Abstract
Plasma quinolone concentrations are not routinely measured in clinical practice. However, in order to optimize quinolone treatment, monitoring of plasma concentrations could sometimes be useful particularly in critically ill patients. In this study, anti-sparfloxacin antibody was obtained by immunizing rabbits with sparfloxacin conjugated with bovine serum albumin using isobutyl chloroformate method. After the assay procedure was optimized, the standard curve of sparfloxacin was established. The practical measuring range of the competitive ELISA extended from 5 ng/mL to 2 μg/mL. The recovery rates and coefficients of variation for rat plasma, urine and tissues were 87.7–106.2% and 4.8–15.3%, respectively. To demonstrate the potential of the ELISA, a preliminary pharmacokinetics and tissue distribution study of sparfloxacin in rats and quantitative analysis of sparfloxacin in several pharmaceuticals were performed and compared with high-performance liquid chromatography (HPLC). The experimental data indicated that the proposed method would be a valuable tool in therapeutic drug monitoring (TDM) for sparfloxacin.
Keywords: Sparfloxacin, Enzyme-linked immunosorbent assay (ELISA), Biological samples, Pharmacokinetics, Tissue distribution
1. Introduction
Sparfloxacin (SPAR), a third-generation quinolone antimicrobial drug, is widely used in the treatment of urinary tract infections due to its excellent activity against various bacteria and good absorption on oral administration [1]. However, based on published results [2] and data on file with the manufacture of sparfloxacin, some adverse effects, including decreased appetite, vomiting, special sense adverse events and so on, would occur in some patients. Plasma quinolone concentrations are not routinely measured in clinical practice. However, in order to optimize quinolone treatment, monitoring of plasma concentrations could sometimes be useful particularly in critically ill patients.
In the past years, several methods, including bioassay [3], high-performance thin-layer chromatography [4], spectrophotometric method [5], high-performance liquid chromatography with ultraviolet [6], [7] or fluorescent detection [8], and liquid chromatography-tandem mass spectrometry [9], have been applied in such a field; however, because of the complexity and diversity of biological samples, these methods have shown some disadvantages for the analysis of sparfloxacin, such as time-consuming, high background and requirement of sample pre-treatment. Moreover, owing to individual characteristics of the method, it is impossible to simultaneously determine multiple samples under the same conditions. So, it is necessary to establish different methods for different test samples, which made high throughput, real-time sparfloxacin detection difficult.
In recent years, because of its lower detection limit, high specificity, low background and no requirement of sample pre-treatment [10], enzyme-linked immunosorbent assay (ELISA) has been applied for the determination of fluoroquinolone antibiotics, such as ofloxacin [11], pefloxacin [12], lomefloxacin, norfloxacin, enrofloxacin [13] and ciprofloxacin [14], [15]. However, few reports on the determination of sparfloxacin by ELISA were reported. Therefore, the aim of this article was to obtain the antibody of sparfloxacin and establish the ELISA method for determination of sparfloxacin by a comparative simple procedure. On such purpose, anti-sparfloxacin antibody was obtained by immunizing rabbits with sparfloxacin conjugated directly with bovine serum albumin (BSA) using isobutyl chloroformate method. A highly simple and sensitive ELISA for the determination of sparfloxacin in biological samples was developed.
2. Materials and methods
2.1. Materials and reagents
Sparfloxacin, enrofloxacin, lomefloxacin, ciprofloxacin, ofloxacin, norfloxacin, spiramycin and amoxicillin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (China). Ovalbumin (OVA), bovine serum albumin (BSA), peroxidase-labeled anti-rabbit IgG, freund's complete and incomplete adjuvants were obtained from Huamei Bioengineering Co. (China). Tetramethybenzidine (TMB) substrate solution was obtained from Amresco Chemical Co. (USA). Polypropylene plate was purchased from Corning incorporated Co. (USA). All other chemicals used were of analytical grade.
Phosphate-buffered saline (PBS, 0.02 M phosphate buffer, pH 7.2, containing 0.15 M NaCl), coating buffer (0.05 M carbonate-hydrogen carbonate, pH 9.6), blocking buffer (0.02 M PBS containing 1% OVA), washing buffer (PBS-T, 0.02 M PBS containing 0.05% Tween 20) were used.
