Abstract
The purpose of this work was to introduce a new concept of coated pellets containing chitosan microspheres loaded with didadosine for oral administration, aiming at reducing the frequency of administration and improving the bioavailability by a suitable release profile. Chitosan microspheres were produced under fluidized bed, followed by extrusion and spheronization to obtain pellets with a mean diameter of about 1 mm. The pellets were then coated with Kollidon® VA64 and Kollicoat® MAE100P in water dispersion to depict a sustained release profile. Conventional hard gelatine capsules were loaded with these pellets and tested in vitro for their release profile of didadosine. Dissolution testing confirmed that chitosan microsphere pellets provides appropriate sustained release up to 2 h behavior for didanosine.
Keywords: Didanosine, Pellets, Gastro-resistance, Coating, Microspheres, Chitosan
1. Introduction
Didanosine is a drug commonly used in acquired immune deficiency syndrome (AIDS) therapy [1], [2]. Although it may suffer hydrolysis in acid pH, which consequently produces hypoxanthine, concomitant administration of didanosine with food may still compromise the stability of the drug due to the acid pH generated by the digestion process. In general, the free drug is administered in buffered tablets to prevent its deactivation when exposed to the low pH of the stomach. The tablet formulation requires the addition of 50% of carbonate or magnesium hydroxide to obtain the buffering effect. However, some collateral effects are referred, such as diarrhea and renal problems, and may also show wide inter-individual bioavailability [3], [4]. Furthermore, the buffered tablets are large; being therefore, difficult to be administered orally. Great benefits in didanosine administration were obtained in 2001 with the commercial formulation Videx® EC composed of gastro-resistant granules. However, Videx® EC still requires frequent administration and lacks in controlled release profile. Furthermore, the lower bioavailability makes didanosine a good candidate for extended release formulations. For pharmacokinetic analysis of didanosine in vivo, we have recently published our first attempt in validating an automated system using on-line solid extraction and high performance liquid chromatography (HPLC) with ultraviolet (UV) detection [5].
In recent years, a continuous interest has been focused on the development of controlled drug release formulations using multiparticulate systems (e.g., pellets), offering various advantages over single dosage forms, namely, an improved bioavailability [6], [7] and easy administration for elderly people and for children [8]. These include a low risk of dose dumping, flexibility of blending units with different release patterns, reproducible gastric residence time, and prevention of high local drug concentration in the gastrointestinal tract [9], [10]. Because of instability of didanosine in acid pH, sustained release pellets have been frequently obtained using different types of coatings [11].
The purpose of this work was to introduce a new concept of coated pellets produced from chitosan microspheres loaded with didadosine. Pellets were coated with different types of formulations of polymers (Kollicoat® MAE100P, Kollidon® VA64) and analyzed for their in vitro gastro-resistance after encapsulation in hard capsules.
2. Materials and methods
2.1. Materials
Didanosine was provided by Labogen Química Fina e Biotecnologia S.A. (Indaiatuba, Brazil); Kollicoat® MAE100P and Kollidon® VA64 were received as gifts from BASF (São Paulo, Brazil); Acetic acid and sodium tripolyphosphate (TPP) were purchased from Synth (São Paulo, Brazil). Chitosan, of MW 296.6 kDa (approx.) and deacetylation degree 82.83±3.63%, was obtained from Polymar (Fortaleza, Brazil). Microcrystalline cellulose was obtained by Henrifarma (São Paulo, Brazil). Distilled water was purified by a Milli-Q system Millipore® (São Paulo, Brazil).
2.2. Methods
2.2.1. Preparation of chitosan microspheres
Chitosan microspheres were prepared by ionotropic gelation of chitosan and sodium tripolyphosphate (TPP) as cross-linking agent, according to Santana et al. [12]. Briefly, the microspheres were formed by dropping an aqueous solution composed of TPP (2 g/L), magnesium hydroxide (6 g/L) and didanosine (39 g/L), to a chitosan aqueous solution (2.0% w/v) previously acidified with acetic acid. The solutions were mixed under stirring (2000 rpm) at 25 °C [5], [13], [14].
