Abstract
The objective of current study was to develop a validated specific stability indicating reversed-phase liquid chromatographic method for the quantitative determination of desvenlafaxine in bulk sample and pharmaceutical dosage form in the presence of degradation products. Forced degradation studies were performed on bulk sample of desvenlafaxine as per ICH prescribed stress conditions using acid, base, oxidative and photolytic degradation to show the stability indicating power of the method. Significant degradation was observed under acidic stress condition and the degradation product formed was identified by LC–MS and a degradation pathway for drug has been proposed. Successful separation of drug from degradation products formed under stress conditions was achieved on a SymmetryShield column C18 (5 μm, 250 mm×4.6 mm, i.d.) using the mobile phase consisting of a mixture of 0.2% (v/v) triethylamine in ammonium acetate (0.05 M; pH 6.5) and methanol using isocratic gradient.
Keywords: Reversed-phase liquid chromatography, Liquid chromatography mass spectrometer (LC/MS), Desvenlafaxine, Stress studies, Validation, Degradation
1. Introduction
Desvenlafaxine, the major active metabolite of venlafaxine, is used in the treatment of depression. Chemically it is 4-[2-(dimethylamino)-1-(1-hydroxycyclohexyl) ethyl] phenol (Fig. 1). Desvenlafaxine is a selective serotonin and norepinephrine reuptake inhibitor [1], [2], [3]. Literature review reveals that methods have been reported for analysis of venlafaxine by UV spectrophotometry [4] and HPLC [5], [6], identification of desvenlafaxine by HPLC [7], estimation of venlafaxine by stability-indicating HPLC [8], [9], determination of venlafaxine and its three metabolites in human plasma by HPLC–MS/ESI [10], [11], analysis of venlafaxine metabolites in rat liver by chiral HPLC [12] and analysis of venlafaxine in tablet by HPTLC [13].
Figure 1.

Structure of desvenlafaxine.
A comprehensive LC and LC–MS study of the degradation behavior of desvenlafaxine under various ICH prescribed stress conditions has been lacking. So we decided to carry out forced degradation studies according to the ICH requirements and develop a selective and validated stability-indicating HPLC method. An integral aim of the study was to identify degradation products and to postulate complete degradation pathway of the drug. The International Conference on Harmonization (ICH) guideline entitled Stability Testing of New Drug Substances and Products requires testing to be performed to elucidate the inherent stability characteristics of the active substance [14]. The proposed method was validated according to ICH guidelines [15] and its updated international convention.
2. Experimental
2.1. Materials
Dr. Reddy's Laboratories Ltd. (Hyderabad, Andhra Pradesh, India), kindly supplied pure drug sample of desvenlafaxine as a gift sample of Batch no.: ADWSP092. Twenty tablets of desvenlafaxine (Brand name: D-VENIZ label claim: 50 mg desvenlafaxine per tablet, Batch no.: SKK0179, Expiry date: November 2012) were procured from a local Pharmacy in Pune, Maharashtra, India. All chemicals and reagents used were of analytical grade and purchased from Merck Chemicals, Mumbai, Maharashtra, India.
2.2. Instrumentation
The LC system included a Jasco Inc. (Easton, MD) Model PU 2080 Intelligent LC Pump with sampler programmed at 20 μL injection and a UV detector (Jasco Model UV 2075) operated at a wavelength of 228 nm. Data were integrated using the Jasco Borwin Version 1.5, LC-Net II/ADC system. The column used was SymmetryShield column C18 (5 μm, 250 mm×4.6 mm i.d.) from Waters, Milford, USA.
LC–MS studies were carried out on a 4000 Q-TRAP Linear Ion Trap Quadrupole Mass Spectrometer (Applied Biosystems Sciex, USA). The mass spectra of desvenlafaxine and the degradation product were taken in ESI (Turbo Ion Spray) positive mode in mass range of 40–600 amu and analyzed in the triple quadrupole analyzer. Samples were dissolved in methanol in a concentration range of 60 μg/mL and injected into the inlet. The whole process was controlled using an Analyst 1.4.2 software. The LC system attached to the MS was an Ultimate 3000 RS HPLC system with an Ultimate 3000 RS pump, Ultimate 3000 RS autosampler and Ultimate 3000 RS column compartment (Dionex, CA, USA) and data were integrated using a Chromeleon v 6.8 SR10 operating software. The mobile phase used was a mixture of 0.2% (v/v) triethylamine in ammonium acetate (0.05 M; pH 6.5) and methanol (40:60) and the column used was C18 (5 μm, 250 mm×4.6 mm i.d.) from Waters, Milford, USA. The flow rate was 1 mL/min and the effluent from the column was introduced into the mass spectrometer through a flow splitter which splits volume of mobile phase and deliver minimum amount of mobile phase in MS. The split ratio was 20:80.
