Abstract
A simple, sensitive, and specific liquid chromatography tandem mass spectrometry (LC-MS/MS) method was developed for the quantification of desloratadine (DL) in human plasma using desloratadine-d5 (DLD5) as an internal standard (IS). Chromatographic separation was performed using an Xbridge C18 column (50 mm×4.6 mm, 5 μm) with an isocratic mobile phase composed of 10 mM ammonium formate: methanol (20:80, v/v), at a flow rate of 0.7 mL/min. DL and DLD5 were detected with proton adducts at m/z 311.2→259.2 and 316.2→264.3 in multiple reaction monitoring (MRM) positive modes, respectively. Liquid–liquid extraction (LLE) method was used to extract the drug and the IS. The method was validated over a linear concentration range of 5.0–5000.0 pg/mL with a correlation coefficient of (r2)≥0.9994. This method demonstrated intra- and inter-day precision within 0.7–2.0% and 0.7–2.7%, and an accuracy within 101.4–102.4%, and 99.5–104.8%. DL was found to be stable throughout the freeze–thaw cycles, bench-top, and postoperative stability studies. This method was successfully applied in the analysis of plasma samples following oral administration of DL (5 mg) in 35 healthy Indian male human volunteers under fasting conditions.
Keywords: Mass spectrometry, Desloratadine, Bioequivalence, Pharmacokinetic study
1. Introduction
Desloratadine is an antihistamine drug used to treat the symptoms of allergies, such as sneezing, teary eyes, and runny nose. It is also used to treat skin hives and itching in people with chronic skin reactions. The molecular formula of desloatadine is C19H19ClN2 with a molecular weight of 310.8. The chemical name of the desloratadine is 8-chloro-6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine and has a structure as described below [1] (Fig. 1). DL is well absorbed and is extensively metabolized in the human body. The major metabolite of DL in human plasma and urine is the glucuronide conjugate of 3-hydroxydesloratadine. We found that the elimination of DL was gradual (mean t(1/2)=19.5 h) and persisted in the plasma for 48–120 h postdose [2], [3], [4]. There were no statistically significant differences in the mean plasma concentrations in any of the main pharmacokinetic parameters of rupatadine, DL, and 3-hydroxydesloratadine when administered in combination with azithromycin or alone [5], [6].
Figure 1.
Chemical structures of desloratadine and desloratadine-D5.
As of now, there have been several methods reported for quantification of DL in rat plasma with LC-MS [7], human plasma with LC-MS [8], [9], [10], [11], [12], LC-MS with nanospray ionization [13], human plasma with HPLC [14], [15], [16], dog plasma with HPLC [17], pharmaceutical formulation with HPLC [18], [19], and pharmaceutical formulation with electrophorsis [20]. Wen et al. [8] reported having developed a method using LLE with a concentration range of 0.1–20 ng/mL. The sensitivity was improved by Xu et al. [9] with a concentration range of 0.05–10 ng/mL. Among all researchers, Yang et al. [12] achieved the best results with high sensitivity at a linearity range of 25–10000 pg/mL with SPE extraction.
The aim of the proposed method is to extract DL in human plasma by the LLE method with high sensitivity. Moreover, the analyte is to be compared with the deuterated internal standard (IS), which is most appropriate to develop in the bioanalytical method in terms of matrix effect and reproducibility. The developed method could be useful for application in clinical and pharmacokinetic studies.
2. Materials and methods
2.1. Chemicals and reagents
DL was obtained from Cadila Pharmaceuticals, India. DLD5 was procured from Clear Synth, India. Ammonium formate and sodium hydroxide (analytical grade) were purchased from Merck, Mumbai, India. Methanol, ethyl acetate, and dichloromethane (HPLC grade) were obtained from J.T. Baker, USA. Human plasma was procured from Navjeevan Blood Blank, Hyderabad. Milli Q water was taken from the in-house Milli-Q system.
2.2. Instrumentation
The 1200 series HPLC system (Agilent Technologies, Germany) was used. Mass spectrometric detection was performed using an API 4000 triple quadrupole instrument (ABI-SCIEX, Toronto, Canada) and using multiple reaction monitoring (MRM). Data processing was performed using the Analyst 1.4.1 software package (SCIEX).
2.3. Detection
Detection was done by turbo ion spray (API) positive mode with unit resolution. For DL, mass transitions were obtained from 311.2 m/z (parent ion) to 259.2 m/z (product ion). Similarly, DLD5 mass transitions were obtained from (316.2 m/z) (parent ion) to 264.3 m/z (product ion).
2.4. Chromatographic conditions
Chromatographic separation was performed using an Xbridge C18 column (50 mm×4.6 mm, 5 μm) at a temperature of 40 °C. The mobile phase was composed of 10 mM ammonium formate:methanol (20:80, v/v) at a flow rate of 0.7 mL/min. Deuterated IS DLD5 was used as the appropriate IS in terms of chromatography and extractability. DL and DLD5 were eluted at 0.9±0.2 min, approximately, with a total run time of 3 min for each sample.
