Abstract
The stereoselective hydrolysis of esmolol in whole blood and in its separated components from rat, rabbit and human was investigated. Blood esterase activities were variable in different species in the order of rat>rabbit>human. Rat plasma showed the high esterase activity and had no stereoselectivity to enantiomers. Rabbit red blood cell (RBC) membrane, RBC cytosol and plasma all hydrolyzed esmolol but with different esterase activity, whereas the hydrolysis in RBC membrane and cytosol showed significant stereoselectivity towards R-(+)-esmolol. Esterase in RBC cytosol from human blood mainly contributed to the esmolol hydrolysis, which was demonstrated with no stereoselctivity. Esterase in human plasma showed a low activity, but a remarkable stereoselectivity with R-(+)-esmolol. In addition, the protein concentration affected the hydrolysis behavior of esmolol in RBC suspension. Protein binding of esmolol enantiomers in human plasma, human serum albumin (HSA) and α1-acid glycoprotein (AGP) revealed that there was a significant difference in bound fractions between two enantiomers, especially for AGP. Our results indicated that the stereoselective protein binding might play a role in the different hydrolysis rates of esmolol enantiomers in human plasma.
Keywords: Esmolol enantiomers, Species-dependent, Stereoselective hydrolysis, Protein binding
1. Introduction
Esmolol, methyl 3-{4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl}propionate hydrochloride, is an ultra-short-acting β-adrenergic receptor antagonist for the rapid control of heart rate in patients with atrial fibrillation and also for the treatment of tachycardia especially with hypertension during surgery and in the postoperative period when indicated [1], [2]. Esmolol is usually used as a racemic mixture of two enatiomers. In general, like most β-adrenergic blocking agents [3], [4], [5], S-(−)-esmolol exhibits blocking effects whereas R-(+)-esmolol is inactive [6].
Similar to many ester-containing drugs [7], [8], [9], esmolol is rapidly metabolized by hydrolysis of the ester linkage to an acid metabolite, 3-{4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl} propionic acid, which has low potency as a β-adrenergic receptor antagonist. In humans, hydrolysis of esmolol is mediated mainly by an esterase in the cytosol of red blood cells and the half-life of esmolol is approximately 9 min in vivo [10]. Species differences of the esterase activity and stereoselectivity for hydrolysis of esmolol were observed [10], [11]. In whole blood, esmolol esterase activity was in the order of guinea pigs>rats>rabbits>dogs>rhesus monkeys>humans. S-(−)-esmolol is hydrolyzed faster than the R-(+)-esmolol with dog and rat blood esterase whereas R-(+)-esmolol is hydrolyzed faster with rhesus monkeys, rabbit, and guinea pig blood esterase. Human esterase did not show stereoselectivity [10]. Inhibition experiments showed that an arylesterase in human and dog blood mediated the hydrolysis of esmolol while an aliphatic esterase mediated the hydrolysis of esmolol in guinea pig and rat blood [11]. Temperature also affected the metabolism of esmolol in vitro. Melendez et al. [12] reported a temperature-dependent decrease in the degradation of esmolol. The half-life for esmolol in human blood was 19.6±3.8 min at 37 °C, 47±10.1 min at 25 °C, 152±46.6 min at 15 °C, and 226.7±60.1 min at 4 °C. This study clearly showed marked reduction of esmolol metabolism with hypothermia, which possibly leads to persistent beta-adrenergic blockade following the discontinuation of cardiopulmonary bypass (CPB). However, few data are available describing the stereoselectivity of hydrolyzing esmolol in the separated components of whole blood, such as plasma, red blood cell (RBC), RBC cytosol, RBC membrane.
The present study focused on investigating the stereoselective hydrolysis of esmolol enantiomers in whole blood and in its separated components from several species including human in vitro. Furthermore, the stereoselective protein binding of esmolol enantiomer was determined in human plasma proteins, human serum albumin (HSA) and α-acid glycoprotein (AGP), which play an important role in the protein binding of many chiral drugs [13], [14], therefore for the first time to explain the underlying reason for the different hydrolyzing behavior of two enantiomers.
