Abstract
Redox behavior of midazolam was studied at a glassy carbon electrode in various buffer systems, supporting electrolytes and pH using differential pulse, square-wave and cyclic voltammetry. Based on its reduction behavior, a direct differential pulse voltammetric method has been developed and validated for the determination of midazolam in parenteral dosage. Three well-defined peaks were observed in 0.1% SLS, Britton–Robinson (BR) buffer of pH 2.5. The effect of surfactants like sodium lauryl sulfate (SLS), cetyl trimethyl ammonium bromide (CTAB) and Tween 20 was studied. Among these surfactants SLS showed significant enhancement in reduction peak. The cathodic peak currents were directly proportional to the concentration of midazolam with correlation coefficient of 0.99.
Keywords: Midazolam, Voltammetry, Surfactant, Glassy carbon electrode, Parenteral dosage form
1. Introduction
Midazolam is used as short-acting sleep inducing agent for sedation for short procedures, induction of an aesthesia, and for prolonged sedation in intensive care units. Chemically midazolam is represented as 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo [1,5–a] benzodiazepine (Fig. 1). Midazolam has strong hypnotic, anxiolytic, anticonvulsant, sedative, amnestic and skeletal muscle relaxant properties. Benzodiazepines appear to intensify the physiological inhibitory mechanisms mediated by gamma-aminobutyric acid (GABA), the most common inhibitory neurotransmitter in the brain [1].
Figure 1.

Structure of midazolam.
Few analytical methods have been reported for the assay of midazolam in pharmaceutical formulations and biological matrices. High performance liquid chromatographic (HPLC) and liquid chromatography-mass spectrometry (LCMS) methods for measuring midazolam in human serum based on liquid–liquid extraction by dichloromethane, isopropyl alcohol and ethyl acetate have been reported [2], [3], [4], [5], [6]. Furthermore, polarographic and voltammetric methods have also been developed and reported for the determination of midazolam in pharmaceutical formulation in organic solvents [7], [8], [9].
Surfactants play important roles in various fields of pharmaceutical industries like formulation development, pharmaceutical analysis, etc. They have already been proven effective in the electroanalysis of many drugs. Surface active agents are often used as selective masking agent to improve sensitivity and selectivity of electro-analytical methods [10], [11].
Literature survey reveals that no electro-analytical method for assay of midazolam has been reported in surfactants. In this paper voltammetric behavior of midazolam has been studied by employing differential pulse voltammetry (DPV), square-wave voltammetry (SWV) and cyclic voltammetry (CV). The effect of various surfactants (anionic, neutral and cationic), pH and concentration of analyte on the voltammetric response has been evaluated. On the basis of response in surfactants, a micelle-enhanced voltammetric method for determination of midazolam has been developed.
2. Experimental
2.1. Materials and methods
Midazolam (98% purity) was obtained from local bulk drug supplier and was used as received. Parenteral dosage form containing midazolam [Mezolam®] labeled 1 mg mL−1 was obtained from commercial source. KCl (0.1 M) solution was prepared in Milli-Q grade water and used as supporting electrolyte. Standard stock solution of midazolam (500 μg/mL) was prepared in 0.1% SLS solution. Sample preparations of midazolam (500 μg/mL) were performed in organic solvents (acetonitrile, dimethylformamide (DMF), 1–4 dioxane) and surfactants (0.1% CTAB, 0.1% SLS and 0.1% Tween 20) by mixing 1 mL of injection with 1 mL of relevant solvents or surfactants. All solutions for recording of voltammograms were prepared by mixing appropriate volume of stock solution, B.R. buffer and 0.1 M KCl. All chemicals used were of analytical reagent grade quality and were employed without further purification.
2.2. Instrumentation
All electrochemical measurements were performed using a MICRO AUTOLAB TYPE III (Eco-Chemie B.V., Utrecht, The Netherlands) potentiostat–galvanostat with 757VA computrace software. The utilized electrodes were glassy carbon as working electrode, Ag/AgCl (3 M KCl) as reference electrode and a graphite rod as auxiliary electrode. All pH-metric measurements were made on a Decible DB-1011 digital pH meter fitted with a glass electrode and a saturated calomel electrode as reference.