2.2. Preparation of an immunogen for sparfloxacin
Firstly, 10 μL of tribytylamine was added to a solution of sparfloxacin (35.7 mg, 0.91 mM) in 1.5 mL of 1,4-dioxane and the resulting solution was allowed to stand with vigorous stirring in the condition of no light and at room temperature for 40 min. 15 μL of isobutyl chloroformate was added slowly to the above solution and kept the solution with vigorous stirring for another 40 min at the same conditions. Subsequently, the reaction mixture was added dropwise to 7 mL BSA (123.9 mg, 1.81 μM) in water (pH 8.0), and under stirring incubated at 4 °C for 24 h. Last, the reaction mixture was dialyzed against deionized water for 3 days at 4 °C. The purified conjugate was lyophilized and used as the sparfloxacin immunogen.
The preparation of the sparfloxacin–OVA conjugate as a coating immunogen was similar to that of the sparfloxacin–BSA conjugate.
2.3. Preparation of sparfloxacin antibody
An aliquot containing 2 mg sparfloxacin–BSA complex was emulsified with an equal volume of Freund's complete adjuvant. Two Zealand white rabbits were each given multiple subcutaneous injections over sites along both sides of their backs. Booster injections were then given four times at bi-weekly intervals, using one-half the amount of the dose of the first immunization emulsified with incomplete Freund's adjuvant in the same ratio. After validation of antibody production by indirect ELISA, blood was collected from carotid artery. The sera were separated by centrifugation at 3000 rpm for 10 min and stored at −70 °C until use.
2.4. Standard solution preparation
The standard stock solution of sparfloxacin (0.5 mg/mL) was prepared in methanol and stored at 4 °C. Working solutions were prepared by appropriate dilution of the stock solution with 0.02 M phosphate buffer (pH 7.2) and eluent for ELISA and HPLC, respectively.
2.5. ELISA procedure
100 μL of the appropriate dilutions of sparfloxacin–OVA in coating buffer was added to each well of a polystyrene microtiter plate and incubated overnight at 4 °C. After incubation, the plates were washed three times with washing buffer. To reduce nonspecific binding, 150 μL of blocking buffer was added to each well and incubated at 37 °C for 2 h to block the unbound sites on the plastic surface. After washing the plate three times with washing buffer, 50 μL of the appropriate dilutions of anti-sparfloxacin antibody and 50 μL of the appropriate dilutions of sample solution were added to each well and incubated for 30 min at 37 °C, the plates were washed three times with washing buffer once more. Finally, 100 μL of TMB peroxidase substrate solution was added to each well. After incubation for 20 min in dark at room temperate, the reaction was terminated by addition of 50 μL of 2 M H2SO4. The activity of enzyme bound to the plate was measured spectrophotometrically at 450 nm using a microplate reader.
2.6. HPLC method
HPCL analysis was performed using an Agilent 1200 system, equipped with an Agilent UV–vis detector setting 290 nm and a Chromeleon software (Agilent) for calculation of peak area. The following conditions were maintained–column: Eclipse XDB-C18 (150 mm×4.6 mm, 5.0 μm); eluent: acetonitrile-0.05 M phosphate buffer (pH 2.4) (15:85, v/v); flow rate: 1 mL/min; injection volume: 20 μL. Under these conditions, the retention time of sparfloxacin was 12.5 min.
2.7. Determination of sparfloxacin in sparfloxacin pharmaceuticals
Two pills of sparfloxacin tablet were ground into powder and 5.0 mg of powder was weighed accurately and added into a calibrated flask with 10 mL of the mobile phase. After 30 min of suspending in an ultrasonic bath, the precipitate was removed by centrifugation at 4000 rpm for 10 min. Five hundred microliters of the suspernatant was pipetted accurately to 10 mL of the mobile phase and filtered through a 0.2 μm membrane and then analyzed by HPLC. The sample was diluted 500-fold with PBS for ELISA assay.
