB has adverse impacts on patient's quality of life, which persists even after microbiological cure has been achieved.[35,36,37,38,39] However, we found only one study focusing on quality of life in treated MDR-TB patients in which 78% of the patients reported impairment in their quality of life using airways questionnaire 20.[33] In our study, the quality of life as assessed by SOLDQ was impaired, and the physical domain was predominantly affected. In another study done in TB patients, also, the main impaired domain was the physical domain.[36]
CONCLUSION
The current study observed that at the completion of MDR-TB treatment, although patients become negative bacteriologically, significant numbers of patients are left with persistent symptoms, CXR abnormality, lung function impairment, and impaired quality of life. Hence, long-term follow-up is important for understanding the overall impact of this disease on patient's physical, functional, and socioeconomic well-being. We need to focus beyond bacteriological cure of MDR-TB patients. Medical management and social support for posttreatment sequelae of MDR-TB should be incorporated in the national programs. The end TB strategy also aims to achieve the target of zero TB-affected families facing catastrophic costs due to TB by 2035.[40]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. Report on the Tuberculosis Epidemic. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Singla N, Singla R, Fernandes S, Behera D. Post treatment sequelae of multi-drug resistant tuberculosis patients. Indian J Tuberc. 2009;56:206–12. [PubMed] [Google Scholar]
- 3.Di Naso FC, Pereira JS, Schuh SJ, Unis G. Functional evaluation in patients with pulmonary tuberculosis sequelae. Rev Port Pneumol. 2011;17:216–21. doi: 10.1016/j.rppneu.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosetti M, Ferrarese M, Codecasa LR, Besozzi G, Sarassi A, Viggiani P, et al. Incidence of venous thromboembolism in tuberculosis patients. Respiration. 2006;73:396. doi: 10.1159/000091188. [DOI] [PubMed] [Google Scholar]
- 5.Bairwa M, Rajput M, Sachdeva S. Modified kuppuswamy's socioeconomic scale: Social researcher should include updated income criteria, 2012. Indian J Community Med. 2013;38:185–6. doi: 10.4103/0970-0218.116358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medical Research Council Committee on Research into Chronic Bronchitis. Instructions for use of the Questionnaire on Respiratory Symptoms. Devon: W.J Holman; 1966. [Google Scholar]
- 7.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–8. [PubMed] [Google Scholar]
- 8.Marjani M, Tabarsi P, Baghaei P, Shamaei M, Biani PG, Mansouri D, et al. Incidence of thromboembolism in hospitalized patients with tuberculosis and associated risk factors. Arch Clin Infect Dis. 2012;7:56–9. [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.de Vallière S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004;8:767–71. [PubMed] [Google Scholar]
- 11.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 12.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 13.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 14.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 15.Tu SP, McDonell MB, Spertus JA, Steele BG, Fihn SD. A new self-administered questionnaire to monitor health-related quality of life in patients with COPD. Ambulatory Care Quality Improvement Project (ACQUIP) Investigators. Chest. 1997;112:614–22. doi: 10.1378/chest.112.3.614. [DOI] [PubMed] [Google Scholar]
- 16.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA, et al. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192:1201–9. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 17.Menon B, Nima G, Dogra V, Jha S. Evaluation of the radiological sequelae after treatment completion in new cases of pulmonary, pleural, and mediastinal tuberculosis. Lung India. 2015;32:241–5. doi: 10.4103/0970-2113.156233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH, et al. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001;21:839–58. doi: 10.1148/radiographics.21.4.g01jl06839. [DOI] [PubMed] [Google Scholar]
- 19.Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, et al. Prior TB, smoking, and airflow obstruction: A cross-sectional analysis of the guangzhou biobank cohort study. Chest. 2010;137:593–600. doi: 10.1378/chest.09-1435. [DOI] [PubMed] [Google Scholar]