Chromatographic experiments were performed on the HPLC apparatus comprising a UV variable wavelength detector (SPD-20AU, Prominence, Shimadzu Corporation, Japan), isocratic pump (LC-20AD, Prominence) and injection valve with a 20 μL sample loop (Model 7125, Rheodyne, Cotati, CA, USA). HPLC separations were performed as per method mentioned in British Pharmacopoeia, using YMC ODS column (250 mm×4.6 mm, 5 μm) at a flow-rate of 1.0 mL/min, detection at 220 nm at room temperature with mobile phase (Methanol: Solution A: 72:28, v/v). Solution A was prepared by mixing equal volume of 0.1 M ortho-phosphoric acid and 0.03 M triethyl, adjusted pH 3.5 by 0.1 M NaOH. Sample injections were performed with a Model 701 syringe (50 μL, Hamilton, Bonaduz, Switzerland). Data acquisition and processing were performed by Spinchrom CFR software.
3. Results and discussion
3.1. Electrochemical behavior of midazolam at glassy carbon electrode
In order to understand the electrochemical process occurring on glassy carbon electrode differential pulse voltammetry, square-wave voltammetry and cyclic voltammetry were carried out in organic solvents as well as surfactants.
3.1.1. Cyclic voltammetric behavior
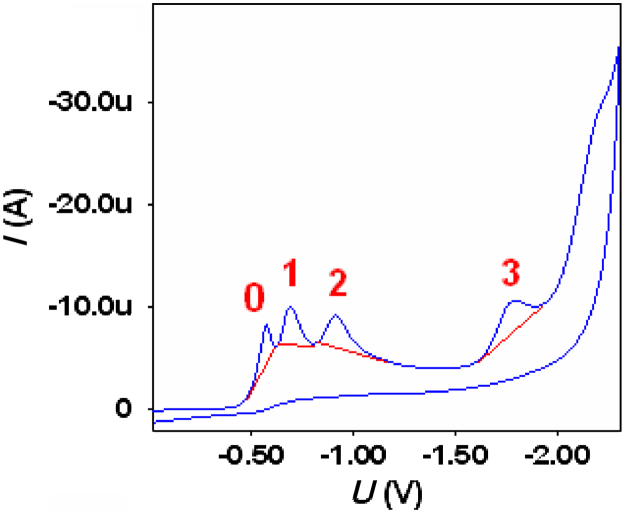
The cyclic voltammogram of midazolam (900 μg/mL) in BR buffer containing 0.1% SLS at glassy carbon electrode exhibits four well defined peaks (Fig. 2). No signal enhancement was observed in peak 0 after spiking of analyte unlike other peaks (peak 1, 2 and 3) hence this peak was labeled as peak 0. Peak labeled as 0 may be a pre-adsorption peak not affected by the concentration of the analyte. Peak 1 may be assigned to the 2-electron reduction of –C=N– bond of the seven membered ring, peak 2 to the 2-electron reduction of –C=N– bond of the five membered ring and peak 3 to the 2-electron reduction of –C=C– of the five membered ring of midazolam. No peak could be observed in the reverse scan indicating the irreversible nature of the electrode process.
Figure 2.
Cyclic voltammogram of midazolam (900 μg/mL) shows peak 0, 1, 2 and 3 in presence of 0.1% SLS indicating the irreversibility of electrode process.
3.1.2. Comparison of peak shape and response obtained by SWV, DPV and CV
Shape and current response of voltammetric peak of midazolam in BR and phosphate buffers at glassy carbon electrode was investigated using SWV, DPV and CV. All the techniques gave comparable results. SWV analysis gave two well-defined peaks. DPV provided voltammograms with high resolution and exhibited four peaks in comparison with two obtained in SWV. Better resolution of all peaks from each other makes DPV best choice for further analysis. The important instrumental parameters such as pulse amplitude, pulse time, sweep rate, etc. were examined using the selected waveform.
