Abstract
A stability-indicating high-performance liquid chromatographic method was developed and validated for the determination of Letrozole in tablet dosage forms. Reversed-phase chromatography was performed on Shimadzu Model LC-Class-Vp with Lichrocart/Lichrosphere 100 C-18 (250 mm×4.6 mm, 5 μm particle size) column with methanol: tetra butyl ammonium hydrogen sulfate (80:20V/V) as mobile phase at a flow rate of 1 mL/min with UV detection at 240 nm. Linearity was observed in the concentration range of 0.5–150 μg/mL (R2=0.9998) with regression equation y=102582x+43185. The limit of quantitation (LOQ) and limit of detection (LOD) were found to be 0.043 and 0.012 μg/mL respectively. The forced degradation studies were performed by using HCl, NaOH, H2O2, thermal and UV radiation. Letrozole is more sensitive towards alkaline conditions and very much resistant towards acidic, oxidative and photolytic degradations. The method was validated as per ICH guidelines. The RSD for intra-day (0.78–0.97) and inter-day (0.86–0.96) precision were found to be lesser than 1%. The percentage recovery was in good agreement with the labeled amount in the pharmaceutical formulations and the method is simple, specific, precise and accurate for the determination of Letrozole in pharmaceutical formulations.
Keywords: Letrozole, Liquid chromatography, Stability-indicating
1. Introduction
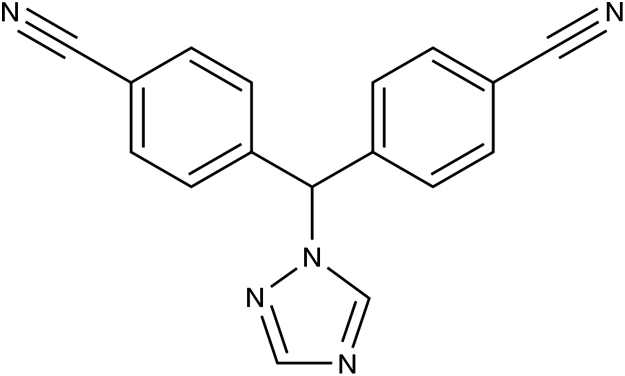
Letrozole (Fig. 1), chemically known as 4-[(4-cyanophenyl)-(1, 2, 4-triazol-1-yl) methyl] benzonitrile [1], is used for the treatment of estrogen-dependent breast cancers [2]. It is an oral non-steroidal aromatase inhibitor that has been introduced for the adjuvant treatment of hormonally-responsive breast cancer. It is readily and completely absorbed from the gastrointestinal tract. It is slowly metabolized in the liver to an inactive carbinol metabolite, which is then excreted as glucoronide in the urine [3].
Figure 1.
Chemical structure of Letrozole.
Analytical methods for Letrozole from pharmaceutical dosage form should be developed and validated. To date, all analytical methods described in literature for the determination of Letrozole in biological and other matrices involve spectrophotometry [4], [5], liquid chromatography [6], [7], [8], [9], [10], [11], the microarray approach [12], capillary gas chromatographic method with flame ionization detector [13] and gas chromatography–mass spectrometry [14] methods. Ping et al. determined the related substances in Letrozole and in its tablet dosage forms using HPLC and TLC [15] methods.
Very few methods are reported in the literature and no stability indicating method is available in the official compendia using HPLC for analyzing of Letrozole in dosage forms. Quality control of pharmaceutical products requires identification and quantification of the active ingredient and its impurities for safety and efficacy reasons. Impurities and potential degradation products that may exist in medicines can change the chemical, pharmacological and toxicological properties of the product.
The reported methods in the literature suffer from one or the other disadvantage such as poor sensitivity, very narrow linearity range, scrupulous control of experimental variables, etc. Since pharmacopoeias do not describe a suitable method for the determination of Letrozole in pharmaceutical formulations, in the present work we developed simple, rapid and accurate reverse phase liquid chromatographic method for the determination of Letrozole tablets as an alternative method. Apart from this, it can be used for assays of Letrozole in biological fluids or in pharmacokinetic investigations.