- 20.Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 2007;131:1817–24. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 21.Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis. 1971;103:625–40. doi: 10.1164/arrd.1971.103.5.625. [DOI] [PubMed] [Google Scholar]
- 22.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–8. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vecino M, Pasipanodya JG, Slocum P, Bae S, Munguia G, Miller T, et al. Evidence for chronic lung impairment in patients treated for pulmonary tuberculosis. J Infect Public Health. 2011;4:244–52. doi: 10.1016/j.jiph.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med. 1989;83:195–8. doi: 10.1016/s0954-6111(89)80031-9. [DOI] [PubMed] [Google Scholar]
- 25.Menezes AM, Hallal PC, Perez-Padilla R, Jardim Jr, Muiño A, Lopez MV, et al. Tuberculosis and airflow obstruction: Evidence from the PLATINO study in Latin America. Eur Respir J. 2007;30:1180–5. doi: 10.1183/09031936.00083507. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculous destroyed lung. Respir Med. 2003;97:1237–42. doi: 10.1016/s0954-6111(03)00255-5. [DOI] [PubMed] [Google Scholar]
- 27.Pefura-Yone EW, Kengne AP, Tagne-Kamdem PE, Afane-Ze E. Clinical significance of low forced expiratory flow between 25% and 75% of vital capacity following treated pulmonary tuberculosis: A cross-sectional study. BMJ Open. 2014;4:e005361. doi: 10.1136/bmjopen-2014-005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: A systematic review. Int J Infect Dis. 2015;32:138–46. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Ralph AP, Kenangalem E, Waramori G, Pontororing GJ, Sandjaja, Tjitra E, et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: Under-recognised phenomena. PLoS One. 2013;8:e80302. doi: 10.1371/journal.pone.0080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakaya J, Kirenga B, Getahun H. Long term complications after completion of pulmonary tuberculosis treatment: A quest for a public health approach. J Clin Tuberc Other Mycobact Dis. 2016;3:10–2. [Google Scholar]
- 31.Ramos LM, Sulmonett N, Ferreira CS, Henriques JF, de Miranda SS. Functional profile of patients with tuberculosis sequelae in a university hospital. J Bras Pneumol. 2006;32:43–7. doi: 10.1590/s1806-37132006000100010. [DOI] [PubMed] [Google Scholar]
- 32.Panda A, Bhalla AS, Sharma R, Mohan A, Sreenivas V, Kalaimannan U, et al. Correlation of chest computed tomography findings with dyspnea and lung functions in post-tubercular sequelae. Lung India. 2016;33:592–9. doi: 10.4103/0970-2113.192871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godoy MD, Mello FC, Lopes AJ, Costa W, Guimarães FS, Pacheco AG, et al. The functional assessment of patients with pulmonary multidrug-resistant tuberculosis. Respir Care. 2012;57:1949–54. doi: 10.4187/respcare.01532. [DOI] [PubMed] [Google Scholar]
- 34.Sivaranjini S, Vanamail P, Eason J. Six minute walk test in people with tuberculosis sequelae. Cardiopulm Phys Ther J. 2010;21:5–10. [PMC free article] [PubMed] [Google Scholar]
- 35.Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis: A systematic review. Health Qual Life Outcomes. 2009;7:14. doi: 10.1186/1477-7525-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhuria M, Sharma N, Ingle G. Impact of tuberculosis on the quality of life. Indian J Community Med. 2008;33:58–9. doi: 10.4103/0970-0218.39249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muniyandi M, Rajeswari R, Balasubramanian R, Nirupa C, Gopi PG, Jaggarajamma K, et al. Evaluation of post-treatment health-related quality of life (HRQoL) among tuberculosis patients. Int J Tuberc Lung Dis. 2007;11:887–92. [PubMed] [Google Scholar]
- 38.Brown J, Capocci S, Smith C, Morris S, Abubakar I, Lipman M, et al. Health status and quality of life in tuberculosis. Int J Infect Dis. 2015;32:68–75. doi: 10.1016/j.ijid.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 39.Atif M, Sulaiman SA, Shafie AA, Asif M, Sarfraz MK, Low HC, et al. Impact of tuberculosis treatment on health-related quality of life of pulmonary tuberculosis patients: A follow-up study. Health Qual Life Outcomes. 2014;12:19. doi: 10.1186/1477-7525-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heit JA, O’Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch Intern Med. 2002;162:1245–8. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]