2.2.2. Preparation of uncoated pellets
The didanosine-loaded microspheres composed of chitosan, as excipients, at a concentration of 4.8% (m/m) [13] were mixed and the granulation was carried out by fluidized bed or extrusion/spheronization. The dry mass was processed by one of the following methods: (a) fluidized bed (Hüttlin Instruments Company, model Microlab, Germany) with the following technical parameters: volumetric flow of air 6 m3/h, inlet air temperature 50 °C, temperature of pellets 40 °C, flow pressure of 0.9 bar, with the microclimate of 0.30 bar (internal pressure in fluidized bed); or (b) passed through a single screw extruder (Caleva Instruments Company, model extruder 20, UK), with a 1.2 mm screen at 20 rpm. The extrudates were processed in a spheronizer (Spheronizer 250, Caleva Instruments Company, UK) fitted with a cross-hatched plate rotated at 100 rpm for about 5 min. The obtained pellets in method (a) or (b) were dried at 40 °C for 2 h in a fluidized bed and sized by passing vibrating sieves (ELML digital plus, Haver & Boecker, Brazil) through a 0.250 mm mesh sieve, during 3 min, 7 s intervals and amplitude 1.
2.2.3. Preparation of coated pellets
The sub-coating and outer-coating solution was prepared using Kollidon® VA64 and Kollicoat® MAE100P, respectively, and according to Table 1. The pellets (40 g) were coated in a fluidized bed with the following technical parameters: volumetric flow of air 6 m3/h, inlet air temperature 50 °C, temperature of pellets 40 °C, flow pressure of 0.9 bar, with the microclimate of 0.30 bar (internal pressure in fluidized bed). The coating dispersions were applied at a rate of 0.5 g/min with the aid of a peristaltic pump (model 323, Watson Marlow, Brazil). The minimum fluidization was calculated using the sphericity of 0.85, diameter of 100 mm, fluid viscosity of 1.927×10−5 kg/ms, fluid density 10.95 kg/m3, bed porosity of 0.216, bed height of 0.4 m and the pressure fluid through the bed of 4559.625 kg/m s2.
Table 1.
Composition of sub-coating and outer-coating solutions used for the production of pellets (% m/v).
| Coatings | Composition | 1* | 2* | 3* | 4* | 5** | 6** | 7** |
|---|---|---|---|---|---|---|---|---|
| Sub-coating | Kollidon® VA64 (%) | 5 | – | 5 | 10 | 10 | 10 | 10 |
| Water (mL) | 30 | – | 60 | 100 | 30 | 30 | 30 | |
| Outer-coating | Kollicoat® MAE 100 P (%) | 10 | 20 | 30 | 20 | 10 | 20 | 30 |
| Propylene glycol (%) | 2.25 | 2.25 | 2.25 | 2.5 | 1.5 | 1.5 | 1.5 | |
| Magnesium trisilicate (%) | 4 | 4 | 4 | 4 | 0.5 | 0.5 | 0.5 | |
| Titanium dioxide (%) | – | – | – | 2 | 0.5 | 0.5 | 0.5 | |
| Alcohol (mL) | – | – | – | – | 100 | 100 | 100 | |
| Water (mL) | 30 | 50 | 60 | 100 | ||||
Pellets produced by fluidized bed.
Pellets produced by extrusion/spheronization.
2.2.4. Characterization of the polymeric coating
The polymeric coatings Kollidon® VA64 and Kollicoat® MAE100P were characterized by surface tension, contact angle and work of adhesion. To assess the surface tension of the coatings, the automatic tensiometer (Future Digital Scientific, OCA-20, USA) was used. The strain was determined from a drop of fluid formed within a glass bottle and its image was projected through a camera, through a specific software, measuring the drop and providing the surface tension. The contact angle measurements were performed by optical method using a manual goniometer (Tantec, Germany). The method consists of depositing a drop of suspension on a flat surface to determine the contact angle. The light focuses on that drop, projecting on a screen graduate. Chitosan microspheres were pressed into a stainless steel flask with the aid of a hydraulic press using 2 t forming a disk. Measurements were performed at 25 °C, 65% relative humidity, and in three different ways: (1) Deposition of Kollidon® VA64 solution directly on the disk; (2) Deposition of Kollicoat® MAE 100 P solution directly on the disk; and (3) Coating the disk press with Kollidon® VA64, following the drying of Kollicoat® MAE100P. The work of adhesion was determined using the Neumann and Good equation [15].
where σLV is the surface tension between liquid and gas phases and θ is the angle of contact. High values indicate a good WAD wetness for each set solid–liquid.
2.2.5. Characterization of coated pellets
The granules were characterized by determining the growth, morphological and geometrical parameters. The geometric parameters were determined by analyzing digital photographs of pellets scattered on a black surface, obtained with a digital camera SONY Cyber-shot 5 megapixel camera. The photographs were processed by UTHSCSA Image Tool 3.0 program. The determined geometric parameters were the area, perimeter and elongation. Sphericity and Feret diameter were determined applying the following equations [16]:
where Ar and Pm stand, respectively, for the area and perimeter of the microspheres. For the morphologies of the external structure, a stereoscopic camera was used coupled with fiber optic lighting (Motic Industrial Instruments, SMZ-168, USA).
