Abstract
A rapid, sensitive and simple spectrofluorimetric method was developed for the estimation of atorvastatin. In this method, the native fluorescence characteristics of atorvastatin have been studied in both acidic and basic media. High sensitivity was obtained with 5% acetic acid at 389 nm using 276 nm for excitation. Regression analysis showed a good correlation coefficient (r=0.9995) between fluorescence intensity and concentration over the range of 1.5–4 μg/mL with detection limit of 0.012 μg/mL. The proposed method was successfully applied to the analysis of atorvastatin in pure and pharmaceutical dosage forms with average recovery of 100.29±0.47%. The results were compared favorably with those of the reported method.
Keywords: Spectrofluorimetric, Atorvastatin, Native fluorescence
1. Introduction
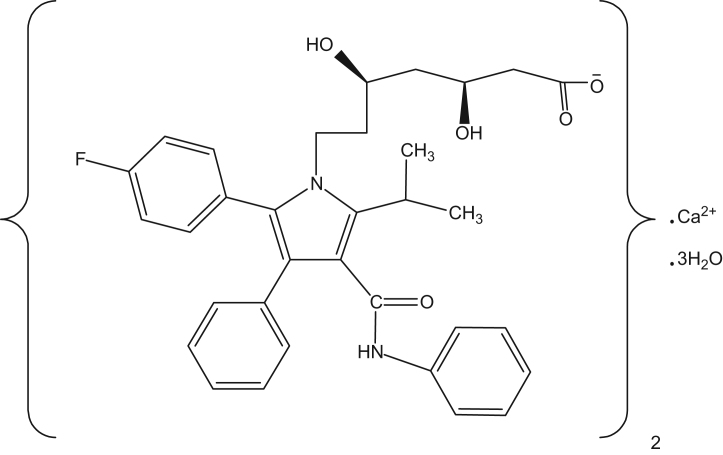
Atorvastatin calcium (Fig. 1) is a calcium (βR, ΔR)-2-(ρ-fluorophenyl)-β, Δ-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl) pyrrole-1-hepatanoic acid(1:2) trihydrate. C66H68CaF2N4O10·3H2O=1209.4 [1], which is one of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (a statin). It is a lipid regulating drug with actions on plasma lipids similar to those of simvastatin. It is used to reduce LDL-cholesterol, apolipoprotein B, and triglycerides, and to increase HDL-cholesterol in the treatment of hyperlipidaemias [2], [3], [4].
Figure 1.
Chemical structure of atorvastatin.
Several studies have been reported for the determination of atorvastatin in pharmaceutical and/or biological fluids including spectrophotometric methods [5], [6], [7], [8], electrophoresis [9], [10], polarographic method [11] and chromatographic methods with different detectors [12], [13], [14], [15], [16], [17], [18], [19], [20]. To the best of our knowledge, none of the reported procedures describe spectrofluorimetric method for the determination of atorvastatin. Thus, an attempt was made to develop a new, rapid, sensitive and validated spectrofluorimetric procedure for the determination of atorvastatin.
2. Experimental
2.1. Materials and reagents
Atorvastatin calcium powder (Batch No. 1617357) was kindly supplied by El-Oboure Pharmaceutical Company. Atorvastatin tablets (each tablet contains 20 mg of atorvastatin), manufactured by Pfizer (Batch No. 084540), were purchased from the Egyptian market. All reagents and chemicals used were of analytical grade and all were purchased from Sigma–Aldrich (Steinheium, Germany). Glass distilled water was further purified using Milli-Q water purification system (Millipore, Bedford, MA, USA). Buffers of different pH values were prepared as prescribed in British Pharmacopeia [21]:
-
•
Acetate buffer (pH 2.45): Mix 200 mL of 1 M hydrochloric acid with 200 mL of 1 M sodium acetate and dilute to 1000 mL with water. Immediately before use adjust the pH to 2.45 by the addition of 1 M hydrochloric acid or 1 M sodium acetate, as required.
-
•
Acetate buffer (pH 2.8): Dissolve 4 g of anhydrous sodium acetate in about 840 mL of water, add sufficient glacial acetic acid to adjust the pH to 2.8 (about 155 mL) and dilute to 1000 mL with water.