2.3. Forced degradation studies
A stock solution (1 mg/mL) containing 100 mg desvenlafaxine in 100 mL methanol was prepared. This solution was used for forced degradation to provide an indication of the stability indicating property and specificity of proposed method. In all degradation studies, the average peak area of desvenlafaxine (60 μg/mL) after application of seven replicates was obtained.
2.3.1. Acid and base induced degradation
Acid decomposition studies were performed by refluxing the solution of drug in 1 M HCl at 80 °C for 8 h. The studies under alkaline conditions were performed in 5 M NaOH and the solution was refluxed for 8 h at 80 °C. The resultant solutions were diluted to a concentration of 60 μg/mL and 20 μL was injected into the LC system.
2.3.2. Hydrogen peroxide induced degradation
To study hydrogen peroxide induced degradation, initial studies were performed in 3% hydrogen peroxide at room temperature for 24 h. Then drug was exposed to 6% hydrogen peroxide at room temperature (25±2 °C) for a period of 8 days and then heated in a boiling water bath for 10 min to completely remove the excess hydrogen peroxide. The resultant solutions were diluted to obtain a concentration of 60 μg/mL and 20 μL was injected into the LC system.
2.3.3. Photochemical degradation
The photochemical stability of the drug was studied by exposing the stock solution (1000 μg/mL) to direct sunlight (60,000–70,000 lx) for 15 days on a wooden plank kept on a terrace.
The photochemical stability of the drug was also performed by keeping the stock solution (1000 μg/mL) in the stability chamber (light providing an overall illumination of 1.2 million lx h and an integrated near ultraviolet energy of not less than 200 W h/m2) for 15 days.
The solution was diluted with methanol to obtain a concentration of 60 μg/mL and then 20 μL of the solution was injected into the LC system.
2.4. Optimization of stability-indicating HPLC method
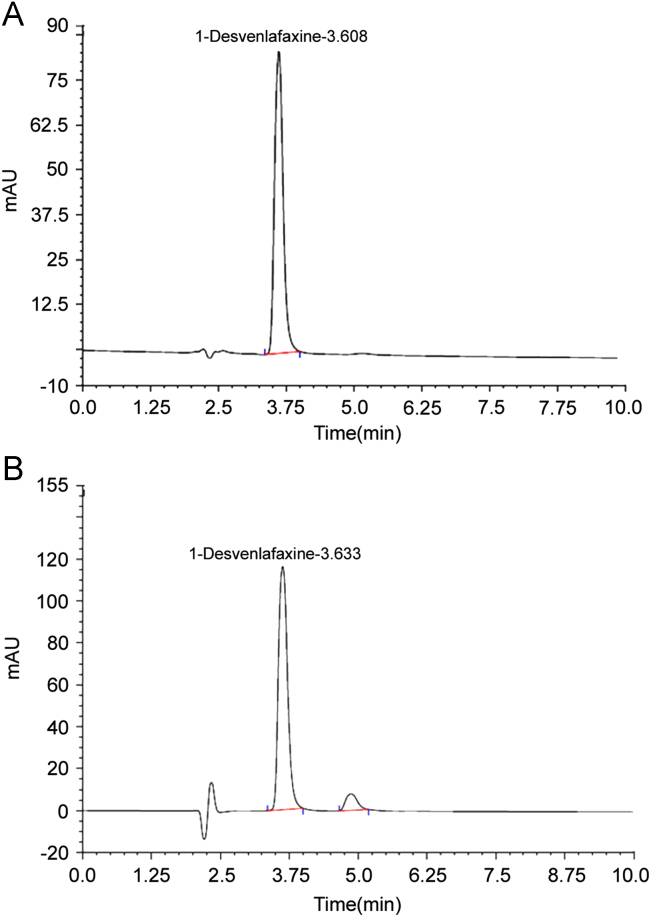
The HPLC procedure was optimized with a view to develop the stability-indicating assay method. The pure drug along with its degraded products was injected and run in different solvent systems. Initially methanol and water in different ratios were tried. It was found that when methanol concentration was increased in the mobile phase, the pure drug started to elute in dead volume. Hence the concentration of methanol was decreased and there was improvement in resolution. It was found that a mixture of 0.2% (v/v) triethylamine in ammonium acetate (0.05 M; pH adjusted to 6.5 with glacial acetic acid) and methanol (40:60) as a mobile phase at a flow rate of 1 mL/min gave acceptable retention time (tR), theoretical plates and good resolution of the drug and degradation products (Fig. 2A and B).