2.2.6. Dissolution test
Dissolution testing of the pellets was performed using USP28-NF26 Apparatus II (paddle) at 75 rpm starting with 750 mL solution at 37±0.5 °C. The preparation method of the dissolution medium was as follows. Firstly, pellets were placed in a 0.1 M HCl solution for 120 min and samples were collected every 30 min repeated to 120 min. Then, 250 mL of 0.05 M phosphate buffer was added and pH was adjusted with a solution sodium hydroxide (NaOH) to pH 6.8. Samples were collected every 30 min up to 120 min. The samples were analyzed at 248 nm (UV-7504, UV/vis spectrophotometer, Vankel Instrument Company, USA), selected on the basis of the obtained highest peak absorbance.
3. Results and discussion
Porosity at minimum fluidization was calculated to fall pressure in the fluidized bed of 5%, and 40 cm of minimum fluidization height. The difference between the real and fluid density was 1481.905 kg/m3, and gravity was 9.81 m/s2. A porosity of 21.6% was obtained, demonstrating the easiness of fluidization for this type of microspheres.
The values obtained by measuring the surface tension of the Kollidon® VA64 and Kollicoat® MAE100P coatings were, respectively, 49.2 mN/m and 34.8 mN/m. Determination of contact angle (representing the mean value of 10 measures) of polymers Kollidon® VA64, Kollicoat® MAE100P, and Kollidon® VA64/Kollicoat® MAE100P were, respectively, 41.80°±1.75, 28.33°±4.63, and 37.60°±3.23.
For the contact angle, the obtained values were below 90°, showing the wetting properties of the disk in all cases. Comparing the two methodologies employed for Kollicoat® MAE100P, the contact angle was greater than those sub-coated with Kollidon® VA64, reflecting lower water absorption due to the decreased superficial energy. The work of adhesion calculated from Kollidon ® VA64 and Kollicoat® MAE100P was, respectively, 86.29 mN/m and 65.42 mN/m. This value was 83.27% of the maximum work of adhesion (W=78.56 mN/m) corresponding to 0°, where complete spreading occurs.
For the formulations 1, 2, 3 and 4 (Table 1), it was not possible to determine the growth of microspheres due to the formation of clusters, whereas for the formulations 5, 6 and 7, the growth was, respectively, 10, 20 and 30% based on the microspheres size without coating. Table 2 shows the geometric parameters determined for the coated pellets, analyzing elongation data. The results approaching the reference (Videx® EC) were obtained for formulation 4. The sphericity values obtained were higher than 0.80, and were considered satisfactory [17], [18]. The Feret diameters were lower in comparison to the value considered suitable (i.e. 1 mm) for filling hard gelatine capsules [19], [20].
Table 2.
Geometric parameters of pellets produced by fluidized bed (Formulations 1*–4*) and by extrusion/spheronization (Formulations 5**–7**) in comparison to a commercial formulation.
| Formulation | Elongation | Sphericity | Feret diameter (mm) |
|---|---|---|---|
| 1* | 1.70±0.82 | 0.90±0.24 | 0.25±0.18 |
| 2* | 1.58±0.71 | 0.92±0.25 | 0.19±0.16 |
| 3* | 1.63±0.72 | 0.81±0.20 | 0.70±0.38 |
| 4* | 1.48±0.46 | 0.81±0.28 | 0.49±0.29 |
| 5** | 1.23±0.26 | 0.76±0.11 | 1.11±0.16 |
| 6** | 1.45±1.20 | 0.66±0.17 | 1.28±0.48 |
| 7** | 1.25±0.48 | 0.74±0.15 | 1.05±0.26 |
| Videx® EC | 1.43±0.31 | 0.86±0.10 | 1.52±0.28 |
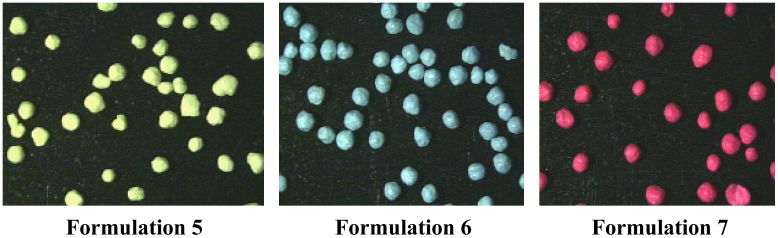
Formulations 1, 2, 3 and 4 showed irregular surface, which has been attributed to the pellets obtained with low adherence of the polymer on the surface pellets, due to the irregular geometry. Fig. 1 shows the pellets obtained with the formulations 5, 6 and 7.