-
•
Acetate buffer (pH 3.4): Mix 5 volumes of 0.1 M sodium acetate with 95 volumes of 0.1 M acetic acid.
-
•
Acetate buffer (pH 4.5): Dissolve 77.1 g of ammonium acetate in water, add 70 mL of glacial acetic acid and dilute to 1000 mL with water.
-
•
Acetate buffer (pH 5.0): Dissolve 13.6 g of sodium acetate and 6 mL of glacial acetic acid in sufficient water to produce 1000 mL.
-
•
Acetate buffer (pH 6.0): Dissolve 100 g of ammonium acetate in 300 mL of water, add 4.1 mL of glacial acetic acid, adjust the pH, if necessary, using 10 M ammonia or 5 M acetic acid and dilute to 500 mL with water
-
•
Phosphate buffers (pH 5.8–8.0): Mix 50 mL of 0.2 M potassium dihydrogen orthophosphate with different quantities of 0.2 M sodium hydroxide and dilute to 200 mL with water.
-
•
Phosphate buffer (pH 9.0): Dissolve 1.74 g of potassium dihydrogen orthophosphate in 80 mL of water, adjust the pH, if necessary, with 1 M potassium hydroxide and dilute to 100 mL with water.
-
•
Borate buffer (pH 10): To 50 mL of a solution containing 0.6189 g of boric acid and 0.7456 g of potassium chloride add 36.85 mL of 0.2 M sodium hydroxide, adjust pH to 10 using 0.2 M sodium hydroxide and dilute with water to 200 mL.
2.2. Standard solutions
Stock solutions of atorvastatin were prepared by dissolving the compound in methanol at concentration of 200 μg/mL.
2.3. Apparatus
Fluorescence spectra and measurements were recorded using PerkinElmer luminescence spectrometer LS 45 (UK), equipped with 150 W Xenon arc lamp. The slit widths for both excitation and emission monochromators were set at 10 nm. A pH-meter (Mettler-Toledo GmbH, Switzerland) was used for pH adjustment.
2.4. Procedures
2.4.1. Spectrofluorimetric method using acetate buffer (pH 3.4)
Different aliquots of atorvastatin stock solution (200 μg/mL in methanol) ranging from 50 to 100 μL were transferred to 5 mL volumetric flask. One milliliter of acetate buffer (pH 3.4) was added to each volumetric flask. The solutions were diluted to volume with ethanol: water (50:50, v/v), shaken vigorously and immediately measured. The fluorescence intensity was measured at 389 nm (λEx=276 nm).
2.4.2. Spectrofluorimetric method using 5% acetic acid (pH 2.5)
Different aliquots of atorvastatin stock solution (200 μg/mL in methanol) ranging from 50 to 200 μL were transferred to 10 mL volumetric flask. The solutions were diluted to volume with acetic acid (5%, v/v), shaken vigorously and immediately measured. The fluorescence intensity was measured at 389 nm (λEx=276 nm).
2.4.3. Spectrofluorimetric method using methanol
Different aliquots of atorvastatin stock solution (200 μg/mL in methanol) ranging from 25 to 125 μL were transferred to a 10 mL volumetric flask. The solutions were diluted to volume with spectroscopic methanol, shaken vigorously and immediately measured. The fluorescence intensity was measured at 389 nm (λEx=276 nm).
2.4.4. Analysis of Lipitor® tablets
Ten Lipitor® tablets were weighed, crushed and mixed in a mortar. An amount of powdered mass equivalent to 20 mg of atorvastatin was weighed and transferred to a 50 mL conical flask, the drug from powder was dissolved and extracted with methanol. To ensure complete extraction of drug it was sonicated for 15 min. The extract was filtered, and residue was washed with methanol. The extract and washing solution were pooled and transferred to a 100 mL volumetric flask and volume was made with methanol. This solution was analyzed as described in the general analytical procedure (Section 2.4.)