Figure 2.
(A) Chromatogram of standard desvenlafaxine. Peak 1, tR: 3.60 min. Mobile phase: a mixture of 0.2 % (v/v) triethylamine in ammonium acetate (0.05 M; pH adjusted to 6.5 with glacial acetic acid) and methanol (40:60). (B) Chromatogram of acid degradation product. Condition: 1 M HCl at 80 °C for 8 h. (Peak 1): desvenlafaxine, tR: 3.63 min. (Peak 2): degraded, tR: 4.87 min.
2.5. Validation of the method
Validation of the optimized LC method was done with respect to following parameters.
2.5.1. Linearity and range
Linearity of the method was studied by injecting seven concentrations of the drug prepared in the mobile phase in the range of 10–80 μg/mL in triplicate into the HPLC system keeping the injection volume constant. The peak areas were plotted against the corresponding concentrations to obtain the calibration graphs.
2.5.2. Precision
Precision of the method was verified by repeatability and intermediate precision studies. Repeatability studies were performed by analysis of three different concentrations (10, 40, and 80 μg/mL) of the drug in hexaplicate (n=6) on the same day. Intermediate precision of the method was checked by repeating studies on three different days.
2.5.3. Limit of detection (LOD) and limit of quantitation (LOQ)
The signal to noise ratio was determined. LOD was considered as 3:1 and LOQ as 10:1. The LOD and LOQ were experimentally verified by diluting known concentrations of standard solution of desvenlafaxine until the average responses were approximately 3 or 10 times the standard deviation of the responses for six replicate determinations.
2.5.4. Robustness of the method
To evaluate robustness of the HPLC method, few parameters were deliberately varied. The parameters included variation of flow rate, percentage of methanol in the mobile phase, pH of mobile phase. Robustness of the method was done at three different concentration levels 10, 40, and 80 μg/mL for desvenlafaxine.
2.5.5. Specificity
The specificity of the method towards the drug was established through study of resolution factor of the drug peak from the nearest resolving peak. Overall selectivity was established through determination of purity for each degradation product peak using PDA detector.
2.5.6. Analysis of marketed formulation
To determine the content of desvenlafaxine in conventional tablets (Brand name: D-VENIZ, Sun Pharmaceuticals Industries Ltd., Batch no. SKK0179, label claim: 50 mg desvenlafaxine per tablet, expiry date: November 2012), the contents of 20 tablets were weighed, their mean weight determined and finely powdered. An equivalent weight of the powder/triturate was transferred to a 50 mL volumetric flask containing 10 mL methanol, sonicated for 30 min and diluted to 50 mL with methanol. The resulting solution was centrifuged at 3000 rpm for 5 min. Supernatant was taken and after suitable dilution the sample solution was then filtered using a 0.45 μm filter (Millipore, Milford, MA). The above stock solution was further diluted to get sample solution at three different concentrations of 10, 40, and 80 μg/mL. A 20 μL volume of each sample solution was injected into the LC system, six times, under the conditions described above. The peak areas were measured at 228 nm and concentrations in the samples were determined using multilevel calibration developed on the same LC system under the same conditions using linear regression equation.
2.5.7. Accuracy
Accuracy of the developed method was determined by applying the method to a drug sample (desvenlafaxine tablets) to which a known amount of desvenlafaxine standard powder corresponding to 80%, 100%, and 120% of label claim was added (standard addition method). The percentage recoveries were calculated from the slope and Y-intercept of the calibration curve.
3. Results and discussion
3.1. Results of forced degradation studies
3.1.1. Acid induced degradation product
The drug was found to be highly labile to acidic degradation as compared to that of alkali. Initially 0.1 M hydrochloric acid was used at 80 °C for 8 h but negligible degradation was observed hence the strength of acid was increased, 10–20% degradation was observed by heating drug solution with 1 M hydrochloric acid at 80 °C for 8 h and associated with rise in a major degradation product at retention time 4.87 min in HPLC (Fig. 2B).