2.8. Pharmacokinetics and tissue distribution of sparfloxacin in rats
2.8.1. Animals and drug administration
Male and female Sprague-Dawley rats (250±5 g) were obtained from the Henan Laboratory Animal Center (Zhengzhou, China). They were kept in an environmentally controlled breeding room for 5 days before starting the experiments and fed with standard laboratory food and water. Animals that received the formulation by oral route were deprived of food 18 h before experimentation. The protocols for animal studies were approved by our Institutional Animal Care and Use Committee. Five rats were orally administered 5 mg/kg sparfloxacin.
2.8.2. Plasma sampling
Thirty minutes before the administration of sparfloxacin, blank blood samples were withdrawn. At predetermined time (0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 9, 12, 24, 36 h) after oral administration, blood samples were withdrawn via orbit vein into heparinized centrifuging tubes and then centrifuged at 4000 rpm for 10 min at 4 °C. The separated plasma was frozen at −20 °C before assay. The plasma was diluted 100-fold with PBS for measurement by ELISA.
2.8.3. Tissue sampling
For tissue distribution study, 30 rats were assigned randomly to 15 groups and were administered 30 mg/kg sparfloxacin by oral administration. After withdrawing the blood, the rats were sacrificed to obtain the tissues (heart, liver, spleen, lung, kidney, stomach, small intestine, and brain) at different time intervals (0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 9, 12, 24, 36 h). The tissue samples were gently blotted with absorbent paper to remove surface blood, frozen and stored at −20 °C until analysis. Samples store period was less than 1 week. On the day of the assay, tissue samples were allowed to thaw and weighted. Sterile 0.9% NaCl saline was added to facilitate homogenization, which was conducted using an apparatus for approximately 1 min in an ice bath. The homogenized samples were transferred to a tube, sonicated for 3 min and centrifuged at 4000 rpm for 10 min. A 100 μL aliquot of the supernatants was transferred to Eppendorf tubes. Tissue samples were treated as described for plasma sample to determine sparfloxacin concentration by ELISA.
3. Results and discussion
3.1. Antiserum production in rabbits
The conjugate of sparfloxacin and BSA was prepared as described above. Rabbits that were inoculated with the sparfloxacin–BSA conjugate obtained by isobutyl chloroformate method, provided antibodies suitable for detection of sparfloxacin. The antibody titer reached a plateau after the sixth booster injection.
3.2. ELISA specificity and sensitivity
Since there are many structurally related compounds present in quinolone antimicrobial drugs, it is extremely important to evaluate the specificity of anti-sparfloxacin antibody with other compounds that are structurally similar to sparfloxacin. In general, cross-reactivity is a critical factor to judge the quality of an antibody and its usefulness. As expected, when the reactivity towards sparfloxacin was referred as 100%, the anti-sparfloxacin antibody showed 51.70% cross-reaction with orbifloxacin, 1.65% cross-reaction with enrofloxacin and 0.25% cross-reaction with lomefloxacin due to their similar sructures. However, under the same conditions, the reactivities of the anti-sparfloxacin antibody with ciprofloxacin, sarafloxacin, ofloxacin, norfloxacin, flumequine, spiramycin, amoxicillin and pipemidic acid were less than 0.01% (shown in Table 1).
Table 1.
Cross-reactivities of anti-sparfloxacin antibodies.
| Compound | Cross-reaction (%)a |
|---|---|
| Sparfloxacin | 100.00 |
| Orbifloxacin | 51.70 |
| Enrofloxacin | 1.65 |
| Ciprofloxacin | <0.01 |
| Lomefloxacin | 0.25 |
| Sarafloxacin | <0.01 |
| Ofloxacin | <0.01 |
| Norfloxacin | <0.01 |
| Flumequine | <0.01 |
| Spiramycin | <0.01 |
| Amoxicillin | <0.01 |
| Pipemidic acid | <0.01 |
Determined as the amount of compound required for 50% inhibition of the binding of the antibodies to the solid-phase antigen, as compared to sparfloxacin itself (100%).