2.5. Preparation of standards and quality control samples
Standard stock solutions of DL (100.0 μg/mL) and DLD5 IS (100.0 μg/mL) were prepared in methanol. The IS spiking solutions (10.0 ng/mL) were prepared in reconstitution solutions (10 mM ammonium formate and methanol in the ratio of 20:80 (v/v) from IS stock solutions. Standard stock solutions and IS spiking solutions were stored in refrigerator conditions of 2–8 °C until analysis. Standard stock solutions of DL (100.0 μg/mL) were added to drug-free screened human plasma to obtain concentration levels of 5.0, 10.0, 200.0, 800.0, 1400.0, 2000.0, 3000.0, 4000.0, and 5000.0 pg/mL for analytical standards, and 5.0, 15.0, 2500.0, and 3500.0 pg/mL for quality control (QC) standards, and stored in the freezer at −30 °C until analysis. The aqueous standards were prepared in a reconstitution solution (10 mM ammonium formate and methanol in the ratio of 20:80 (v/v) and stored in the refrigerator at 2–8 °C until analysis.
2.6. Sample preparation
The LLE method was used to isolate DL and DLD5 from human plasma. For this purpose, 100 μL of DLD5 (10 ng/mL) and 400 μL of plasma sample were added to the labeled polypropylene tubes and vortexed briefly for about 5 min. Thereafter, 100 μL of 0.1 M NaOH solution and 3 mL of extraction solvent (in the ratio of ethyl acetate:dichloromethane 80:20(v/v)) were added and vortexed for about 10 min. Next, the samples were centrifuged at 4000 rpm for approximately 5 min at ambient temperature. From each, a supernatant sample was transferred into labeled polypropylene tubes and evaporated to a dryness of 40 °C briefly, and then reconstituted with a reconstitution solution (10 mM ammonium formate and methanol in the ratio of 20:80 (v/v), and the sample was transferred into auto sampler vials and injected into the LC-MS for study.
2.7. Selectivity and sensitivity
Selectivity was performed by analyzing human blank plasma samples from six different sources (donors) with an additional hemolyzed group and lipedimic group to test for interference at the retention times of analytes. The sensitivity was compared with the lower limit of quantification (LLOQ) of the analyte with its blank plasma sample. The peak area of blank samples should not be more than 20% of the mean peak area of the limit of quantification (LOQ) of DL and 5% of the mean peak area of DLD5.
2.8. Precision and accuracy
It was determined by replicate analysis of QC samples (n=6) at LLOQ, low QC (LQC), medium QC (MQC), high QC (HQC), and upper limit of quantification (ULOQ) levels. The % coefficient of variation (CV) should be less than 15%, and accuracy within 15%, except for LLOQ, where it should be within 20%.
2.9. Matrix effect
The matrix effect caused due to the plasma matrix was used to evaluate the ion suppression/enhancement in a signal when comparing the absolute response of QC samples after pretreatment (LLE) with the reconstitution samples extracted from the blank plasma sample spiked with analyte. Experiments in triplicates were performed at MQC levels with 6 different plasma lots with the acceptable precision (% CV) of ≤15%.
2.10. Recovery
The extraction recovery of analyte and IS from human plasma was determined by analyzing QC samples. Recovery at 3 concentrations (15.0, 2500.0, and 3500.0 pg/mL) was determined by comparing peak areas obtained from the plasma sample and the standard solution spiked with the blank plasma residue. A recovery of more than 50% was considered adequate to obtain required sensitivity.
2.11. Stability
LQC and HQC samples (n=6) were retrieved from deep freeze after 3 freeze-thaw cycles according to clinical protocol. Samples were stored at −30 °C in 3 cycles of 24, 36, and 48 h. In addition, the long-term stability of DL in QC samples was also evaluated by analysis after 105 days of storage at −30 °C. Auto-sampler stability was studied following a 53-h storage period in the auto sampler tray with control concentrations. Room temperature stability was studied over a 24.5-h period with control concentrations. Stability samples were processed and extracted along with the freshly spiked calibration curve standards. The precision and accuracy for the stability samples must be within ≤15 and ±15%, respectively, of their nominal concentrations.
2.12. Analysis of Human samples
The bioanalytical method described above was used to determine DL concentrations in plasma following oral administration of healthy human volunteers. These volunteers were contracted by the APL Research Pvt. Ltd., Hyderabad, India, and each subject was administered a 5-mg dose (one 5 mg tablet). This was performed in 35 healthy volunteers through oral administration along with 240 mL of drinking water. The reference product, Clarinex tablets (Merck) 5 mg, USA, and test product, DL tablets (test tablet) 5 mg, were used. Study protocol was approved by IEC (Institutional Ethical committee) as per ICMR (Indian Council of Medical Research). Blood samples were collected at the predose 0 h 5 min prior to dosage followed by further samples at 0.333, 0.667, 1, 1.333, 1.667, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 7, 8, 10, 12, 18, 24, 48, and 72 h. After dosage, 2 mL blood was collected each time in vaccutainers containing K2EDTA. A total of 44 (22 time points for test, and 22 time points for reference) time points were collected using centrifugation at 3200 rpm, 10 °C, for 10 min, and kept stored at −30 °C until sample analysis. Test and reference were administered separately to the same human volunteers under fasting conditions with proper washing periods as per protocol approved by the IEC.