3. Results ad discussion
3.1. Spectrofluorimetric method using acetate buffer (pH 3.40)
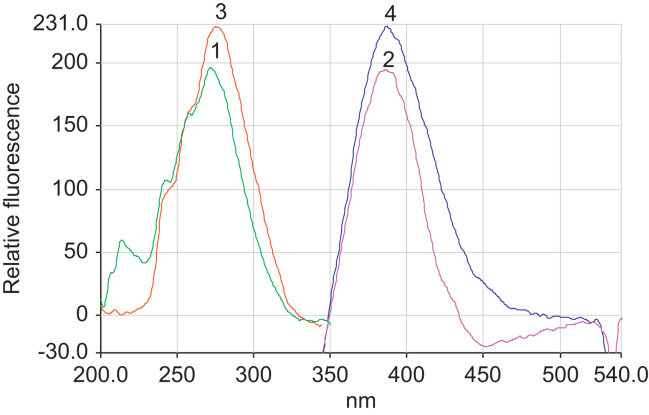
Atorvastatin presents a native fluorescence with slight differences in both acidic and basic media as shown in Fig. 2. The excitation and emission spectra at pH 3.4 using acetate buffer (spectra 3, 4) and at pH 9.0 using phosphate buffer (spectra 1, 2) are shown in Fig. 2. As can be seen the fluorescence of atorvastatin in acetate buffer (pH 3.4) is slightly higher than that in phosphate buffer (pH 9.0). The emission and excitation wavelengths were recorded at 389 and 276 nm, respectively.
Figure 2.
The excitation and emission spectra at pH 9 using phosphate buffer (spectra 1, 2) and at pH 3.4 using acetate buffer (spectra 3, 4) of 2 μg/mL atorvastatin.
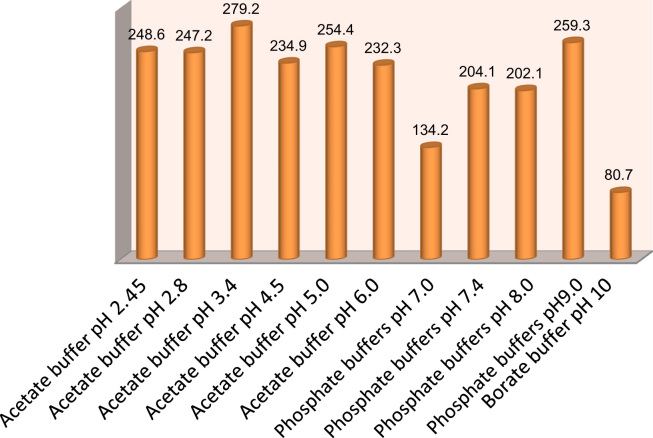
To regulate the pH, different buffers; acetate, phosphate and borate buffers prepared as described in British Pharmacopoeia, were tested. The variation of the emission intensity at 389 nm (λEx=276 nm) of atorvastatin versus the pH of the different buffers is shown in Fig. 3. The maximum fluorescence intensity was recorded with acetate buffer; therefore acetate buffer was chosen as the best buffer to regulate the pH of atorvastatin.
Figure 3.
Effect of different buffers on the fluorescence intensity of 2.5 μg/mL atorvastatin.
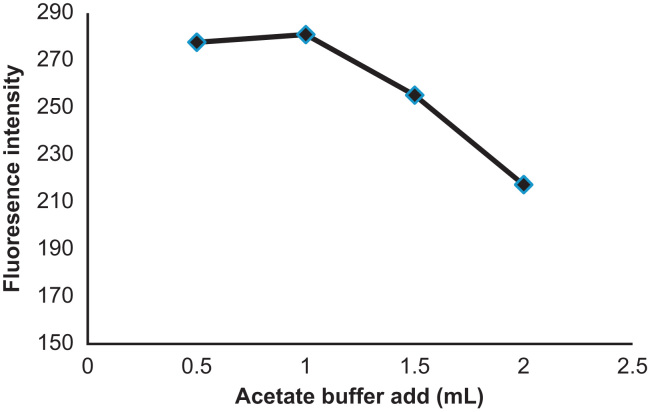
The effect of acetate buffer (pH 3.4) on the fluorescence intensity of atorvastatin expressed by the number of milliliters of buffer added to a 5 mL volumetric flask is shown in Fig. 4. As can be seen one milliliter of acetate buffer is sufficient to produce the maximum fluorescence of atorvastatin. Also the effect of the measuring time was examined between 5 and 30 min. The fluorescence slightly decreased as the time increased. Therefore, immediate measurement is preferable. The influence of the temperature was studied between 20 and 50 °C and it could be observed that the fluorescence intensity decreased when the temperature increased. For that reason 20 °C was chosen for further experiences.