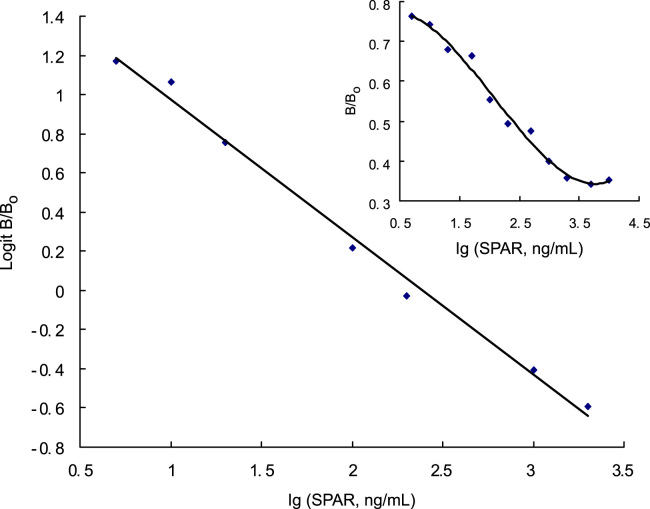
After the assay procedure was optimized, the standard curve of sparfloxacin was established. When the absorbance for each standard was plotted versus its sparfloxacin concentration on a linear-log scale, a sigmoidal curve was obtained and plotting logit (B/B0) versus Ig standard sparfloxacin concentration yielded a linear response, the linear regression equation and correlation coefficient were y=−0.7019x+1.6756 and 0.996, respectively (Fig. 1). The practical measuring range of the competitive ELISA extended from 5 ng/mL to 2 μg/mL samples.
Figure 1.
ELISA standard curve of sparfloxacin. B and B0 are the percentages of binding in the presence and absence of sparfloxacin, respectively. The logit–log plot is obtained from ln[(B/B0)/(1−B/B0)]. Coating antigen concentration: 1 μg/mL, rabbit anti-sparfloxacin antibody dilution: 1/25600, peroxidase labeled anti-rabbit IgG dilution: 1/5000.
The rat plasma, urine and tissue samples to which known concentration of sparfloxacin (20, 100, 200 μg/mL) had been spiked were 100-fold diluted with PBS and subjected to ELISA assay without further treatment. The recovery rates and coefficients of variation for rat plasma, urine and tissues at two sparfloxacin levels were 87.7–106.2% and 4.8–15.3%, respectively (shown in Table 2). Comparison of the proposed method with other methods is listed in Table 3. These data proved that the ELISA is a rapid, simple and sensitive method and is suitable for the analysis of the biological samples, even in therapeutic drug monitoring.
Table 2.
Percent recovery of sparfloxacin from plasma, urine and tissues (n=5).
| Samples | SPAR in spike (ng/mL) | Measured (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Plasma | 200 | 211.21±10.03 | 105.6 | 4.8 |
| 1000 | 1062.03±67.97 | 106.2 | 6.4 | |
| Urine | 200 | 194.05±15.66 | 97.0 | 8.1 |
| 1000 | 981.03±94.27 | 98.1 | 9.6 | |
| Liver | 200 | 200.56±18.04 | 100.3 | 9.0 |
| 2000 | 1785.10±163.76 | 89.3 | 9.2 | |
| Kidney | 200 | 175.32±12.46 | 87.7 | 7.1 |
| 2000 | 2015.31±248.96 | 102.6 | 12.1 | |
| Lung | 200 | 196.17±24.44 | 98.1 | 12.5 |
| 2000 | 1960.73±245.30 | 98.0 | 12.5 | |
| Spleen | 200 | 197.81±22.03 | 98.9 | 11.1 |
| 2000 | 2004.75±182.83 | 100.2 | 9.1 | |
| Heart | 200 | 185.78±26.12 | 92.9 | 14.1 |
| 2000 | 1839.84±200.93 | 92.0 | 10.9 | |
| Brain | 200 | 194.32±26.14 | 97.2 | 13.5 |
| 2000 | 1819.89±138.93 | 91.0 | 7.6 | |
| Stomach | 200 | 186.82±23.27 | 93.4 | 12.5 |
| 2000 | 2083.33±319.31 | 104.2 | 15.3 | |
| Small intestine | 200 | 193.92±25.77 | 96.9 | 13.3 |
| 2000 | 2040.39±242.11 | 102.0 | 11.9 |
Table 3.