3.1.2. Base induced degradation product
Initially 1 M and 2 M sodium hydroxide solutions were used at 80 °C for 8 h but no degradation was observed hence the strength of alkali was increased. Subsequently, studies were performed in 5 M sodium hydroxide which was refluxed at 80 °C for 8 h. It was found that the drug was highly stable to alkali condition.
3.1.3. Hydrogen peroxide induced degradation product
In 6% hydrogen peroxide at room temperature the drug was found to be stable to degradation as negligible degradation was seen after exposing drug to 6% hydrogen peroxide for 8 days.
3.1.4. Photochemical degradation product
The drug was found to be stable against photochemical degradation as negligible degradation was seen after exposing the drug solution to direct sunlight as well as in photo-stability chamber for 15 days.
3.2. Identification of major degradation product formed under acidic stress condition
LC–MS analysis was carried out for the acid stress sample of desvenlafaxine using a 4000 Q-TRAP Linear Ion Trap Quadrupole Mass Spectrometer with suitable volatile buffer ammonium acetate (0.05 M, pH 6.5) as mobile phase. Satisfactory separation of degradation product was achieved using a C18 column. The degradation product formed shows the m/z of 246.5 which has 18 lesser m/z value than desvenlafaxine m/z 264.4 (Fig. 3). The fragmentation for the degradant was also carried out for degradation product and desvenlafaxine using product ion scan by LCMS/MS. Based on the molecular weight and the fragmentation pattern, the presence of degradation product was confirmed and also, the structure could be proposed. The fragmentation pattern (Fig. 4) clearly indicates that there is one possible structure of degradation product formed under acidic condition. The fragment 246.5 formed from the cleavage of –OH bond attached to cyclohexane ring and loss of water molecule (m/z 18). So the probable structure is 4-[2-(dimethylamino)(1-cyclohexylidene) ethyl]phenol as shown in Fig. 5.
Figure 3.
Representative positive ESI-Quadrupole (+Q1) mass spectra of desvenlafaxine and acid degradation product.
Figure 4.
Fragmentation mass spectrum of degradation product formed in acidic degradation of desvenlafaxine.
Figure 5.
Structure of degradation product (m/z: 246.5) formed in acidic degradation of desvenlafaxine.
3.3. Results of method validation
The results of validation studies on the stability-indicating method developed for desvenlafaxine in the current study involving a mixture of 0.2% (v/v) triethylamine in ammonium acetate (0.05 M; pH adjusted to 6.5 with glacial acetic acid) and methanol (40:60) as a mobile phase are given below.
3.3.1. Linearity
The response for the drug was linear in the concentration range of 10-80 μg/mL. The mean (±RSD) values of slope, intercept and correlation coefficient were 8658.1 (±1.30), 21789 (±1.57) and 0.9998 (±0.876), respectively.
3.3.2. Precision
The results of the repeatability and intermediate precision experiments are shown in Table 1. The developed method was found to be precise as the RSD values for repeatability and intermediate precision studies were <2%, respectively, as recommended by ICH guideline. Separation of the drug and degradation product in stressed sample was found to be similar when analysis was performed on different chromatographic systems on different days.
Table 1.
Precision studies for desvenlafaxine.
| Concentration (μg/mL) | Intra-day precision (n=6) |
Inter-day precision (n=6) |
||||
|---|---|---|---|---|---|---|
| Measured concentration (μg/mL) | RSD (%) | Recovery (%) | Measured concentration (μg/mL) | RSD (%) | Recovery (%) | |
| 10 | 9.87 | 1.13 | 98.70 | 9.89 | 0.23 | 98.90 |
| 40 | 39.92 | 1.01 | 99.80 | 40.01 | 0.03 | 100.02 |
| 80 | 78.99 | 1.22 | 98.73 | 79.88 | 0.38 | 99.85 |
3.3.3. LOD and LOQ
The signal:noise ratios of 3:1 and 10:1 were considered as LOD and LOQ, respectively. The LOD and LOQ were found to be 5 μg/mL and 10 μg/mL, respectively.