Figure 4.
Effect of acetate buffer (pH 3.4) on the fluorescence intensity of 2.5 μg/mL atorvastatin.
3.2. Spectrofluorimetric method using 5% acetic acid
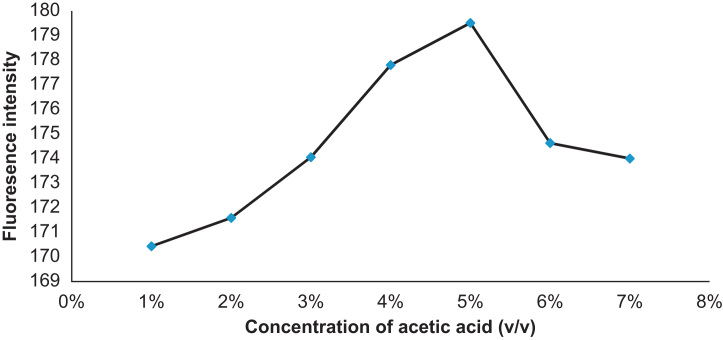
The different experimental parameters that affect the fluorescence intensity of atorvastatin in acetic acid were carefully studied and optimized. The effect of acetic acid concentration on the fluorescence intensity of atorvastatin expressed as percentage (v/v) is shown in Fig. 5. As can be seen 5% acetic acid is sufficient to produce the maximum fluorescence of atorvastatin. Also the effect of the measuring time was examined between 5 and 30 min. The fluorescence slightly decreased as the time increased. Therefore, immediate measurement is preferable. The influence of the temperature was studied between 20 and 50 °C and it could be observed that the fluorescence intensity decreased when the temperature increased. For that reason 20 °C was chosen for further experiences.
Figure 5.
Effect of acetic acid concentration on the fluorescence intensity of 1.5 μg/mL atorvastatin.
3.3. Spectrofluorimetric method using methanol
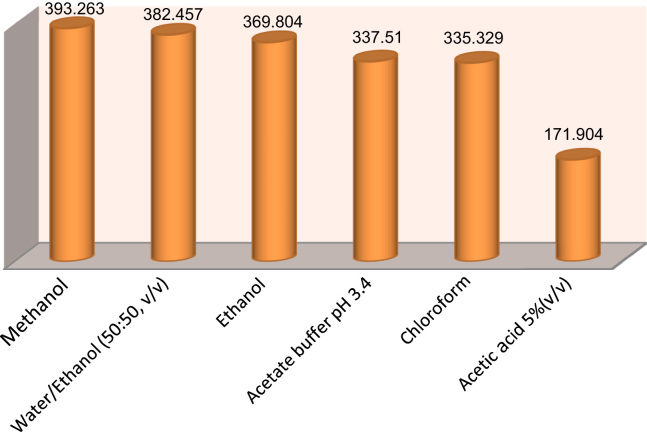
The fluorescence intensities of atorvastatin in different solvents are shown in Fig. 6. It could be observed that methanol or ethanol increases the signal of fluorophore.
Figure 6.
Effect of different solvents on the fluorescence intensity of 1.5 μg/mL atorvastatin.
The fluorescence characteristics of atorvastatin have been established in acidic, basic and neutral media. When comparing the three methods with each other, they showed a good linearity in all cases. A comparison also revealed that the using of 5% acetic acid or methanol as a co-solvent was much better than the using of acetate buffer due to their higher sensitivity.
3.4. Validation of the method
3.4.1. Linearity, detection and quantification limits
Satisfactory linearity (r>0.999) was obtained for the three proposed spectrofluorimetric methods. The analytical parameters of the proposed methods are summarized in Table 1. The detection limit and the quantification limit were calculated using the following equation [22]:
where F is the factor of 3.30 and 10 for DL and QL, respectively. SD is the standard deviation of the blank and b is the slope of the regression line. The estimated limits were verified by analyzing a suitable number of samples containing the analyte at the corresponding concentrations.