2.13. Pharmacokinetics and statistical analysis
Pharmacokinetics parameters from the human plasma samples were calculated by a noncompartmental statistics model using WinNon-Lin5.0. software (Pharsight, USA). Blood samples were taken during a period of 3–5 times the terminal elimination half-life (t1/2), and it was considered as the area under the concentration time curve (AUC) ratio higher than 80%, as per FDA guidelines. Plasma DL concentration-time profiles were visually inspected, and Cmax and tmax values were determined. The AUC0–t was obtained by the trapezoidal method. AUC0–∞ was calculated up to the last measureable concentration, and extrapolations were obtained using the last measureable concentration and the terminal elimination rate constant (Ke). The terminal elimination rate constant, (Ke), was estimated from the slope of the terminal exponential phase of the plasma of DL concentration time curve (by means of the linear regression method). The terminal elimination half-life, t1/2, was then calculated as 0.693/Ke. Regarding AUC0−t and Cmax, bioequivalence was assessed by means of analysis of variance (ANOVA) and the standard 90% confidence intervals (CIs 90%) of the ratios' test/reference (logarithmically transformed data) was calculated. The bioequivalence was considered when the ratio of averages of log transformed data was within 80–125% for AUC0−t, AUC0−∞, and Cmax [21], [22].
3. Results and discussion
3.1. Method development
During development of the method, different options were evaluated to optimize mass spectrometry detection parameters, chromatography, and sample extraction.