3.2. Optimization of operational parameters for DPV
3.2.1. Effect of pH
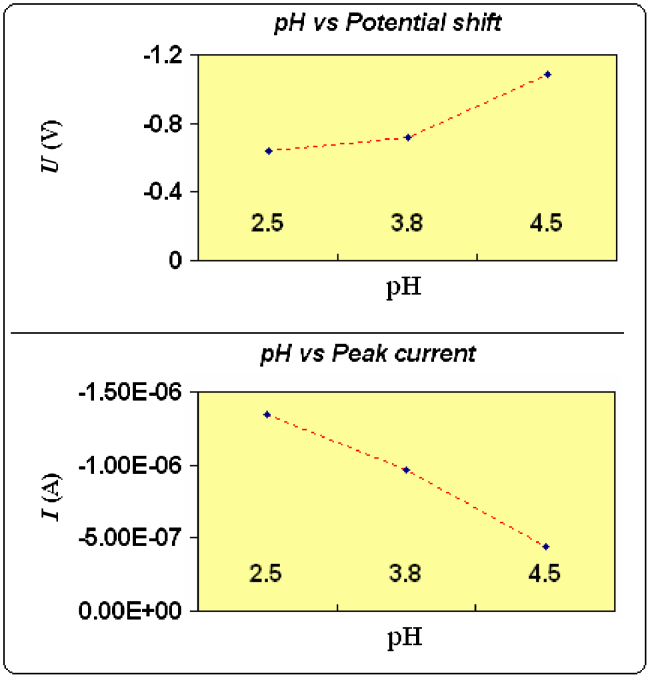
In all voltammograms well defined peaks are obtained, which are strongly dependent on buffers and pH of the medium. Britton–Robinson, acetate and phosphate buffers were used in the study and the best results in terms of peak shape were obtained in BR buffers of pH 2.5, 3.8 and 4.5 but most intense peaks were observed at pH 2.5. From the plot of U (V) vs. pH (Fig. 3), it is clear that with increase in pH from 2.5 to 3.8 there is slight shift in peak potential towards negative potential but after pH 3.8 potential shifts towards negative side are more marked indicating participation of protons in the electrode process. Further, a plot of I (A) vs. pH revealed that as pH was raised from 2.5 to 4.5, the peak current decreased rapidly indicating difficult reduction with increase in pH. It may be due to the less availability of protons at higher pH. Therefore, pH 2.5 was chosen as optimum one for the study of midazolam reduction.
Figure 3.
Influence of pH on the cathodic peak current I (A) and potential U (V) shift of midazolam (150 μg/mL) in 0.1% SLS, BR buffer (pH-2.5, 3.8 and 4.5).
3.2.2. Effect of surfactants on voltammetric response
Voltammetric current response of midazolam (500 μg/mL) has been studied in various organic solvents and surfactants. It is observed that addition of cationic surfactant (SLS) into the midazolam containing electrolyte enhanced the reduction current signal while other surfactants like CTAB (anionic) and tween-20(neutral) showed an opposite effect. Cationic surfactants might combine with the substrate and strengthen their adsorption on the electrode surface, which facilitated the electron or the substance transfer between the electrode and the solution. The effect of SLS concentration was also examined and it was observed that midazolam with 0.1% SLS gave well-defined reduction peaks. Limit of detection and limit of quantitation were also found to be lower in 0.1% SLS in comparison with organic solvent used under same experimental conditions.
Increase in the peak current in SLS may be due to aggregation of SLS on the electrode surface in the form of bilayers, cylinders or surface micelles. Midazolam forms self-micelle aggregate with the SLS that affects the mass transport, which results in changing the overpotential of the electrochemical process and rate of its corresponding charge transfer. It is also observed that surfactants are highly effective in stabilizing the voltammetric response of analyte by protecting the electrode surface from fouling. Peak current responses of midazolam in various media (organic solvent as well as surfactants) are given in Fig. 4.
Figure 4.
(A) Peak current response I (A) comparison of midazolam (500 μg/mL) in different medium (Acetonitrile (ACN), 0.1% CTAB, 0.1% SLS, and 0.1% Tween 20). (B) Inset picture represents midazolam response in acetonitrile where x-axis shows potential U (V) and y-axis represents cathodic peak current I (A). (C) Inset picture shows significant enhancement in intensity of peak in presence of 0.1% SLS in comparison with ACN.
Various others operational parameters have also been optimized and compiled in Table 1.
Table 1.