Table 1.
Regression characteristics and assay parameters of the proposed methods.
| Parameters | Spectrofluorimetric method using acetate buffer (pH 3.4) | Spectrofluorimetric method using 5% acetic acid | Spectrofluorimetric method using methanol |
|---|---|---|---|
| Linearity range (μg/mL) | 1.50–4.00 | 1.00–4.00 | 0.50–3.00 |
| Detection limit (DL) (μg/mL) | 0.03 | 0.01 | 0.01 |
| Quantification limit (QL) (μg/mL) | 0.75 | 0.13 | 0.13 |
| Regression equation* | |||
| Slop (b) | 97.14 | 51.60 | 111.39 |
| Intercept (a) | 46.09 | 16.14 | 23.32 |
| Correlation coefficients (r) | 0.9996 | 0.9998 | 0.9995 |
y=a+bx where y is the fluorescence and x is the concentration.
3.4.2. Precision and accuracy
Samples of pure atorvastatin were prepared and tested using the proposed procedures. The results obtained for pure drugs are given in Table 2. To ascertain the accuracy of the proposed procedures, they were successfully applied for the determination of atorvastatin in Lipitor® tablets, as presented in Table 3.
Table 2.
Determination of atorvastatin by the proposed spectrofluorimetric methods.
| Spectrofluorimetric method using acetate buffer (pH 3.4) |
Spectrofluorimetric method using 5% acetic acid |
Spectrofluorimetric method using methanol |
||||||
|---|---|---|---|---|---|---|---|---|
| Known concentration (μg/mL) | Concentration found (μg/mL) | Recovery (%) | Known concentration (μg/mL) | Concentration found (μg/mL) | Recovery (%) | Known concentration (μg/mL) | Concentration found (μg/mL) | Recovery (%) |
| 2.00 | 1.96 | 98.04 | 1.50 | 1.48 | 98.87 | 0.50 | 0.51 | 102.46 |
| 2.50 | 2.54 | 101.57 | 2.00 | 2.03 | 101.35 | 1.00 | 1.02 | 101.68 |
| 3.00 | 3.01 | 100.40 | 2.50 | 2.48 | 99.13 | 1.50 | 1.49 | 99.11 |
| 3.50 | 3.52 | 100.44 | 3.00 | 3.02 | 100.63 | 2.00 | 1.97 | 98.65 |
| 4.00 | 3.97 | 99.32 | 3.50 | 3.51 | 100.23 | 2.50 | 2.48 | 99.21 |
| 4.00 | 3.99 | 99.66 | 3.00 | 3.04 | 101.32 | |||
| Mean | 99.95 | 100.20 | 99.99 | |||||
| N | 5.00 | 6.00 | 6.00 | |||||
| SD | 1.78 | 0.73 | 1.95 | |||||
| R.S.D. (%) | 1.33 | 0.86 | 1.40 | |||||
Table 3.
Determination of atorvastatin in Lipitor® tablets (20 mg) by the proposed spectrofluorimetric methods.
| Spectrofluorimetric method using acetate buffer (pH 3.4) |
Spectrofluorimetric method using 5% acetic acid |
Spectrofluorimetric method using methanol |
||||||
|---|---|---|---|---|---|---|---|---|
| Known concentration (μg/mL) | Concentration found (μg/mL) | Recovery (%) | Known concentration (μg/mL) | Concentration found (μg/mL) | Recovery (%) | Known concentration (μg/mL) | Concentration found (μg/mL) | Recovery (%) |
| 2.00 | 1.97 | 98.62 | 2.00 | 1.99 | 99.75 | 1.00 | 1.03 | 102.92 |
| 2.50 | 2.59 | 103.44 | 3.00 | 3.02 | 100.60 | 1.50 | 1.47 | 98.03 |
| 3.00 | 3.15 | 105.10 | 4.00 | 4.02 | 100.53 | 2.00 | 1.96 | 98.18 |
| 3.50 | 3.59 | 102.67 | 2.50 | 2.54 | 101.47 | |||
| 4.00 | 3.99 | 99.95 | 3.00 | 3.12 | 104.09 | |||
| Mean | 101.96 | 100.29 | 100.94 | |||||
| N | 5.00 | 3.00 | 5.00 | |||||
| SD | 2.64 | 0.47 | 2.75 | |||||
| R.S.D. (%) | 1.16 | 0.27 | 1.22 | |||||
3.5. Statistical analysis of the results in comparison with the official methods
The results of the analysis of the drug were compared statistically by the Student's t-test and the variance ratio F-test with those obtained by the reported methods [5]. The Student's t-values at 95% confidence level did not exceed the theoretical values, indicating that there was no significant difference between the proposed methods and the reported method. It was also noticed that the variance ratio F-values calculated for p=0.05 did not exceed the theoretical values, indicating that there was no significant difference between the precision of the proposed methods and the reported method. The results are given in Table 4.