Analytical methods for determination of sparfloxacin in biological samples and pharmaceutical formulations.
| Methods | Linear range | Limit of detection (ng/mL) | Recovery (%) | Analytical samples | References |
|---|---|---|---|---|---|
| Spectrophotometric | 2–12 mg/mL | – | 92.70–100.00 | Tablets | [5] |
| HPLC | 50–2000 ng/mL | 50 | 83.36–113.17 | Human serum | [7] |
| LC/MS | 10–1000 ng/mL | 2 | 96.20–110.90 | Rat plasma | [9] |
| ELISA | 5–2000 ng/mL | 5 | 87.70–106.20 | Rat plasma | This work |
3.3. Comparison of ELISA and HPLC
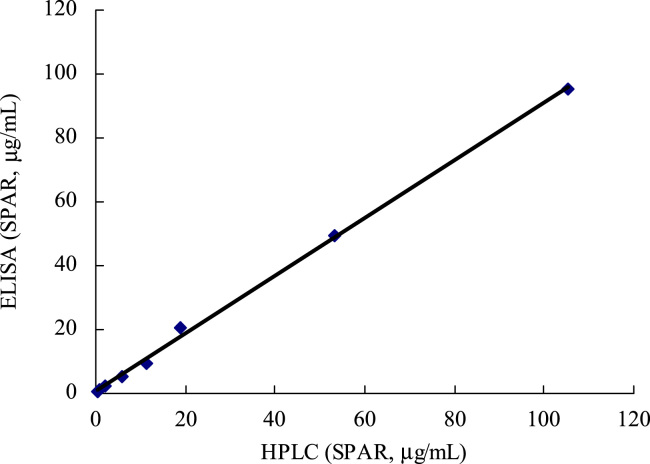
The ELISA method was compared with an HPLC method for measuring standard samples and pharmaceutical samples. The HPLC technique analyzed the 8 samples of various sparfloxacin concentrations ranging from 0.5 to 100 μg/mL, showing a linear relationship between the peak area and the added sparfloxacin dose. ELISA determination was carried out using these sparfloxacin samples, properly diluted to adjust the drug-concentration in the measurable range of the ELISA. Fig. 2 shows that there was a good correlation between the values determined by the two methods, and the plot was linear as predicted by the equation y=0.9073x+0.5471, where y is the concentration value determined by ELISA analysis and x is that determined by HPLC, the correlation coefficient was 0.998 (n=3).
Figure 2.
Correlation between the values of sparfloxacin obtained by ELISA and HPLC.
Table 4 indicates the concentrations of total sparfloxacin in sparfloxacin tablets analyzed by ELISA and HPLC. Although the concentrations of total sparfloxacin determined by ELISA were a little higher than the values determined by HPLC, most likely due to the cross-reactivity with the matrix in samples, the concentrations of sparfloxacin determined by ELISA and HPLC correlated well in general and were in accordance with China Pharmacopoeia (content 90–110%). Thus, the immunoassay is comparable, in terms of accuracy and reproducibility, to the currently available HPLC method.
Table 4.
Results of sample determination by ELISA and HPLC (n=5).