2. Experimental
2.1. Reagents and apparatus
All solvents used were HPLC grade and all chemicals were analytical grade. Esmolol hydrochloride (purity>99.5%) was kindly provided by Zhan Wang Chemical Pharmaceutical Company (Huzhou, Zhejiang, China). 3-{4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl}propionic acid (purity>99.5%) was synthesized by our laboratory according to the method provided by Zhan Wang Chemical Pharmaceutical Company. 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate (GITC), the internal standard (I.S.) (−)-S-propranolol, HSA and AGP were purchased from Sigma (St. Louis, MO, USA). Triethylamine (TEA) was obtained from Shanghai Chemical Reagent Plant (Shanghai, China).
The HPLC system consisted of an LC-10ATvp pump, a manual injector with a 20 μL fixed loop and an SPD-10Avp UV–Vis detector (Shimadzu, Japan). The separation was performed on a 5 μm reversed-phase Aglient Zorbax C18 column (250 mm×4.6 mm I.D.), equipped with a C18 guard column (10 mm×5 mm I.D.) at ambient temperature. The mobile phase consisted of a mixture of acetonitrile/0.02 M phosphate buffer (pH 4.5) (55:45, v/v) and was delivered at a flow rate of 0.75 mL/min. Eluted peaks were detected at 224 nm.
2.2. Collection of whole blood and its separated components
Whole blood was taken from Sprague-Dawley rats, New Zealand rabbits (Laboratory Animal Center of Zhejiang University, Hangzhou, China), and five healthy volunteers aged 20–30 years, who took no medication for a week before blood sampling. (This study was approved by the Ethics Committee of Zhejiang University) The blood samples, collected in heparinized tubes were centrifuged at 4 °C at 1800g for 10 min to separate the plasma from the blood cells. The resultant blood cells were washed for five times with an equal volume of isotonic saline (pH 7.4) and centrifuged at 1800g for 5 min. The supernatant, including the white blood cells and the platelets seen at the top of the RBC, was discarded, and the RBC was suspended in adequate volumes of isotonic saline or isotonic saline containing 600 μM HSA and 24 μM AGP to obtain the same haematocrit values as the blood samples. The RBC was further separated into the membrane and cytosol, according to the previously reported method [15]. In brief, the RBC suspension was diluted in a 10-fold volume of 0.005 M phosphate buffer (pH 7.4) and centrifuged at 20,000g for 30 min at 4 °C to prepare the cytosol. The membrane precipitate was then washed for three times with a 10-fold volume of isotonic saline, and suspended in the buffer to make it equal volume with the original blood samples.
2.3. Hydrolysis study
Blood, plasma, RBC suspended in isotonic saline or isotonic saline containing 600 μM HSA and 24 μM AGP, cytosol and membrane suspended in phosphate buffer were pre-incubated for 10 min at 37 °C. The incubation volume was 0.7 mL in all experiments. Esmolol enantiomers were added to incubation solution to give an initial concentration of 4 μg/mL. Incubation was performed at 37 °C. At different time intervals, 0.5 mL of samples were taken from the shaking water bath and immediately mixed with 250 μL of 6% perchloric acid, which immediately stopped the hydrolysis of esmolol. Then the samples were stored at −20 °C until analysis.
2.4. Analytical methods
A RP-HPLC method was used to analyze the concentrations of the enantiomers of esmolol and its metabolites according to a method previously described [16], [17], [18]. Briefly, the samples prepared were placed on ice, and the internal standard was immediately added. After centrifugation at 4 °C at 5000g for 10 min to precipitate proteins, the clear supernatant was transferred to a clean tube and mixed with 100 μL of 1.0 M sodium hydroxide and 1.5 mL of 0.02 M sodium phosphate buffer (pH 7.0). The mixture was applied to LC-18 SPE column (1.0 mL tube, Supelco Park, Bellefonte, PA, USA). The sample solution was allowed to run through by gravity and the cartridge was washed with 1.0 mL water. Excess of water was removed by leaving the cartridge in a vacuum system for about 30 min. The analytes were eluted from the cartridge with 3 mL of acetonitrile containing 1% glacial acetic acid. The eluent was evaporated to dryness under a gentle stream of air. Then 70 μL of GITC (1.02 mg/mL in acetonitrile) and 5 μL of TEA (1.25% in acetonitrile) were then added to the dried residue. The reaction was made at ambient temperature for 20 min. After chiral derivatization was completed, the reaction mixture was evaporated to dryness under a gentle stream of air and the residue was reconstituted with 100 μL of mobile phase. Finally, an aliquot of 20 μL of the resulting solution was injected into the HPLC system.