Table 4.
Statistical analysis of pure atorvastatin by the proposed spectrofluorimetric methods.
| Items | Spectrofluorimetric method in acetate buffer (pH 3.4) | Spectrofluorimetric method using 5% acetic acid | Spectrofluorimetric method using methanol | Reported method [5] |
|---|---|---|---|---|
| Mean (%) | 99.95 | 99.98 | 100.41 | 100.97 |
| N | 5.00 | 6.00 | 6.00 | 7.00 |
| V | 1.78 | 0.88 | 2.57 | 1.47 |
| SD | 1.33 | 0.94 | 1.60 | 1.21 |
| R.S.D. (%) | 0.60 | 0.38 | 0.65 | 0.45 |
| F-test | 1.23 (4.53)a | 1.63 (4.95)a | 1.77 (4.39)a | |
| Student's t-test | 1.34 (2.228)a | 1.57 (2.179)a | 0.70 (2.179)a |
The figures in parenthesis are the corresponding tabulated values at p=0.05.
4. Conclusion
In this work three spectrofluorimetric methods for the determination of atorvastatin are proposed. All of them present several advantages, such as rapid preparation of the samples and short time of analysis where all the measurements can be made just after preparation.
References
- 1.Reents S., Seymour J. Gold Standard Multimedia Inc.; Tampa, FL: 1998. Clinical Pharmacology; An Electronic Reference and Teaching Guide CD-ROM. [Google Scholar]
- 2.Lea A.P., McTavish D. Atrovastatin; a review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs. 1997;53:828–847. doi: 10.2165/00003495-199753050-00011. [DOI] [PubMed] [Google Scholar]
- 3.Malinowski J.M. Atorvastatin; a hydroxylmethylglutaeryl-coenzyme a reductase inhibitor. Am. J. Health Syst. Pharm. 1998;55:2253–2267. doi: 10.1093/ajhp/55.21.2253. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra H.S., Goa K.L. Atorvastatin; an updated review of its pharmacological properties and use in dyslipidaemia. Drugs. 2001;61:1835–1881. doi: 10.2165/00003495-200161120-00012. [DOI] [PubMed] [Google Scholar]
- 5.Nagaraj, Vipul K., Rajshree M. Simultaneous quantitative resolution of atorvastatin calcium and fenofibrate in pharmaceutical preparation by using derivative ratio spectrophotometry and chemometric calibrations. Anal. Sci. 2007;23(4):445–451. doi: 10.2116/analsci.23.445. [DOI] [PubMed] [Google Scholar]
- 6.Darwish H.W., Hassan S.A., Salem M.Y. Three different spectrophotometric methods manipulating ratio spectra for determination of binary mixture of amlodipine and atorvastatin. Spectrochim. Acta, Part A. 2011;83(1):140–148. doi: 10.1016/j.saa.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Maher H.M., Youssef R.M., Hassan E.M. Enhanced spectrophotometric determination of two antihyperlipidemic mixtures containing ezetimibe in pharmaceutical preparations. Drug Test. Anal. 2011;3(2):97–105. doi: 10.1002/dta.165. [DOI] [PubMed] [Google Scholar]
- 8.Joseph L., George M., Rao B.V.R. Simultaneous estimation of atorvastatin and ramipril by RP-HPLC and spectroscopy. Pak J. Pharm. Sci. 2008;21(3):282–284. [PubMed] [Google Scholar]
- 9.Kiya Y., Miura S., Zhang B. Effect of levothyroxine on total lipid profiles as assessed by analytical capillary isotachophoresis in a patient with hypothyroidism. Endocrinol. J. 2006;53(6):865–868. doi: 10.1507/endocrj.k05-181. [DOI] [PubMed] [Google Scholar]
- 10.Guihen E., Sisk G.D., Scully N.M. Glennon, Rapid analysis of atorvastatin calcium using capillary electrophoresis and microchip electrophoresis. Electrophoresis. 2006;27(12):2338–2347. doi: 10.1002/elps.200500899. [DOI] [PubMed] [Google Scholar]