3.1.1. Mass spectrometry detection parameters optimization
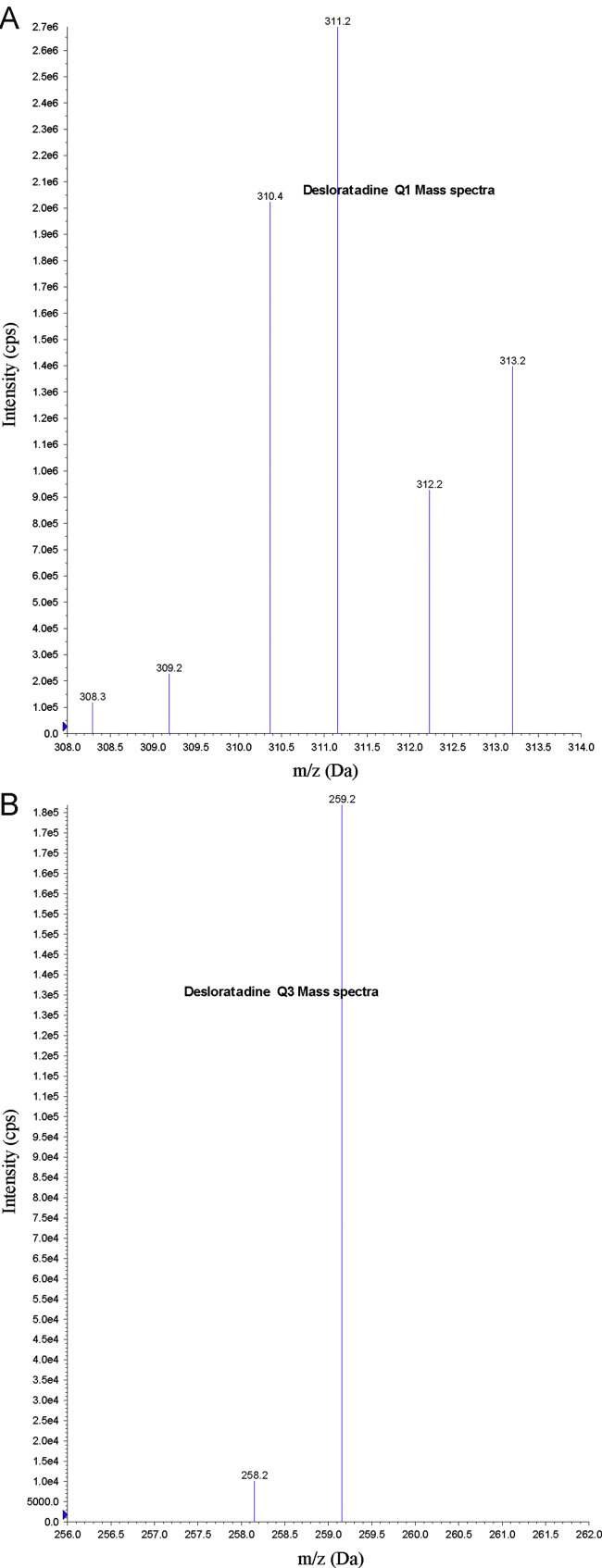
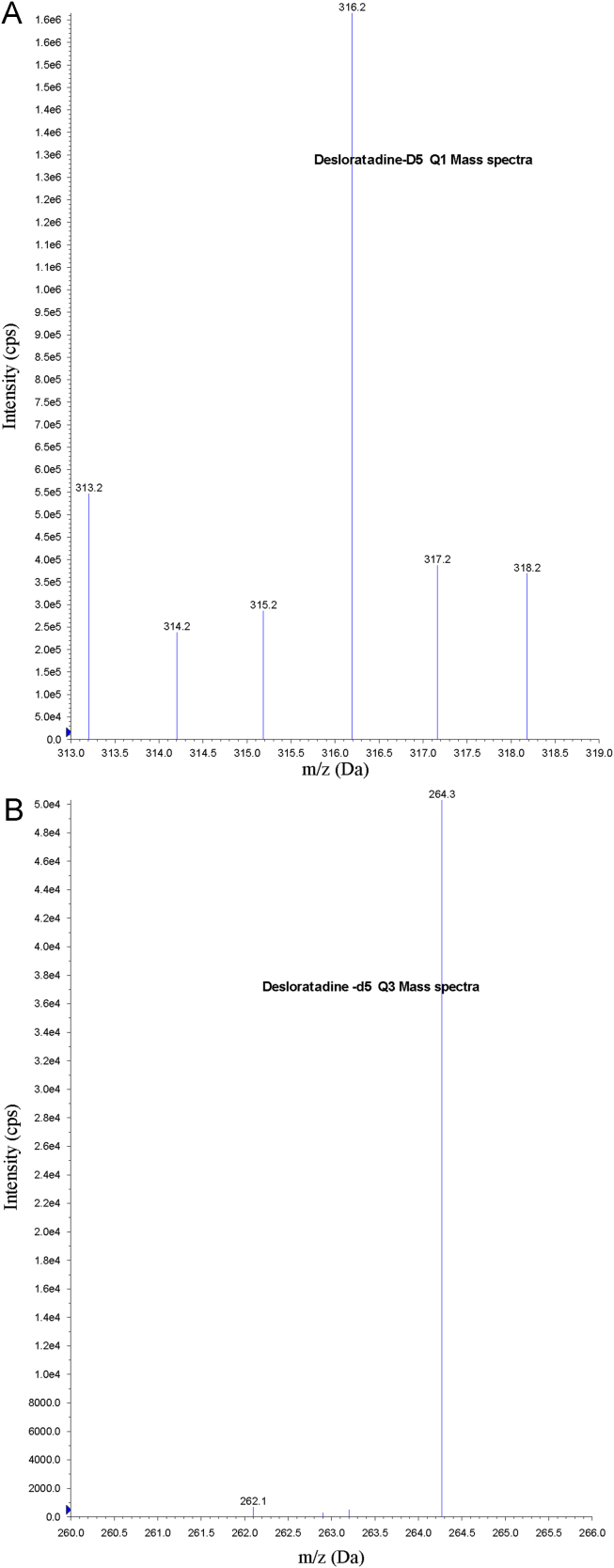
Electrospray ionization (ESI) provided a maximum response over atmospheric pressure chemical ionization (APCI) mode, and was chosen for this method. The instrument was optimized to obtain sensitivity and signal stability during infusion of the analyte in the continuous flow of mobile phase to electrospray ion source operated at both polarities at a flow rate of 5 μL/min. DL gave a better response when in the positive ion mode as compared to the negative ion mode. The predominant peaks in the primary ESI spectra of DL and DLD5 correspond to the (M+H)+ ions at m/z 311.2 and 316.2, respectively (Figs. 2(A) and 3(A)). Product ions of DL and DLD5 scanned in quadrupole 3 after a collision with nitrogen in quadrupole 2 had an m/z of 259.2 and 264.3, respectively (Figs. 2(B) and 3(B)). Mass parameters were optimized as source temperature 500 °C, heater gas 45 psi (nitrogen), nebulizer gas 30 psi (nitrogen), curtain gas 20 psi (nitrogen), CAD gas 5 psi (nitrogen), ion spray (IS) voltage 5500 V, source flow rate 600 μL/min without split, entrance potential 10 V, declustering potential 70 V, collision energy 30 V, and collision cell exit potential 15 V for both analyte and IS.
Figure 2.
(A) Parent ion mass spectra of desloratadine and (B) product ion mass spectra of desloratadine.
Figure 3.
(A) Parent ion mass spectra of desloratadine-D5 and (B) product ion mass spectra of desloratadine-D5.