2.5. Protein binding study
2.5.1. Nonspecific adsorption of esmolol enantiomers to ultrafilter
Esmolol enantiomers were added to 0.7 mL Sorensen phosphate buffer, which had been pre-incubated for 10 min at 37 °C. After vertically mixing, 0.5 mL phosphate buffer was taken into the Amicon centrifree micropartition systems fitted with the filter membrane with molecular weight cut-off value of 30,000, and centrifuged at 2000g for 5 min at 37 °C. Approximately 150 μL of the ultrafiltrate was collected, then the ultrafiltrate and the phosphate buffer without ultrafiltration were analyzed by HPLC without derivatization according to the method previously described in Section 2.4.
Adsorption rate of the ultrafilter was calculated according to the following equation:
where Aultrafilter is the peak area of esmolol enantiomers in the ultrafilter and APBS is the peak area of esmolol enantiomers in the phosphate buffer.
2.5.2. Protein binding study with human plasma, HSA and AGP
Esmolol enantiomers were added to 0.7 mL the human plasma or 600 μM HSA or 24 μM AGP solutions, which were prepared with Sorensen phosphate buffer. To achieve equilibrium between the drugs and proteins, the spiked protein samples containing esmolol were incubated at 37 °C for 15 min. An aliquot of the protein samples (0.5 mL) was used for the determination of the total concentration of the enantiomers, while the remainder was transferred to Amicon centrifree micropartition systems. The samples mixed with human plasma, HSA and AGP were centrifuged at 37 °C at 9000g for 15 min, 9000g for 5 min and 3000g for 5 min, respectively. Approximately 150 μL of the ultrafiltrate was collected, then the ultrafiltrate and the phosphate buffer without ultrafiltration were analyzed by HPLC method.
2.6. Data analysis
The enzyme activities for hydrolysis of esmolol enantiomer in whole blood and its components were assumed to be the initial slopes of the linear regression lines of plots of the production of acid metabolite enantiomers vs. time. The activities were corrected for dilution and expressed as the rate of the metabolite generation in a unit time per equivalent volume of whole blood.
The in vitro half- lives of esmolol enantiomers (t1/2) were determined by equation:
where k is the hydrolysis rate constant, which was calculated by linear regression analysis of log-transformed plasma concentrations of the enantiomers of esmolol vs. time.
The percentage bound was calculated using the following equation:
where Cu and Ct are the unbound concentration and the total concentration of the enantiomer of esmolol, and P% is the nonspecific adsorbtion rate of the ultrafilter.
The drug–protein interactions are analyzed assuming that the drug is bound to m class of identical independent binding sites. The fraction r of bound drug molecules per protein molecule is given by:
where Cf is the concentration of free drugs, ni is the number of sites of class i and Ki is the corresponding association constant.
3. Results and discussion
3.1. Hydrolysis of esmolol in whole blood
The stereoselective hydrolysis of racemic esmolol is summarized in Table 1. The in vitro hydrolysis of esmolol in blood was rapid and stereoselective, although the hydrolysis rate and stereoselectivity from the three species were totally different, which was probably due to a species variation in the expression of the esterase in the blood. In this study, the order of activity for hydrolyzing esmolol was rat>rabbit>humans. And the esterase activity in rat whole blood was approximately 100 and 1000 times higher than that in rabbit and human whole blood, respectively. The high esterase activity in rat was consistent with previous observations on other ester containing compounds, such as clevidipine [19], remifentanil [20] and isocarbacyclin methyl ester (TEI-9090) [21]. The hydrolysis of esmolol was stereoselective with the esterases from rabbit and human blood, R-(+)-enantiomer faster than S-(−)-enantiomer, whereas no stereoselectivity with the esterases from rat blood was observed. The mean R-(+)/S-(−)-esmolol ratio in rabbit and human whole blood was 1.67 and 1.18, respectively.