Figure 1.
Photographs of pellets obtained by stereoscopy.
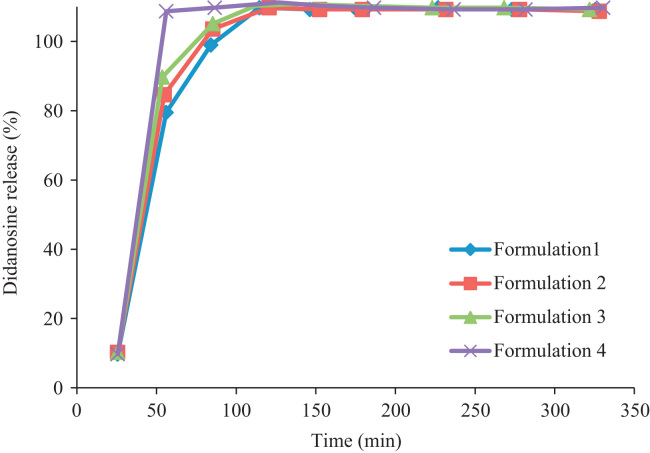
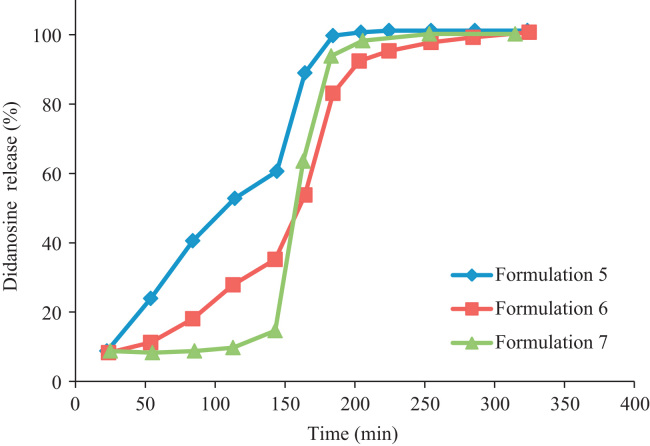
The drug release profiles of formulations 1, 2, 3 and 4 are depicted in Fig. 2 showing a burst didanosine release. Those obtained with formulations 5, 6 and 7 depicted a controlled release and gastro-resistant properties (Fig. 3), possibly attributed to the type of solvent used. For the formulations 1, 2, 3, 4 water was used to solubilize Kollicoat ® MAE 100 P, while for 5, 6, 7 alcohol was used. That prevented the agglomeration of the pellets and the formation of a complete and uniform coverage.
Figure 2.
Release profile of didanosine vs time from hard gelatine capsules containing pellets produced by fluidized bed (Formulations 1–4).
Figure 3.
Release profiles of didanosine vs time from hard gelatine capsules containing pellets produced by extrusion/spheronization (Formulations 5–7).
4. Conclusions
Controlled release and gastro-resistant pellets loading didanosine were successfully prepared using microspheres of chitosan and coating of polymers by fluidized bed following extrusion/spheronization. Results showed satisfactory morphological and dissolution properties for didanosine and this technology can be useful for other drugs, promoting higher compliance to HIV patients. The pellets have suitable shape and appropriate hardness for the coating process and may be suitable for subsequent in vivo experiments.
Acknowledgments
The authors acknowledge the financial support from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP/Brazil) and the Conselho Nacional de Pesquisa (CNPq, Brazil). The authors also wish to acknowledge Fundação para a Ciência e Tecnologia do Ministério da Ciência e Tecnologia, under the reference ERA-Eula/0002/2009.
Contributor Information
Eliana B. Souto, Email: eliana@ufp.edu.pt.
Maria H.A. Santana, Email: lena@feq.unicamp.br, souto.eliana@gmail.com.