| Batch no. | HPLC |
ELISA |
||
| Content of total sparfloxacin (%) | RSD (%) | Content of total sparfloxacin (%) | RSD (%) | |
| 090901 | 90.34 | 3.4 | 94.83 | 6.5 |
| 100402 | 88.67 | 2.6 | 94.77 | 7.4 |
| 20090804 | 89.12 | 3.8 | 93.73 | 8.9 |
3.4. Pharmacokinetic study and tissue distribution of sparfloxacin in rats
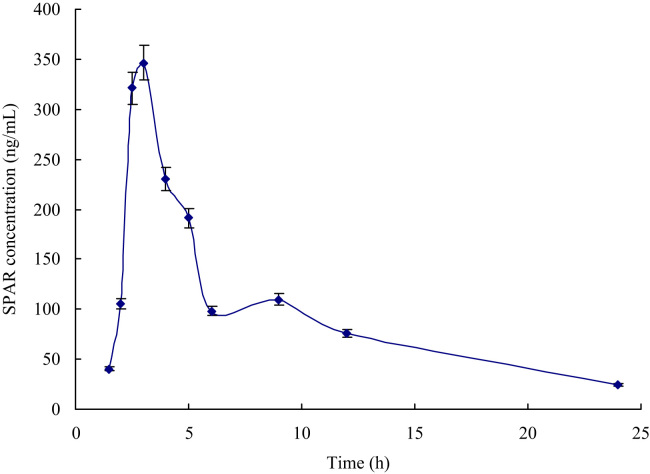
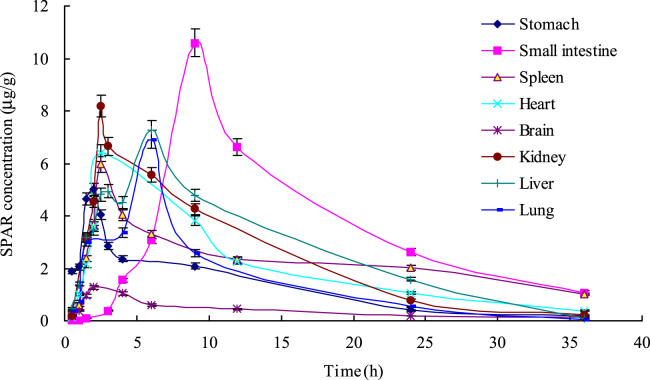
After oral administration of sparfloxacin, blood and tissue were obtained at the different intervals and the sparfloxacin content in the plasma and tissues was determined by this ELISA. The mean plasma and tissue concentration-time curve profiles are illustrated in Figure 3, Figure 4, respectively. The main pharmacokinetics parameters of sparfloxacin in rats after oral administration are summarized in Table 5.
Figure 3.
Mean plasma concentration-time profiles of sparfloxacin after a single oral administration to rats (n=5).
Figure 4.
Distribution of sparfloxacin in tissues after a single oral administration to rats (n=5).
Table 5.
Pharmacokinetic parameters of sparfloxacin in rats after an oral dose of 5 mg/kg a.
| Parameter | Mean (n=5) |
|---|---|
| t1/2α (h) | 1.81 |
| t1/2β (h) | 12.86 |
| AUC(0–t) (mg h/L) | 1.96 |
| AUC(0–∞) (mg h/L) | 2.17 |
| CL (L/h kg) | 2.31 |
| Cmax (ng/mL) | 346.06 |
| tmax (h) | 3.00 |
Two-compartment model analysis.
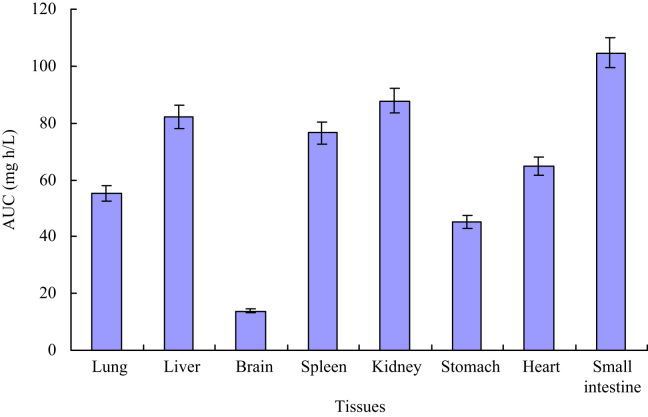
In order to further understand tissues distribution of sparfloxacin, the AUC of sparfloxacin was also calculated (shown in Fig. 5). The AUC of sparfloxacin in different tissues showed that sparfloxacin was mainly distributed in heart, kidney and liver, which implied that the distribution of sparfloxacin depends on the blood flow or perfusion rate of the organ. The high distribution in small intestine and kidney confirms the reports that sparfloxacin has a good absorption and has good curative effect on urinary system infection [1], [2]. Meanwhile, the high level in kidney demonstrated that kidney might be the primary excretion organ of prototype sparfloxacin. The lowest level founded in brain implied that sparfloxacin had difficulty crossing the blood–brain barrier.
Figure 5.
Area under curve (AUC) of sparfloxacin in various tissues.
4. Conclusion
In conclusion, the ELISA procedure for sparfloxacin reported here is sensitive, specific, reproducible, simple and adaptable for analysis of a large number of biological samples. To some extent, the ELISA not only can overcome the disadvantages of some methods, but also can test all the samples under the same experimental conditions without further sample pre-treatment. This ELISA will be a valuable tool in TDM and pharmacokinetic studies.