Table 1.
The stereoselective hydrolysis of esmolol enantiomers in while blood and in its separated components from rat, rabbit and human (mean±SD, n=5).
| Matrix | Hydrolysis activity (pmol/min/mL blood) |
|
|---|---|---|
| S-(−)-esmolol | R-(+)-esmolol | |
| Rat | ||
| Whole blood | 119,606±38,116 | 117,889±37,512 |
| Plasma | 153,312±41,235 | 148,285±40,589 |
| RBC | 307.96±12.31 | 298.58±13.89 |
| RBC cytosol | 98.21±9.25 | 93.10±8.36 |
| RBC membrane | 105.62±10.61 | 106.32±13.65 |
| Rabbit | ||
| Whole blood | 783.12±80.32 | 1310.11±124.53 |
| Plasma | 284.74±31.20 | 278.72±28.01 |
| RBC | 408.28±48.49 | 863.26±90.47 |
| RBC cytosol | 118.11±8.27 | 180.78±11.75 |
| RBC membrane | 235.62±16.79 | 397.13±30.33 |
| Human | ||
| Whole blood | 109.97±9.78 | 129.62±10.25 |
| Plasma | 16.87±1.35 | 31.23±2.42 |
| RBC | 106.97±10.16 | 109.69±11.21 |
| RBC cytosol | 85.82±9.45 | 82.50±8.78 |
| RBC membrane | 7.75±0.35 | 8.15±0.41 |
| RBC with HSA and AGP | 92.50±9.45 | 101.42±8.78 |
3.2. Hydrolysis of esmolol in the separated components of whole blood
The hydrolysis activities in separated components of whole blood from rats, rabbits and humans were measured following addition of 4 μg/mL of racemic esmolol (Table 1). In rats, plasma esterases mainly contributed to esmolol hydrolysis and showed no selectivity towards two enantiomers. Quon et al. [11] reported a S-(−)-preferential hydrolysis in rat plasma, but in our experiments, the enantioselectivity disappeared. This difference might result from the individual variation.
In contrast to the rat blood, the hydrolysis activity in the rabbit blood was involved in all the RBC cytosol, RBC membrane and plasma. RBC membrane activity was similar with plasma activity and about 2 times higher than RBC cytosol activity. However, the stereoselective hydrolysis of esmolol was found only in RBC cytosol and membrane, which showed significant stereoselectivity toward S-(−)-esmolol. The mean R-(+)/S-(−)-esmolol ratio in RBC cytosol and membrane was 1.53 and 1.68, respectively. These data suggested that the hydrolysis of esmolol in RBC cytosol and membrane might be catalyzed by different esterases.
The hydrolysis of esmolol in human blood was largely mediated by esterase in RBC cytosol and did not show any stereoselectivity, which was also previously reported by Quon et al. [11]. Human plasma esterase exhibited a low activity but a remarkable stereoselectivity with the R-(+)/S-(−)-esmolol ratio of 1.85.
Although the in vitro half-life of esmolol in RBC was lower than that in human whole blood, the addition of plasma proteins to human RBC suspension slightly elongated the half-life of esmolol and promoted the stereoselectivity of hydrolysis (Table 2). The mean half-life of S-(−)-esmolol in human RBC suspension containing HSA and AGP was 29.91 min, and the corresponding half-life of R-(+)-esmolol was 26.07 min. Similar findings with aspirin [22], clevidipine and TEI-9090 indicated that protein molecules protect the drugs from RBC esterases in human whole blood. This machinery could also be applied to esmolol.
Table 2.