References
- 1.Pasqualone F. Bristol-Myers Squibb clarification on didanosine supply. Lancet. 2010;376:1054. doi: 10.1016/S0140-6736(10)61488-2. [DOI] [PubMed] [Google Scholar]
- 2.Velasque L.S., Estrela R.C., Suarez-Kurtz G. A new model for the population pharmacokinetics of didanosine in healthy subjects. Braz. J. Med. Biol. Res. 2007;40:97–104. doi: 10.1590/s0100-879x2007000100013. [DOI] [PubMed] [Google Scholar]
- 3.DePestel D.D., Kazanjian P.H., Cinti S.K. Magnitude and duration of elevated gastric pH in patients infected with human immunodeficiency virus after administration of chewable, dispersible, buffered didanosine tablets. Pharmacotherapy. 2004;24:1539–1545. doi: 10.1592/phco.24.16.1539.50959. [DOI] [PubMed] [Google Scholar]
- 4.Lin M.Y., Lin S.J., Chan L.C. Impact of food and antacids on the pharmacokinetics of anti-tuberculosis drugs: systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2010;14:806–818. [PubMed] [Google Scholar]
- 5.Severino P., Silva H., Souto E.B. Analysis of in vivo absorption of didanosine-loaded chitosan microspheres based pellets in male adult dogs by HPLC. J. Pharm. Anal. 2011 doi: 10.1016/j.jpha.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdul S., Chandewar A.V., Jaiswal S.B. A flexible technology for modified-release drugs: multiple-unit pellet system (MUPS) J. Control. Release. 2010;147:2–16. doi: 10.1016/j.jconrel.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Mohamad A., Dashevsky A. Development of pulsatile multiparticulate drug delivery system coated with aqueous dispersion Aquacoat ECD. J. Pharm. 2006;318:124–131. doi: 10.1016/j.ijpharm.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Varum F.J., Merchant H.A., Basit A.W. Oral modified-release formulations in motion: the relationship between gastrointestinal transit and drug absorption. Int. J. Pharm. 2011;395:26–36. doi: 10.1016/j.ijpharm.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 9.Pan X., Chen M., Han K. Novel compaction techniques with pellet-containing granules. Eur. J. Pharm. Biopharm. 2010;75:436–442. doi: 10.1016/j.ejpb.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Tuleu C., Andrieux C., Boy P. Gastrointestinal transit of pellets in rats: effect of size and density. Int. J. Pharm. 1999;180:123–131. doi: 10.1016/s0378-5173(98)00400-1. [DOI] [PubMed] [Google Scholar]
- 11.Fu J., Wang X., Xu L. Preparation and in vitro-in vivo evaluation of double layer coated and matrix sustained release pellet formulations of diclofenac potassium. Int. J. Pharm. 2011;406:84–90. doi: 10.1016/j.ijpharm.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 12.M.H. Santana, C. Da Silva, F. Martins, Mucoadhesive granules containing chitosan nano-and/or microspheres and process for obtaining said mucoadhesive granules, in: PCT/BR2008/000123 (Ed.), 2008.
- 13.da Silva C.F., Severino P., Martins F. The intestinal permeation of didanosine from granules containing microspheres using the everted gut sac model. J. Microencapsul. 2009;26:523–528. doi: 10.1080/02652040802466691. [DOI] [PubMed] [Google Scholar]
- 14.da Silva C.F., Severino P., Martins F. Didanosine-loaded chitosan microspheres: optimization of fabrication process. Lat. Am. J. Pharm. 2011;30:1037–1040. [Google Scholar]
- 15.A.W. Neumann, R.J. Good, Techniques of measuring contact angles, in: R.J. Good, R.R. Stromberg (Eds.), Surface and Colloid Science, vol. 11, Plenum Press, New York, 1979, pp. 31–91.
- 16.M.E. Aulton, Pharmaceutics: The science of dosage form design, in: Johen Fell (Eds.), Surface and Interfacial Phenomena, 2nd ed., Churchill Livingstone, Livingstone, 2010, pp. 59–70.
- 17.Pauli-Bruns A., Knop K., Lippold B.C. Preparation of sustained release matrix pellets by melt agglomeration in the fluidized bed: influence of formulation variables and modelling of agglomerate growth. Eur. J. Pharm. Biopharm. 2010;74:503–512. doi: 10.1016/j.ejpb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Gajdziok J., Bernatoniene J., Muselik J. The evaluation of formulations for the preparation of new formula pellets. Pharm. Dev. Technol. 2011;16:520–528. doi: 10.3109/10837450.2010.502174. [DOI] [PubMed] [Google Scholar]
- 19.Podczeck F., Rahman S.R., Newton J.M. Evaluation of a standardised procedure to assess the shape of pellets using image analysis. Int. J. Pharm. 1999;192:123–138. doi: 10.1016/s0378-5173(99)00302-6. [DOI] [PubMed] [Google Scholar]
- 20.Dey N.S., Majumdar S., Rao M.E.D. Multiparticulate drug delivery systems for controlled release. Trop. J. Pharm. Res. 2008;7:1067–1075. [Google Scholar]