- 11.Korany M.A., Hewala I.I., Abdel-Hay K.M. Determination of etofibrate, fenofibrate, and atorvastatin in pharmaceutical preparations and plasma using differential pulse polarographic and square wave voltammetric techniques. J. AOAC Int. 2008;91(5):1051–1058. [PubMed] [Google Scholar]
- 12.Bahrami G., Mohammadi B., Mirzaeei S. Determination of atorvastatin in human serum by reversed-phase high-performance liquid chromatography with UV detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005;826(1–2):41–45. doi: 10.1016/j.jchromb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Shah Y., Iqbal Z., Ahmad L. Simultaneous determination of rosuvastatin and atorvastatin in human serum using RP-HPLC/UV detection: method development, validation and optimization of various experimental parameters. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879(9–10):557–563. doi: 10.1016/j.jchromb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri R.K., Desai M.M., Raghavaraju T.V. Simultaneous quantitative determination of Metoprolol, Atorvastatin and Ramipril in capsules by a validated stability-indicating RP-UPLC method. Sci. Pharm. 2010;78(4):821–834. doi: 10.3797/scipharm.1004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farahani H., Norouzi P., Beheshti A. Quantitation of atorvastatin in human plasma using directly suspended acceptor droplet in liquid-liquid-liquid microextraction and high-performance liquid chromatography-ultraviolet detection. Talanta. 2009;80(2):1001–1006. doi: 10.1016/j.talanta.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Nováková L., Vlcková H., Satínský D. Ultra high performance liquid chromatography tandem mass spectrometric detection in clinical analysis of simvastatin and atorvastatin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009;877(22):2093–2103. doi: 10.1016/j.jchromb.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Guillén D., Cofán F., Ros E. Determination of atorvastatin and its metabolite ortho-hydroxyatorvastatin in human plasma by on-line anion-exchange solid-phase extraction and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2009;394(6):1687–1696. doi: 10.1007/s00216-009-2852-3. [DOI] [PubMed] [Google Scholar]
- 18.Nirogi R., Mudigonda K., Kandikere V. Chromatography-mass spectrometry methods for the quantitation of statins in biological samples. J. Pharm. Biomed. Anal. 2007;44(2):379–387. doi: 10.1016/j.jpba.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Zarghi A., Shafaati A., Foroutan S.M. A simple and rapid HPLC method for the determination of atorvastatin in human plasma with UV detection and its application to pharmacokinetic studies. Arzneimittelforschung. 2005;55(8):451–454. doi: 10.1055/s-0031-1296887. [DOI] [PubMed] [Google Scholar]
- 20.Ertürk S., Sevinç A.E., Ersoy L. An HPLC method for the determination of atorvastatin and its impurities in bulk drug and tablets. J. Pharm. Biomed. Anal. 2003;33(5):1017–1023. doi: 10.1016/s0731-7085(03)00408-4. [DOI] [PubMed] [Google Scholar]
- 21.British Pharmacopoeia CD-ROM, Volume I & II, Monographs: Medicinal and Pharmaceutical Substances, Stationary Office, London, 2004
- 22.Joachim E., Miller J.H.McB. WILEY-VCH Verlag GmbH & Co. KGaA; Weinheim: 2005. Method Validation in Pharmaceutical Analysis. A Guide to Best Practice. p. 101. [Google Scholar]