3.1.2. Chromatography optimization
Initially, a mobile phase consisting of ammonium acetate and acetonitrile in varying combinations was tried, but a low response was observed. The mobile, phase containing 5 mM ammonium formate, methanol (20:80 v/v), gave a better response, but poor peak shape was observed. A mobile phase of 0.1% formic acid in water in combination with methanol and acetonitrile with varying combinations was tried. Finally, a mobile, phase containing 5 mM ammonium formate, acetonitrile (20:80 v/v), gave the best signal along with a marked improvement in the peak shape observed for DL and DLD5. Short-length columns, such as Symmetry Shield RP18 (50 mm×2.1 mm×3.5 μm), Inertsil ODS-2V (50 mm×4.6 mm, 5 μm), Hypurity C18 (50 mm×4.6 mm, 5 μm), and Hypurity Advance (50×4.0 mm, 5 μm), Xbridge C18 (50 mm×4.6 mm, 5 μm), were tried during development of the method. Xbridge C18 (50 mm×4.6 mm, 5 μm) column gave a relatively good peak shape with the best signal being obtained. It gave satisfactory peak shapes for both DL and DLD5. A flow rate of 0.7 mL/min without splitter was utilized and the run time was reduced to 2.5 min. Both drug and IS were eluted in a shorter time at 0.9±0.2 min. For an LC-MS/MS analysis, utilization of stable isotope-labeled, or suitable analog drugs as an IS proved helpful when a significant matrix effect is possible. In our case, DLD5 was found to be best suited for the present purpose. The column oven temperature was kept at a constant temperature of about 40 °C. Injection volume of a 5 μL sample is adjusted for better ionization and chromatography.
3.1.3. Extraction optimization
Prior to loading the sample for LC injection, the coextracted proteins should be removed from the prepared solution. For this purpose, initially, we tested with different extraction procedures like protein precipitation (PPT), LLE, and solid phase extraction (SPE). We found an ion-suppression effect in the PPT method for the drug and IS. Further, we also tried this with SPE and LLE. Out of all these, we observed that LLE is suitable for extraction of the drug and IS. We tried with several organic solvents (ethyl acetate, chloroform, n-hexane, dichloromethane, and methyl tertiary butyl ether) individually as well as with various combinations of LLE to extract analyte from the plasma sample. In our case, an ethyl acetate:dichloromethane (80:20) combination served as a good extraction solvent. The auto sampler wash is optimized as 80% methanol. High recovery and selectivity was observed in the LLE method. These optimized detection parameters, chromatographic conditions, and extraction procedures resulted in reduced analysis time with accurate and precise detection of DL in human plasma.
3.2. Method validation
A thorough and complete method of validation of metaxalone in human plasma was carried out following USFDA guidelines [23]. The method was validated for selectivity, sensitivity, matrix effect, linearity, precision and accuracy, recovery, and stability.
3.2.1. Selectivity and specificity
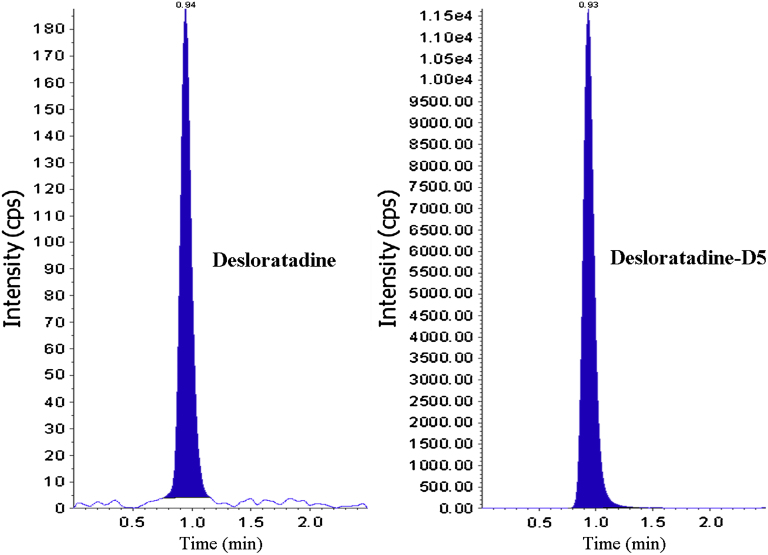
The analysis of DL and DLD5 using MRM function was highly selective with no interfering compounds (Fig. 4). Specificity was performed using 6 different lots of human plasma. Chromatograms obtained from plasma spiked with DL (5.0 pg/mL) and DLD5 (10.0 ng/mL) are shown in Fig. 5.
Figure 4.
MRM chromatogram of blank human plasma.
Figure 5.
Chromatogram of desloratadine, desloratadine-D5 at LOQ level.
3.2.2. Matrix effect
The overall precision of the matrix factor is expressed as CV% and was determined to be 1.34% for DL and 1.41% for DLD5.
3.2.3. Linearity
Calibration curves were plotted as the peak area ratio (DL/DLD5) vs. (DL) concentration. Calibration was found to be linear over the concentration range of 5.0–5000.0 pg/mL. The % CV was less than 3.2%, and the accuracy ranged from 97.6% to 102.2%. The determination coefficients (r2) were greater than 0.9994 for all curves (Table 1).