Half-lives of esmolol enantionmers in RBC suspension with or without HSA and AGP (mean±SD, n=3).
| Matrix | Half-lives (min) |
|
|---|---|---|
| S-(−)-esmolol | R-(+)-esmolol | |
| RBC | 24.27±1.17 | 23.88±1.33 |
| RBC with HSA and AGP | 29.91±1.39 | 26.07±1.06 |
3.3. Protein binding of esmolol
3.3.1. Nonspecific adsorption of esmolol enantiomers to ultrafilter
The nonspecific adsorption of esmolol enantiomers at different concentrations to ultrafilter is shown in Table 3. All nonspecific adsorption rates are less than 3%, indicating that the ultrafilter can be used for studying protein binding.
Table 3.
The nonspecific adsorption of esmolol with ultrafilter (n=3).
| Spiked amount (μg/mL) | APBS | Aultrafiltrate | P (%) | (%) |
| 0.5 | 12,063 | 11,582 | 3.81 | 2.80 |
| 3 | 71,873 | 70,551 | 1.84 | |
| 15 | 358,945 | 349,038 | 2.76 | |
3.3.2. Esmolol enantiomers binding with human plasma proteins
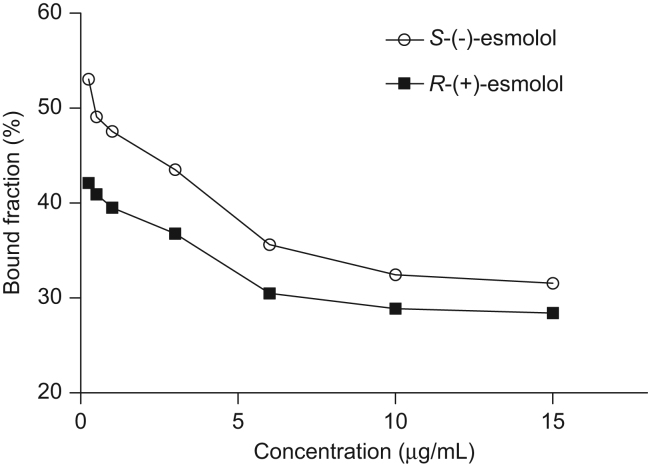
The binding of esmolol enantiomers to plasma proteins using ultrafiltration method is given in Fig. 1. The percentages of protein binding of esmolol in the concentration range between 0.25 and 15 μg/mL were 31.06–53.04% for S-(−)-esmolol and 28.41–42.12% for R-(+)-esmolol, and presented a concentration-dependent manner. Furthermore, the protein binding rates of S-(−)-esmolol and R-(+)-esmolol showed significant difference (P<0.01), and the binding of human plasma proteins with esmolol enantiomers showed stereoselectivity toward S-(−)-esmolol.
Figure 1.
The binding of esmolol enantiomers to plasma proteins.
3.3.3. Esmolol enantiomers binding with HSA