Operational parameters of proposed voltammetric method.
| Parameter | Value |
|---|---|
| Medium | 0.1% SLS |
| Buffer | BR buffer |
| pH | 2.5 |
| Purge time (s) | 30 |
| Stirring rate (rpm) | 2000 |
| Equilibration time (s) | 10 |
| Pulse amplitude (V) | 0.050 |
| Pulse time (s) | 0.04 |
| Voltage step (V) | 0.00059 |
| Voltage step time (s) | 0.4 |
| Sweep rate (V/s) | 0.0149 |
3.3. System suitability
For verification of constant and consistent response system, suitability tests (SST) were performed. The role of surfactant in stabilizing the voltammetric response of analyte is already known [12]. Stable response is key ingredient for success of any analytical method. The significance of SST in this study is to ensure that the whole analytical system (including instrument, reagents and electrodes) is suitable for the intended application. Five replicate voltammetric readings of midazolam standard solution (500 μg/mL) were recorded and used for system suitability evaluation. RSD has been calculated from five replicate voltammetric readings. Again 0.1% SLS emerged as a suitable solvent due to lesser value of % RSD in comparison with other surfactants and organic solvents.
3.4. Midazolam assay calculation in parenteral dosage form
Midazolam assay calculation in parenteral dosage form is performed by employing the following expression:
where HT is the height of midazolam peak in the test voltammogram or chromatogram, HS is the height of midazolam peak in the standard voltammogram or chromatogram, DS is the dilution factor for standard solution, DT is the dilution factor for test solution, P is the potency of midazolam standard, on as is basis and C is the label claim of midazolam in mg per mL.
3.5. Validation of DPV method
Method validation study was conducted for assay of midazolam in midazolam injection as per ICH guidelines [13].
3.5.1. Specificity
The specificity of the assay method for determination of midazolam in midazolam injection was evaluated from benzyl alcohol as a major compound. No interfering peaks are found in presence of benzyl alcohol. Additionally different concentrations of benzyl alcohol were added to solution containing midazolam (500 μg/mL) and analyzed by proposed method. The obtained percentage recoveries based on the three replicate measurements showed no significant interference from benzyl alcohol. Thus the proposed procedure has specificity to quantitate midazolam in midazolam injection.
3.5.2. Linearity
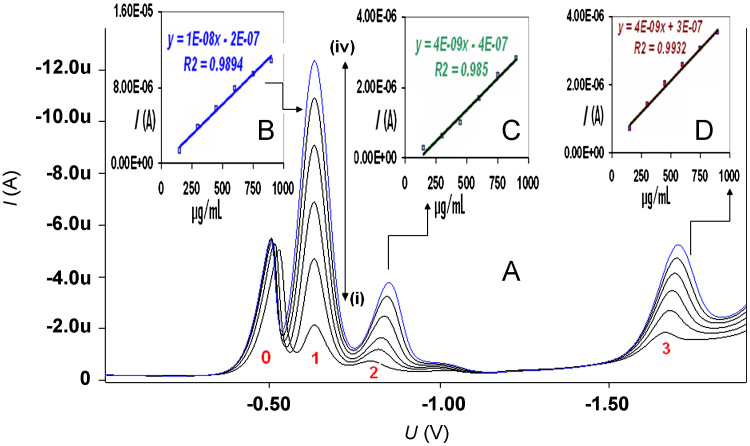
Linearity was performed by standard addition method. Linearity of response for midazolam was determined in the range of 150–900 μg/mL. Data in Fig. 5 and Table 2 indicate that the responses are linear over the specified range of all mentioned peaks. Various statistical parameters for linear regression equation like slope, standard deviation, intercept, standard deviation, correlation coefficient, standard error of estimation, sum of square of regression and sum of square of residual have been calculated for peak 1, peak 2 and peak 3.
Figure 5.
(A) Differential pulse voltammograms of midazolam at various concentrations (i) 150 μg/mL, (ii) 300 μg/mL, (iii) 450 μg/mL, (iv) 600 μg/mL, (v) 750 μg/mL and (vi) 900 μg/mL. (B) Inset picture represents linearity curve of peak 1 at different concentrations. (C) Inset picture represents linearity curve of peak 2 at different concentrations. (D) Inset picture represents linearity curve of peak 3 at different concentrations.