Table 1.
Calibration curve details from one batch of the validation section.
| Spiked plasma concentration (pg/mL) | Concentration measured (mean, pg/mL) | SD | CV (%) (n=5) | Accuracy (%) |
|---|---|---|---|---|
| 5.0 | 5.1 | 0.1 | 1.9 | 102.0 |
| 10.0 | 9.7 | 0.3 | 3.0 | 97.6 |
| 200.0 | 199.6 | 6.4 | 3.2 | 99.8 |
| 800.0 | 817.2 | 15.2 | 1.8 | 102.2 |
| 1400.0 | 1389.5 | 19.5 | 1.4 | 99.3 |
| 2000.0 | 2006.6 | 23.1 | 1.1 | 100.3 |
| 3000.0 | 2999.7 | 19.6 | 0.6 | 100.0 |
| 4000.0 | 3968.9 | 75.1 | 1.8 | 99.2 |
| 5000.0 | 5026.8 | 91.6 | 1.8 | 100.5 |
3.2.4. Precision and accuracy
Precision and accuracy for this method were controlled by calculating the intra- and inter-batch variations at four concentrations (5.0, 15.0, 2500.0, and 3500.0 pg/mL) of QC samples in 6 replicates. As shown in Table 2, the intrabatch % CV was less than 2.0%, and the accuracy ranged from 101.4% to 102.4%. Interbatch % CV was less than 2.7%, and the accuracy ranged from 99.5% to 104.8%. These results indicate the adequate reliability and reproducibility of this method within the analytical range.
Table 2.
Precision and accuracy (analysis with spiked plasma samples at four different concentrations).
| Spiked plasma concentration (pg/mL) | Within-run |
Between-run |
||||
|---|---|---|---|---|---|---|
| Concentration measured (n=6, pg/mL, mean±SD) | CV (%) | Accuracy (%) | Concentration measured (n=30, pg/mL, mean±SD) | CV (%) | Accuracy (%) | |
| 5.0 | 5.1±0.1 | 2.0 | 102.4 | 5.2±0.1 | 1.9 | 104.8 |
| 15.0 | 15.2±0.3 | 2.0 | 101.9 | 14.9±0.3 | 2.0 | 99.5 |
| 2500.0 | 2535.0±21.4 | 0.8 | 101.4 | 2495.2±25.1 | 1.0 | 99.8 |
| 3500.0 | 3577.1±25.2 | 0.7 | 102.2 | 3550.7±25.5 | 0.7 | 101.4 |
3.2.5. Recovery
The recovery following the sample preparation using the LLE method was calculated by comparing the peak area ratios of DL in plasma samples with the peak area ratios of solvent samples, and was estimated at control levels of DL. The recovery of DL was determined at three different concentrations of 15.0, 2500.0, and 3500.0 pg/mL, and was found to be 89.6%, 90.2%, and 91.2%, respectively. The overall average recovery of DL and DL D5 was found to be 90.3% and 92.5%, respectively.
3.2.6. LOQ and limit of detection
The LOQ was determined at 5 pg/mL. The limit of detection (LOD) was determined at 50 fg/10 μL injection volume.
3.2.7. Stability
Quantification of the DL in plasma which was subjected to 3 freeze-thaw (−30 °C up to room temperature) cycles showed the stability of the analyte. The concentrations ranged from 99.3% to 98.5% of the theoretical values. No significant degradation of the DL was observed even after a 53-h storage period in the auto sampler tray, and the final concentration of DL was between 97.3% and 103.2% of the theoretical values. Room temperature stability at 24.5 h was between 98.0% and 99.4% of the theoretical values. In addition, the long-term stability of DL in QC samples after 105 days of storage at −30 °C was also evaluated. The concentrations ranged from 94.6% to 101.8% of the theoretical values. These results confirmed the stability of DL in human plasma for at least 105 days at −30 °C (Table 3).
Table 3.
Stability of the desloratadine in plasma samples at different conditions.