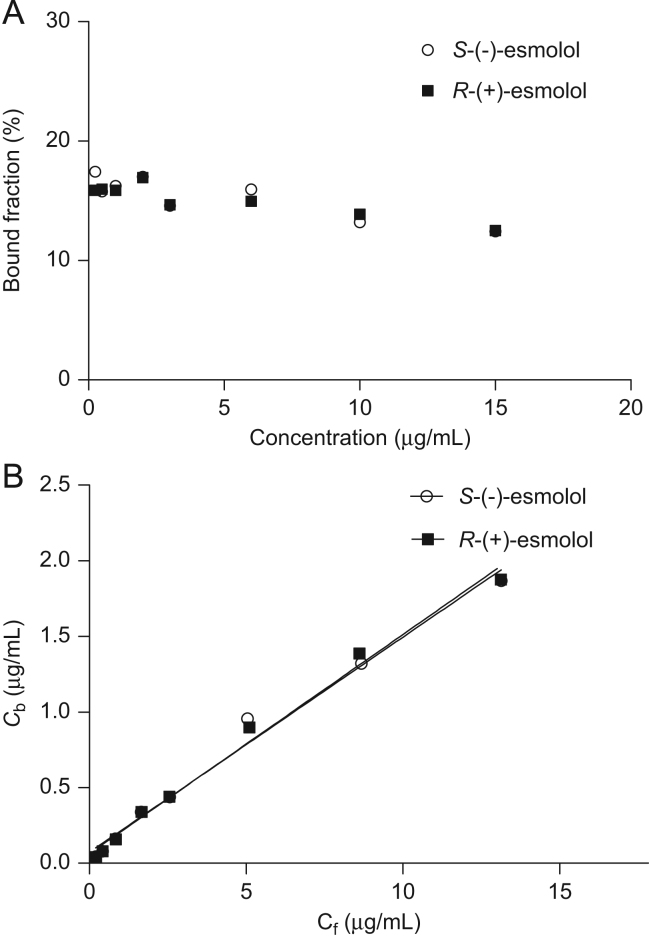
The binding of esmolol enantiomers to HSA is given in Fig. 2(A). The percentages of protein binding of esmolol in the concentration range between 0.25 and 15 μg/mL were 12.45–17.45% for S-(−)-esmolol and 12.51–15.89% for R-(+)-esmolol, and did not show any significant difference. As shown in Fig. 2(B), the binding of esmolol enantiomers to HSA was non-specific, and the concentration of free esmolol and bound esmolol was positively correlated (r>0.99). Therefore the binding activity (nK) of S-(−)-esmolol and R-(+)-esmolol was 241 and 237 L/mol, respectively, according to Henry’s isotherm equation: Cb=nKPtCf, where Pt is the concentration of protein and Cb is the concentration of bound drugs.
Figure 2.
The binding of esmolol enantiomers to HSA. (A) The bound fraction of esmolol enantiomers vs. concentration in HSA; (B) the bound concentration vs. unbound concentration of esmolol enantiomers in HSA.
3.3.4. Esmolol enantiomers binding with AGP
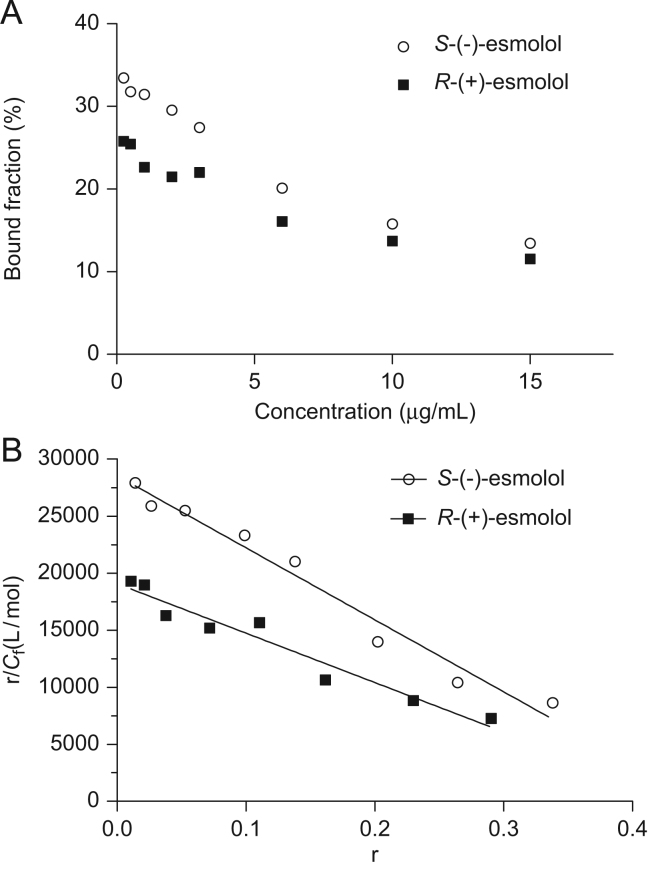
The binding of esmolol enantiomers to AGP is given in Fig. 3(A). The percentages of protein binding of esmolol in the concentration range between 0.25 and 15 μg/mL were 13.45–33.45% for S-(−)-esmolol and 11.55–25.78% for R-(+)-esmolol, higher than that to HSA. As shown in Fig. 3(B), esmolol enantiomers bound to a single site of AGP, and m=1. The binding parameters of esmolol enantiomers to AGP are presented in Table 4 according to the following equation: r=nKCf/(1+KCf). The ratio of binding activity was 1.5, indicating that the binding of esmolol with AGP had stereoselectivity toward S-(−)-esmolol.