References
- 1.Efthimiadou E.K., Sanakis Y., Raptopoulou C.P. Crystal structure, spectroscopic, and biological study of the copper (II) complex with third-generation quinolone antibiotic sparfloxacin. Bioorg. Med. Chem. Lett. 2006;16:3864–3867. doi: 10.1016/j.bmcl.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Jerome J.S. Sparfloxacin: a review. Clin. Ther. 2000;22:372–387. doi: 10.1016/S0149-2918(00)89007-4. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura S., Kurobe N., Ohue T. Pharmacokinetics of a novel quinolone, AT-4140, in animals. Antimicrob. Agents Chemother. 34 (1) 1990:89–93. doi: 10.1128/aac.34.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody V.D., Pandya K.K., Satia M.C. High performance thin-layer chromatographic method for the determination of sparfloxacin in human plasma and its use in pharmacokinetic studies. J. Pharm. Biomed. Anal. 1998;16:1289–1294. doi: 10.1016/s0731-7085(97)00156-8. [DOI] [PubMed] [Google Scholar]
- 5.Marona H.R.N., Schapoval E.E.S. Spectrophotometric determination of sparfloxacin in pharmaceutical formulations using bromothymol blue. J. Pharm. Biomed. Anal. 2001;26:501–504. doi: 10.1016/s0731-7085(01)00429-0. [DOI] [PubMed] [Google Scholar]
- 6.Kamberi M., Kamberi P., Hajime N. Determination of sparfloxacin in plasma and urine by a simple and rapid liquid chromatographic method. Ther. Drug. Monitoring. 1999;21:411–415. doi: 10.1097/00007691-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cho H.Y., Park S.A., Lee Y.B. Improvement and validation of an HPLC method for examining the effects of the MDR1 gene polymorphism on sparfloxacin pharmacokinetics. J. Chromatogr. B. 2006;834:84–92. doi: 10.1016/j.jchromb.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Borner K., Borner E., Lode H. Determination of sparfloxacin in serum and urine by high-performance liquid chromatography. J. Chromatogr. 1992;579:285–289. doi: 10.1016/0378-4347(92)80393-5. [DOI] [PubMed] [Google Scholar]
- 9.Noh K., Kwon K.I., Jeong T.C. Quantitative determination of sparfloxacin in rat plasma by liquid chromatography/tandem mass spectrometry. Biomed. Chromatogr. 2010;24:1199–1202. doi: 10.1002/bmc.1427. [DOI] [PubMed] [Google Scholar]
- 10.Zeng H.J., Yu B.Y., Liu J.H. Determination of glycyrrhizin in Chinese prescriptions and biological samples by enzyme-linked immunosorbent assay. Anal. Chim. Acta. 2006;564:173–178. [Google Scholar]
- 11.Sun W.Y., Liu W.Y., Qu L.B. Development of ELISA and immunochromatographic assay for ofloxacin. Chin. Chem. Lett. 2007;18:1107–1110. [Google Scholar]
- 12.Cao L.M., Sui J.X., Kong D.X. Generic immunoassay of quinolones: production and characterization of anti-pefloxacin antibodies as broad selective receptors. Food Anal. Method. 2011;4:517–524. [Google Scholar]
- 13.Kato M., Ihara Y., Nakata E. Development of enrofloxacin ELISA using a monoclonal antibody tolerating an organic solvent with broad cross-reactivity to other new quinolones. Food Agr. Immunol. 2007;18:179–187. [Google Scholar]
- 14.Hu K., Huang X.Y., Jiang Y.S. Monoclonal antibody based enzyme-linked immunosorbent assay for the specific detection of ciprofloxacin and enrofloxacin residues in fishery products. Aquaculture. 2010;310:8–12. [Google Scholar]
- 15.Francisco B.M.B.G., Riedstra S., Ferreira J.P.M. Development of an immunoassay for ciprofloxacin based on phage-displayed antibody fragments. J. Immunol. Methods. 2010;358:17–22. doi: 10.1016/j.jim.2010.03.021. [DOI] [PubMed] [Google Scholar]