Table 2.
Linearity parameters comparison of Peak 1, Peak 2 and Peak 3.
| Linearity parameters | Peak 1 | Peak 2 | Peak 3 |
|---|---|---|---|
| Slope | 1.27×10−08 | 3.54×E−09 | 3.74×E−09 |
| Standard deviation | 6.59×E−10 | 2.19×E−10 | 1.54×E−10 |
| Intercept | −1.79×E−07 | −4.17×E−07 | 2.52×E−07 |
| Standard deviation | 3.85×E−07 | 1.28×E−07 | 9.01×E−08 |
| Correlation coefficient | 0.989 | 0.985 | 0.993 |
| Standard error of estimation | 4.14×E−07 | 1.37×E−07 | 9.68×E−08 |
| Sum of squares of regression | 6.36×E−11 | 4.94×E−12 | 5.48×E−12 |
| Sum of squares of residuals | 6.84×E−13 | 7.54×E−14 | 3.75×E−14 |
On the basis of maximum response, peak 1 has been selected for analytical method validation exercise.
3.5.3. Precision
3.5.3.1. System precision
Six replicate peak current responses of peak 1 of standard solution were recorded. Relative standard deviation was calculated and result shown in Table 3 indicates an acceptable level of system precision for the DPV method.
Table 3.
Analytical method validation parameters.
| Parameters | Results |
|---|---|
| Precision (% RSD) | |
| System precision | 2.83 |
| Method precision | 3.01 |
| Accuracy (% Recovery) | |
| Recovery level-1 | 98.1 |
| Recovery level-2 | 103.4 |
| Recovery level-3 | 102.9 |
| Stability (h) | |
| Standard solution | At least 5.5 |
| Sample solution | At least 5.5 |
| Robustness | 3.97 (Overall %RSD) |
3.5.3.2. Method precision
Six samples from midazolam injection were prepared by adding equal amount of 0.1% SLS and analyzed. Data are mentioned in Table 3. The % RSD value has been calculated from results of six different preparations. It indicates that this method has an acceptable level of method precision.
3.5.4. Accuracy
Benzyl alcohol was taken and spiked with known amount of midazolam standard at three different concentration levels each in triplicates. All samples were prepared and analyzed as per methodology; results shown in Table 3 indicate that accuracy of the proposed voltammetric method is acceptable.
3.5.5. Stability
For stability of analytical solution standard and test solutions were prepared and kept at 1–10 °C. Solutions were analyzed initially and at different time intervals. Cumulative % RSD of 5 time point up to 5.5 h shows that the solutions were stable at 1–10 °C. Results are compiled in Table 3.
3.5.6. Robustness
The robustness was also investigated by evaluating the influence of slight change in some of the most important method parameters including pH of BR buffer, concentration of KCL, purging time, equilibration time and strength of SLS solution. Solutions were prepared and analyzed under each modified condition, and assay of midazolam in midazolam injection was determined. % RSD of different determination is tabulated in Table 3. Robustness of method is indicated by the cumulative RSD value.
3.6. Application and comparison of DPV method with HPLC method
3.6.1. Assay of midazolam by DPV method
The developed and validated DPV method for the midazolam determination is applied to parenteral dosage form. Results obtained by the DPV method are shown in Table 4 and the results show that the proposed method could be applied to determination of midazolam.
Table 4.
Assay of midazolam by the voltammetric method and comparison with reference HPLC method.
| Proposed method |
Reference method |
|||||
| Label claim (mg/mL) | Mean (mg/mL) | Label claim (%) | RSD (%) | Mean (mg/mL) | Label claim (%) | RSD (%) |
| 1 | 0.97 | 97 | 3.78 | 1.03 | 103.0 | 1.02 |
Proposed method—voltammetric method developed in-house.
Reference method—HPLC method for midazolam assay mentioned in British Pharmacopoeia (BP).
Label claim—label claim of midazolam in midazolam injection.
Mean—mean of assay results obtained in three determinations.
RSD—% RSD of assay results obtained in three determinations.