Figure 3.
The binding of esmolol enantiomers to AGP. (A) The bound fraction of esmolol enantiomers vs. concentration in AGP; (B) scatchard plots for the binding of esmolol enantiomers in AGP.
Table 4.
Binding parameters of esmolol enantiomers in AGP.
| Binding parameters | S-(−)-esmolol | R-(+)-esmolol |
|---|---|---|
| N | 0.45 | 0.44 |
| K (L/mol) | 6.31×104 | 4.33×104 |
Therefore, the results showed that the binding of esmolol enantiomers to human plasma was stereoselective toward S-(−)-esmolol, which was consistent with the previously reported results by dialysis [17]. The underlying reason for this selectivity may be the significantly stereoselective binding of esmolol enantiomers to AGP, not to HSA. The stereoselective binding might interpret the difference of the in vitro hydrolysis of esmolol enantiomer in human blood. Furthermore, the stereoselectivity of hydrolyzing esmolol in human plasma is more significant than that in whole blood and RBC suspension containing HSA and AGP. This discrepancy might be caused by the relative low protein concentration in whole blood or the different esterase in human plasma.
4. Conclusion
In the present study, the stereoselective hydrolysis of esmolol in whole blood and in its separated components including RBC membrane, RBC cytosol and plasma of rat, rabbit and human was investigated. Blood esterase activities were in the order of rat>rabbit>human, and species-dependent in separated components of blood. The esmolol hydrolysis in human blood are mainly catalyzed by the esterase in RBC cytosol and without stereoselctivity. And the addition of protein affected the hydrolysis rate of esmolol in RBC suspension. Esterase in human plasma showed a low activity, but a remarkable stereoselectivity toward R-(+)-esmolol. Therefore, the protein binding study was performed to determine which protein contributed to this phenomenon. The binding of esmolol enantiomers to HSA and AGP showed that the bound fractions of two enantiomers was significantly different, especially for AGP, which might be one of the reasons for the different hydrolysis rates of esmolol enantiomers in human plasma in vitro.
Acknowledgement
Project was supported by National Major Projects of Ministry Science and Technology of China (2011CB710800, 2012ZX09506001-004) and Zhejiang Education Department (Y200909571).
References
- 1.Van Besouw J. The use of short-acting beta-blockers in cardiac surgery. Esp. Anestesiol. Reanim. 2001;48(10):482–648. [PubMed] [Google Scholar]
- 2.Malovaná S., Gajdosová D., Benedík J. Determination of esmolol in serum by capillary zone electrophoresis and its monitoring in course of heart surgery. J. Chromatogr. B: Biomed. Sci. Appl. 2001;760(1):37–43. doi: 10.1016/s0378-4347(01)00235-3. [DOI] [PubMed] [Google Scholar]
- 3.Ariëns E.J., Wuis E.W. Bias in pharmacokinetics and clinical pharmacology. Clin. Pharmacol. Ther. 1987;42(4):361–363. doi: 10.1038/clpt.1987.163. [DOI] [PubMed] [Google Scholar]
- 4.Nathanson J.A. Stereospecificity of beta adrenergic antagonists: R-enantiomers show increased selectivity for beta-2 receptors in ciliary process. Pharmacol. Exp. Ther. 1988;245(1):94–101. [PubMed] [Google Scholar]
- 5.Mehvar R., Brocks D.R. Impact of stereoselectivity on the pharmacokinetics and pharmacodynamics of antiarrhythmic drugs. J Pharm. Pharmaceut. Sci. 2001;4(2):185–200. [PubMed] [Google Scholar]
- 6.Wiest D. Esmolol: a review of its therapeutic efficacy and pharmacokinetic characteristics. Clin. Pharmacokinet. 1995;28(3):190–202. doi: 10.2165/00003088-199528030-00002. [DOI] [PubMed] [Google Scholar]