3.3.4. Robustness of the method
Each factor selected (except columns from different manufacturers and solvents of different lots) was changed at three levels (−1, 0 and 1). One factor at a time was changed to estimate the effect. Thus, replicate injections (n=6) of mixed standard solution at three concentration levels were performed under small changes of three chromatographic parameters (factors). Insignificant differences in peak areas and less variability in retention time were observed (Table 2).
Table 2.
Robustness testing for desvenlafaxinea (n=6).
| Factorb | Level | tRc | kd | Te |
|---|---|---|---|---|
| Flow rate (mL/min) | ||||
| 0.9 | −1 | 3.71 | 1.62 | 1.20 |
| 1.0 | 0 | 3.60 | 1.75 | 1.01 |
| 1.1 | +1 | 3.47 | 2.04 | 1.11 |
| Mean±SD | 3.59±0.12 | 1.80±0.21 | 1.80±0.09 | |
| Percent of methanol in the mobile phase (v/v) | ||||
| 59 | −1 | 3.70 | 2.40 | 0.98 |
| 60 | 0 | 3.60 | 2.89 | 1.24 |
| 61 | +1 | 3.61 | 1.23 | 1.13 |
| Mean±SD | 3.63±0.05 | 2.17±0.85 | 1.116±0.13 | |
| pH of mobile phase | ||||
| 6.4 | −1 | 3.32 | 2.45 | 1.23 |
| 6.5 | 0 | 3.60 | 2.87 | 1.02 |
| 6.6 | +1 | 3.67 | 2.99 | 1.01 |
| Mean±SD | 3.53±0.18 | 2.77±0.28 | 1.08±0.12 | |
Average of three concentrations: 10, 40 and 80 μg/mL.
Three factors were slightly changed at three levels (−1, 0, and +1).
tR=retention time (min).
k=retention factor.
T=tailing factor.
3.3.5. Specificity
The specificity of the LC method is illustrated in Fig. 3, where complete separation of desvenlafaxine in the presence of its degradation product was seen. The peaks obtained were sharp and had clear baseline separation. The resolution factor for the drug from its nearest peak was >3 (Fig. 2B). The photodiode array detector scanned all of the components present in a mixture in the wavelength range from 200 to 400 nm. Results indicated that there were no degradation peak hiding under, or unresolved from the analyte peak (pure drug), which also reflected the specificity of the method.
3.3.6. Analysis of marketed formulation
Two different lots of commercially available desvenlafaxine tablet were analyzed using the proposed procedures and the results are summarized in Table 3.
Table 3.
Analysis of commercial formulation (D-VENIZ, 50 mg) (n=6).
| Commercial formulation D-VENIZ (50 mg) | Drug found (mean±SD, mg) | Recovery (mean±SD, %) |
|---|---|---|
| 1st lot | 50.09±0.17 | 100.18±0.21 |
| 2nd lot | 49.91±0.55 | 99.82±0.49 |
3.3.7. Recovery studies
As shown from the data in Table 4 good recoveries of the drug in the range from 98.9% to 100.18% were obtained at various added concentrations.
Table 4.
Accuracy (% recovery) of desvenlafaxine in tablet formulation at three concentration levels (n=6).
| Label claim (mg per tablet) | Amount added (%) | Total amount (mg) | Amount recovered (mg)±RSD (%) | Recovery (%) |
|---|---|---|---|---|
| 50 | 80 | 90 | 89.21±0.12 | 99.12 |
| 100 | 100 | 98.90±0.11 | 98.90 | |
| 120 | 110 | 110.20±0.11 | 100.18 |
4. Conclusion
In this paper a sensitive, specific, accurate, validated and well-defined stability indicating LC method for the determination of desvenlafaxine in the presence of degradation product was described. The behavior of desvenlafaxine under various stress conditions was studied; the acid degradants were identified by LC–MS. The drug was found to be stable in basic, oxidative and photolytic conditions. The degradation product was well separated from the drug substance demonstrating the stability indicating capability of the method. The method was validated for parameters like linearity, precision, accuracy, specificity, etc. and was also applied to real marketed samples. The information presented herein could be very useful for quality monitoring of bulk samples and also employed to check the quality of drug during stability studies.
Acknowledgment
The authors thank Dr. Reddy's Laboratories Ltd. (Hyderabad, Andhra Pradesh, India) for providing gift sample of standard desvenlafaxine and Dr. K.R. Mahadik, Principal, Poona College of Pharmacy (Pune, Maharashtra, India) for providing necessary facilities to carry out the work.