| Stability | Spiked plasma concentration (pg/mL) | Concentration measured (n=6, pg/mL, mean±S.D.) | CV (%, n=6) |
|---|---|---|---|
| Room temperature stability (24.5 h) | 15.0 | 14.7±0.5 | 3.4 |
| 3500.0 | 3480.3±43.1 | 1.2 | |
| Autosampler sample stability (53 h) | 15.0 | 14.6±0.5 | 3.4 |
| 3500.0 | 3614.4±256.6 | 7.1 | |
| Long-term stability (105 days) | 15.0 | 14.2±0.5 | 3.5 |
| 3500.0 | 3563.7±63.4 | 1.8 | |
| Freeze and thaw stability (cycle 3, 48 h) | 15.0 | 14.9±0.2 | 1.3 |
| 3500.0 | 3450.2±39.1 | 1.1 |
3.3. Application to biological samples
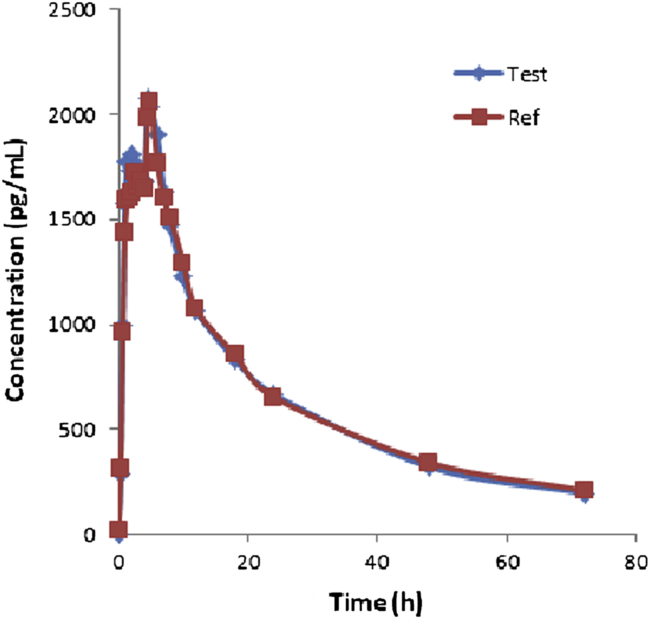
The method validated above was used in the determination of DL in plasma samples for establishing the bioequivalence of a single 5 mg dose (one 5 mg tablet) in 35 healthy volunteers. Typical plasma concentration vs. time profiles is shown in Fig. 6. All the plasma concentrations of DL were in the standard curve region and retained above the 5.0 pg/mL LOQ for the entire sampling period. Pharmacokinetic details are represented in Table 4. Test vs. reference Cmax, AUC0−t, AUC0−∞ were 101.1%, 99.3%, and 98.6%, respectively. On the basis of the pharmacokinetic data obtained from the test and reference values, it can be concluded that the two DL formulations (reference and test) analyzed are bioequivalent in terms of rate and extent of absorption.
Figure 6.
Mean plasma concentrations of test vs. reference after a 5 mg dose (one 5 mg Tablet) single oral dose (35 healthy volunteers).
Table 4.
Pharmacokinetic data of test vs. reference after a 5 mg dose (one 5 mg Tablet) single oral dose (35 healthy volunteers).
| Pharmacokinetic parameter | Test | Reference |
|---|---|---|
| Cmax (pg/mL) | 2079.2 | 2058.1 |
| AUC0−t (pg h/mL) | 46,361.8 | 46,696.0 |
| aUC0−α (pg h/mL) | 53,250.1 | 54,025.6 |
| tmax (h) | 4.5 | 5 |
| t1/2 (h) | 24.5 | 24.3 |
| Ke | 0.02822 | 0.02849 |
AUC0−∞: Area under the curve extrapolated to infinity.
AUC0−t: Area under the curve up to the last sampling time.
Cmax: The maximum plasma concentration.
tmax: The time to reach peak concentration.
Ke: Elimination rate.
4. Conclusions
The proposed method was five times higher in sensitivity than the method reported by Yang et al. [12] and the analyte was compared with deuterated IS. The method described here is quick (it requires less than 2.5 min of analysis time), is rugged, is a reproducible bioanalytical method, and was successfully applied to the bioequivalence and pharmacokinetic study of DL in 35 human volunteers after oral administration of 5 mg tablets under fasting conditions.
Acknowledgments
The authors wish to thank the support received (for providing literature survey) from IICT (Indian Institute of Chemical Technology), Hyderabad, India, and the APL Research Center Pvt Ltd, Hyderabad, India, to carry out this research work.
Contributor Information
Venkata Suresh Ponnuru, Email: sureshpharma78@gmail.com.
B.R. Challa, Email: baluchalla_99@yahoo.com.