3.6.2. Assay of midazolam by reference HPLC method
The reference reverse phase HPLC method is also used for comparison to evaluate the validity of the developed method. Fig. 6 shows the representative chromatogram of midazolam. Table 4 depicts the results obtained using the proposed voltammetric and reference HPLC methods for three determinations. The results are compared and there are no significant differences between the results obtained from both the methods.
Figure 6.
HPLC chromatogram of midazolam (100 μg/mL) using YMC ODS column (250 mm×4.6 mm, 5 μm) at a flow rate of 1.0 mL/min, detection at 220 nm with mobile phase (Methanol: Solution A: 72:28, v/v). Solution A was prepared by mixing equal volume of 0.1 M ortho-phosphoric acid and 0.03 M triethyl, adjusted pH 3.5 by 0.1 M NaOH.
3.7. Controlled potential coulometry
Controlled potential coulometry was employed for determining number of electrons (n) transferred in electrode process. Number of electrons n, was calculated from the charge consumed by the desired concentration of midazolam. The charge consumed was determined in acidic medium. For this purpose 2 mL of 300 μg/mL midazolam in acetonitrile with supporting electrolyte was electrolysed at −1.2 V and at 1.7 V against reference electrode.
During the electrolysis, solutions were continuously stirred and purged with nitrogen. Number of electrons n was calculated using the equation Q=nFN, where Q is the charge in coulombs, F is the Faraday constant, and N is the number of moles of substrate. Number of electrons involved in the electrode process was found four at −1.2 V and six at −1.7 V.
3.8. Reaction mechanism
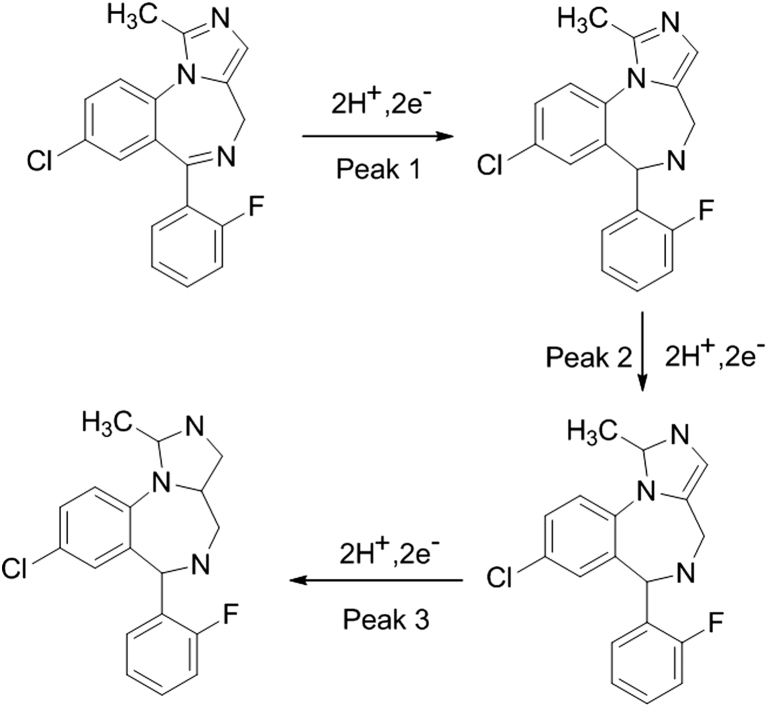
On the basis DPV, CV, square wave voltammetry and coulometry following mechanism may be postulated for the reduction of midazolam (Scheme 1). Peak labeled as 0 may be a pre-adsorption peak not affected by the concentration of the analyte. Peak 1 may be assigned to the 2-electron reduction of –C=N– bond of the seven membered ring, peak 2 to the 2-electron reduction of –C=N– bond of the five membered ring and peak 3 to the 2-electron reduction of –C=C– of the five membered ring of midazolam [14], [15], [16].
Scheme 1.
Reaction mechanism for reduction of midazolam.