References
- 1.Liebowitz M.R., Tourian K.A. Efficacy, safety, and tolerability of desvenlafaxine 50 mg/d for the treatment of major depressive disorder: a systematic review of clinical trials. Prim. Care Companion J. Clin. Psychiatry. 2010;12(3):e1–e10. doi: 10.4088/PCC.09r00845blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols A.I., Richards L.S., Behrle J.A. The pharmacokinetics and safety of desvenlafaxine in subjects with chronic renal impairment. Int. J. Clin. Pharmacol. Ther. 2011;49(1):3–13. doi: 10.5414/cpp49003. [DOI] [PubMed] [Google Scholar]
- 3.Nichols A.I., Focht K., Jiang Q. Pharmacokinetics of venlafaxine extended release 75 mg and desvenlafaxine 50 mg in healthy cyp2d6 extensive and poor metabolizers: a randomized, open-label, two-period, parallel-group, crossover study. Clin. Drug Invest. 2011;31(3):155–167. doi: 10.2165/11586630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Sundaraganapathy R., Jambulingam M., Ananda Thangadurai S. Development and validation of UV spectrophotomeric method for the determination of venlafaxine hydrochloride in bulk and solid dosage forms. Int. J. Pharm. Ind. Res. 2011;1(1):28–31. [Google Scholar]
- 5.Somasekhar V., Gowrisankar D., Shivakumar H.N. Development and validation of a rapid RP-HPLC method for the determination of venlafaxine hydrochloride in pharmaceutical dosage forms using experimental design. Eur. J. Chem. 2009;6(4):1091–1102. [Google Scholar]
- 6.Baldania S.L., Bhatt K.K., Mehta R.S. RP-HPLC estimation of venlafaxine hydrochloride in tablet dosage forms. Indian J. Pharm. Sci. 2008;70(1):124–128. doi: 10.4103/0250-474X.40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carneiro W.J., Andrade C.H., Braga R.C. Identification of desvenlafaxine, the major active metabolite of venlafaxine, in extended-release capsules. Revista Eletrônica de Farmácia. 2010;7(1):39–53. [Google Scholar]
- 8.Ebenezer B.A., Patrick J.F., Mobin A.T. Validation and application of a stability-indicating HPLC method for the in vitro determination of gastric and intestinal stability of venlafaxine. J. Pharm. Biomed. Anal. 2007;43:1854–1859. doi: 10.1016/j.jpba.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Makhija S.N., Vavia P.R. Stability indicating LC method for the estimation of venlafaxine in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2002;28(6):1055–1059. doi: 10.1016/s0731-7085(01)00701-4. [DOI] [PubMed] [Google Scholar]
- 10.Kingback M., Josefsson M., Karlsson L. Stereoselective determination of venlafaxine and its three demethylated metabolites in human plasma and whole blood by liquid chromatography with electrospray tandem mass spectrometric detection and solid phase extraction. J. Pharm. Biomed. Anal. 2010;53:583–590. doi: 10.1016/j.jpba.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Liu W., Cai H.L., Li H.D. High performance liquid chromatography–electrospray ionization mass spectrometry (HPLC–MS/ESI) method for simultaneous determination of venlafaxine and its three metabolites in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;850(1–2):405–411. doi: 10.1016/j.jchromb.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Da Fonseca P., Bonato P.S. Chiral HPLC analysis of venlafaxine metabolites in rat liver microsomal preparations after LPME extraction and application to an in vitro biotransformation study. Anal. Bioanal. Chem. 2010;396(2):817–824. doi: 10.1007/s00216-009-3271-1. [DOI] [PubMed] [Google Scholar]
- 13.Shirvi V.D., Channabasavaraj K.P., Vijaya Kumar G. HPTLC analysis of venlafaxine hydrochloride in the bulk drug and tablets. J. Planar Chromatogr. 2010;23(5):369–372. [Google Scholar]
- 14.ICH. Topic Q1A (R2): stability testing of new drugs substances and products, in: Proceedings of the International Conference on Harmonization (ICH), IFPMA, Geneva, Switzerland, 2003.
- 15.ICH. Topic Q2 (R1): validation of analytical procedures: text and methodology international conference on harmonization, Geneva, Switzerland, 2005.