- 7.Bojarski J., Oxelbark J., Andersson C. Enantioselective lipase-catalyzed ester hydrolysis: effects on rates and enantioselectivity from a variation of the ester structure. Chirality. 1993;5(3):154–158. doi: 10.1002/chir.530050309. [DOI] [PubMed] [Google Scholar]
- 8.Fedtke N., Wiegand H.J. Hydrolysis of vinyl acetate in human blood. Arch. Toxicol. 1990;64(5):428–429. doi: 10.1007/BF01973471. [DOI] [PubMed] [Google Scholar]
- 9.Kageyama Y., Yamazaki Y., Afify A.S. Stereoselective hydrolysis of xenobiotic esters by different cell lines from rat liver and hepatoma and its application to chiral prodrugs for designated growth suppression of cancer cells. Chirality. 1995;7(4):297–304. doi: 10.1002/chir.530070418. [DOI] [PubMed] [Google Scholar]
- 10.Quon C.Y., Mai K., Patil G. Species differences in the stereoselective hydrolysis of esmolol by blood esterases. Drug Metab. Dispos. 1988;16(3):425–428. [PubMed] [Google Scholar]
- 11.Quon C.Y., Stampfli H.F. Biochemical properties of blood esmolol esterase. Drug Metab. Dispos. 1985;13(4):420–424. [PubMed] [Google Scholar]
- 12.Melendez J.A., Stone J.G., Delphin E. Influence of temperature on in vitro metabolism of esmolol. J. Cardiothorac. Anesth. 1990;4(6):704–706. doi: 10.1016/s0888-6296(09)90007-2. [DOI] [PubMed] [Google Scholar]
- 13.Guo C.C., Tang Y.H., Hu H.H. Analysis of chiral non-steroidal anti-inflammatory drugs flurbiprofen, ketoprofen and etodolac binding with HSA. J. Pharm. Anal. 2011;1(3):184–190. doi: 10.1016/j.jpha.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertucci C., Domenici E. Reversible and covalent binding of drugs to human serum albumin: methodological approaches and physiological relevance. Curr. Med. Chem. 2002;9(15):1463–1481. doi: 10.2174/0929867023369673. [DOI] [PubMed] [Google Scholar]
- 15.Dodge J.T., Mitchell C., Hanahan D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y.H., He Y., Yao T.W. Simultaneous determination of the enantiomers of esmolol and its acid metabolite in human plasma by reversed phase liquid chromatography with solid-phase extraction. J. Chromatogr. B. 2004;805(2):249–254. doi: 10.1016/j.jchromb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y.H., He Y., Yao T.W. Stereoselective RP-HPLC determination of esmolol enantiomers in human plasma after pre-column derivatization. J. Biochem. Biophy. Meth. 2004;59(2):159–166. doi: 10.1016/j.jbbm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y.H., Wang X.J., Jin Y.X. RP-HPLC analysis of the enantiomers of esmolol and its metabolite in human blood with solid-phase extraction. Chin. J. Pharm. Anal. 2005;25(10):1165–1168. [Google Scholar]
- 19.Ericsson H., Tholander B., Regårdh C.G. In vitro hydrolysis rate and protein binding of clevidipine, a new ultrashort-acting calcium antagonist metabolised by esterases, in different animal species and man. Eur. J. Pharm. Sci. 1999;8(1):29–37. doi: 10.1016/s0928-0987(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 20.Haidar S.H., Moreton J.E., Liang Z. The pharmacokinetics and electroencephalogram response of remifentanil alone and in combination with esmolol in the rat. Pharm. Res. 1997;14(12):1817–1823. doi: 10.1023/a:1012156502624. [DOI] [PubMed] [Google Scholar]
- 21.Minagawa T., Kohno Y., Suwa T. Species differences in hydrolysis of isocarbacyclin methyl ester (TEI-9090) by blood esterases. Biochem. Pharmacol. 1995;49(10):1361–1365. doi: 10.1016/0006-2952(95)00071-7. [DOI] [PubMed] [Google Scholar]
- 22.Costello P.B., Green F.A. Aspirin survival in human blood modulated by the concentration of erythrocytes. Arthritis. Rheum. 1982;25(5):550–555. doi: 10.1002/art.1780250509. [DOI] [PubMed] [Google Scholar]