References
- 1.Zheng J., Rustum A.M. Rapid separation of desloratadine and related compounds in solidpharmaceutical formulation using gradient ion-pair chromatography. J. Pharm. Biomed. Anal. 2010;51(1):146–152. doi: 10.1016/j.jpba.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Ramanathan R., Reyderman L., Kulmatycki K. Disposition of loratadine in healthy volunteers. Xenobiotica. 2007;37(7):753–769. doi: 10.1080/00498250701463317. [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan R., Alvarez N., Su A.D. Metabolism and excretion of loratadine in male and female mice, rats and monkeys. Xenobiotica. 2005;35(2):155–189. doi: 10.1080/00498250500038906. [DOI] [PubMed] [Google Scholar]
- 4.Ghosal A., Yuan Y., Hapangama N. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of 3-hydroxydesloratadine. Biopharm. Drug Dispos. 2004;25(6):243–252. doi: 10.1002/bdd.405. [DOI] [PubMed] [Google Scholar]
- 5.Yeh G.C., Deng S.T., Lo C.Y. Pharmacokinetics and bioequivalence study of a generic desloratadine tablet formulation in healthy male volunteers. Arzneimittelforschung. 2004;54(3):166–170. doi: 10.1055/s-0031-1296954. [DOI] [PubMed] [Google Scholar]
- 6.Solans A., Izquierdo I., Donado E. Pharmacokinetic and safety profile of rupatadine when coadministered with azithromycin at steady-state levels: a randomized, open-label, two-way, crossover, Phase I study. Clin. Ther. 2009;30(9):1639–1650. doi: 10.1016/j.clinthera.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Chen G., Daaro I., Pramanik B.N. Structural characterization of in vitro rat liver microsomal metabolites of antihistamine desloratadine using LTQ-Orbitrap hybrid mass spectrometer in combination with online hydrogen/deuterium exchange HR-LC/MS. J. Mass Spectrom. 2009;44(2):203–213. doi: 10.1002/jms.1498. [DOI] [PubMed] [Google Scholar]
- 8.Wen J., Hong Z., Wu Y., Wei H. Simultaneous determination of rupatadine and its metabolite desloratadine in human plasma by a sensitive LC-MS/MS method: application to the pharmacokinetic study in healthy Chinese volunteers. J. Pharm. Biomed. Anal. 2009;49(2):347–353. doi: 10.1016/j.jpba.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Xu H.R., Li X.N., Chen W.L. Simultaneous determination of desloratadine and its active metabolite 3-hydroxydesloratadine in human plasma by LC/MS/MS and its application to pharmacokinetics and bioequivalence. J. Pharm. Biomed. Anal. 2007;45(4):659–666. doi: 10.1016/j.jpba.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Shen J.X., Xu Y., Tama C.I. Simultaneous determination of desloratadine and pseudoephedrine in human plasma using micro solid-phase extraction tips and aqueous normal-phase liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21(18):3145–3155. doi: 10.1002/rcm.3187. [DOI] [PubMed] [Google Scholar]
- 11.Lu X.Y., Sheng-Tu J.Z., Chen Z.G. Study on determination of desloratadine in human serum and its pharmacokinetics by HPLC/MS. J. Zhejiang Univ. Med. Sci. 2005;34(4):372–374. doi: 10.3785/j.issn.1008-9292.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Clement R.P., Kantesaria B. Validation of a sensitive and automated 96-well solid-phase extraction liquid chromatography-tandem mass spectrometry method for the determination of desloratadine and 3-hydroxydesloratadine in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;792(2):229–240. doi: 10.1016/s1570-0232(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan R., Zhong R., Blumenkrantz N. Response normalized liquid chromatography nanospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2007;18(10):1891–1899. doi: 10.1016/j.jasms.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Shen J.X., Wang H., Tadros S. Orthogonal extraction/chromatography and UPLC, two powerful new techniques for bioanalytical quantitation of desloratadine and 3-hydroxydesloratadine at 25 pg/mL. J. Pharm. Biomed. Anal. 2006;40(3):689–706. doi: 10.1016/j.jpba.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Ghosal A., Gupta S., Ramanathan R. Metabolism of loratadine and further characterization of its in vitro metabolites. Drug Metab. Lett. 2009;3(3):162–170. doi: 10.2174/187231209789352067. [DOI] [PubMed] [Google Scholar]
- 16.El-Enany N., El-Sherbiny D., Belal F. Spectrophotometric, spectrofluorometric and HPLC determination of desloratadine in dosage forms and human plasma. Chem. Pharm. Bull. (Tokyo) 2007;55(12):1662–1670. doi: 10.1248/cpb.55.1662. [DOI] [PubMed] [Google Scholar]
- 17.Liu L., Qi M., Wang P. High-performance liquid chromatographic method for the bioequivalence evaluation of desloratadine fumarate tablets in dogs. J. Pharm. Biomed. Anal. 2004;34(5):1013–1019. doi: 10.1016/j.jpba.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J., Rustum A.M. Rapid separation of desloratadine and related compounds in solid pharmaceutical formulation using gradient ion-pair chromatography. J. Pharm. Biomed. Anal. 2010;51(1):146–152. doi: 10.1016/j.jpba.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 19.El-Sherbiny D.T., El-Enany N., Belal F.F. Simultaneous determination of loratadine and desloratadine in pharmaceutical preparations using liquid chromatography with a microemulsion as eluent. J. Pharm. Biomed. Anal. 2007;43(4):1236–1242. doi: 10.1016/j.jpba.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Kubacák P., Mikus P., Valásková I. Desloratadine Electrophorsis. Ceska Slov Farm. 2005;54(6):266–269. [PubMed] [Google Scholar]
- 21.Guidance for industry food—effect bio availability and fed bio equivalence studies, US Department of Health and Human services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), December 2002.
- 22.Guidance for industry bio availability and fed bio equivalence studies for orally administered drug products—general considerations, US Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), March 2003.
- 23.Guidance for industry: bioanalytical method validation, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), May 2001.