4. Conclusion
The electrochemical behavior of midazolam at glassy carbon electrode is studied using SWV, CV and DPV techniques in the presence of surfactants for the first time. This work has demonstrated that midazolam has three reduction peaks at glassy carbon electrode in presence of 0.1% SLS solution. It showed irreversible process corresponding to reduction of midazolam. On the basis of SWV, CV, DPV and coulometric studies a six electron mechanism has been suggested for the reduction of the drug. Peak current could be enhanced by the addition of cationic surfactant SLS. From the analytical point of view largest peak was used to assay determination of midazolam. After optimizing various operational parameters analytical method for quantitation of midazolam with good stability, high sensitivity and selectivity was developed and validated. In addition, analysis was performed with good recoveries without any interference from the excipient. Owing to the simple solution preparation and shorter run time of the proposed procedure, the method can be suggested as a good alternative for the assay determination of midazolam over HPLC method.
References
- 1.Agrawal N., Usmani A., Sehgal R. Effect of intrathecal midazolam bupivacaine combination on post operative analgesia. Indian J. Anaesth. 2005;49:37–49. [Google Scholar]
- 2.Odou P., Robert H., Luyckx M. A routine HPLC method for monitoring midazolam in serum. Biomed. Chromatogr. 1998;11:19–21. doi: 10.1002/(SICI)1099-0801(199701)11:1<19::AID-BMC611>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Lee T.Ch., Charles B. Measurement by HPLC of midazolam and its major metabolite 1-hydroxymidazolam in plasma of very premature neonates. Biomed. Chromatogr. 1999;10:65–68. doi: 10.1002/(SICI)1099-0801(199603)10:2<65::AID-BMC555>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Lepper E.R., Hicks J.K., Verweij J. Determination of midazolam in human plasma by liquid chromatography with mass-spectrometric detection. J. Chromatogr. B. 2004;806:305–310. doi: 10.1016/j.jchromb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.British Pharmacopoeia, The Stationery Office, London, 2007.
- 6.European Pharmacopoeia, sixth ed, Council of Europe, Strasbourg, France, 2007.
- 7.Kir S., Onar A.N., Temizer A. Adsorptive stripping voltammetric determination of midazolam as a method for quality control. Anal. Chim. Acta. 1990;229:145–147. [Google Scholar]
- 8.Santos C.d., Famila V., Gonçalves S.M. Voltammetric techniques for determination of psychoactive 1,4-benzodiazepine drugs. Anal. Bioanal. Chem. 2002;374:1074–1081. doi: 10.1007/s00216-002-1535-0. [DOI] [PubMed] [Google Scholar]
- 9.Vire J.C., Patriarche G.J., Hermosa B.G. Polarographic behaviour and hydrolysis of midazolam and its metabolites. Anal. Chim. Acta. 1987;196:205–212. [Google Scholar]
- 10.Connors T.F., Rusling J.F., Owlia A. Determination of standard potentials and electron-transfer rates for halobiphenyls from electrocatalytic data. Anal. Chem. 1985;57:170–174. doi: 10.1021/ac00279a042. [DOI] [PubMed] [Google Scholar]
- 11.Stadiober M., Kalcher K., Raber G. Anodic stripping voltammetric determination of titanium(IV) using a carbon paste electrode modified with cetyltrimethylammonium bromide. Talanta. 1996;43:1915–1924. doi: 10.1016/0039-9140(96)01977-7. [DOI] [PubMed] [Google Scholar]
- 12.Hoyer B., Jensen N. Stabilization of the voltammetric serotonin signal by surfactants. Electrochem. Commun. 2006;8:323–328. [Google Scholar]
- 13.ICH topic Q2(R), Validation of Analytical Procedure: Methodology, ICH Harmonized Tripartite Guidelines, November 6, 1996, CPMP/ICH/281/95, November, 2005.
- 14.El-Sayed G.O., Yasin S.A., El Badawy A.A. Adsorptive voltammetric determination of chlordiazepoxide in pure and dosage forms. J. Chem. Pharm. Res. 2009;1:225–232. [Google Scholar]
- 15.Dryhurst G. Insights provided by electrochemical techniques into the biological redox chemistry of purines. Bioelectrochem. Bioenerg. 1985;14:251–274. [Google Scholar]
- 16.Subramanian P., Heeg M.J., Dryhurst G. Electrochemical oxidation of 1,3,7,9-tetramethyluric acid. J. Electroanal. Chem. 1986;197:279–304. [Google Scholar]