2. Experimental
2.1. Chemicals and solutions
Letrozole standard (purity 99.80%) was obtained from Shantha Biotech (India). Methanol (HPLC grade), tetra butyl ammonium hydrogen sulfate (TBAHS), sodium hydroxide (NaOH) and hydrochloric acid (HCl) and hydrogen peroxide (H2O2) were obtained from Merck (India). HPLC grade water was obtained from Gen Pure system (TKA, Germany). Letrozole is available commercially with brand names LETOCOR® (Chandrabhagat Pharma), LETORIPE® (Miracallis) as tablets with a label claim of 2.5 mg drug was purchased commercially. All chemicals were of an analytical grade and used as received.
2.2. HPLC instrumentation and conditions
Chromatographic separation was achieved using a Lichrocart/Lichrosphere 100 C-18 (250 mm×4.6 mm i.d., 5 μm particle size) column of Shimadzu Model LC-Class-Vp version 6.12 SPI, equipped with UV–VIS detector Model SPD-10 A maintained at 25 °C. Isocratic elution was performed using methanol and 10 mM TBAHS (80:20, v/v). The overall run time was 10 min and the flow rate was 1.0 mL/min .20 μl of sample was injected into the HPLC system.
The mobile phase was prepared by accurately weighing and transferring 3.3954 g of TBAHS (10 mM) (pH 3.4) in to a 1000 mL volumetric flask, dissolving and diluting to volume with HPLC grade water.
Letrozole stock solution (1000 μg/mL) was prepared by accurately weighing 25 mg of Letrozole in a 25 mL amber volumetric flask and making up to volume with mobile phase. Working solutions for HPLC injections were prepared on a daily basis from the stock solution in a solvent mixture of methanol and 10 mM TBAHS (80:20, v/v) (mobile phase). Solutions were filtered through a 0.45 μm membrane filter prior to injection.
Twenty tablets were purchased from the local market, weighed and crushed to a fine powder. An aliquot of powder equivalent to the weight of 25 mg Letrozole was accurately weighed into a 25 mL volumetric flask and made up to volume with methanol. The volumetric flask was sonicated for 30 min to enable complete dissolution of Letrozole. The solution was filtered and aliquots of filtrate were transferred using a pipette into 5 mL volumetric flasks and made up to volume with mobile phase to yield a concentration of 20 μg/mL. These solutions were filtered through a 0.45 μm nylon filter before injections.
2.3. Forced degradation studies/specificity
Forced degradation studies were performed to evaluate the stability indicating properties and specificity of the method [16]. All solutions for use in stress studies were prepared at an initial concentration of 1 mg/mL of Letrozole and refluxed for 30 min at 80 °C. All samples were then diluted in mobile phase to give a final concentration of 20 μg/mL and filtered before injection.
2.3.1. Acid and alkali degradation studies
Acid decomposition was carried out in 0.1 M HCl at a concentration of 1.0 mg/mL Letrozole and after refluxation for 30 min at 80 °C the stressed sample was cooled, neutralized and diluted with mobile phase.
Similarly stress studies in alkaline conditions were conducted using a concentration of 1.0 mg/mL in 0.1 M NaOH and refluxed for 30 min at 80 °C. After cooling the solution was neutralized and diluted with mobile phase.
2.3.2. Oxidation
Solutions for oxidative stress studies were prepared using 3% H2O2 at a concentration of 1 mg/mL of Letrozole and after refluxation for 30 min at 80 °C on the thermostat the sample solution was cooled and diluted accordingly with the mobile phase.
2.3.3. Thermal degradation study
For thermal stress testing, the drug solution (1 mg/mL) was heated in thermostat at 80 °C for 30 min, cooled and used.
2.3.4. Photo stability
The drug solution (1 mg/mL) for photo stability testing was exposed to UV light for 1 h (365 nm) UV light chamber and analyzed.
2.4. Method validation
The method was validated for the following parameters: system suitability, linearity, limit of quantitation (LOQ), limit of detection (LOD), precision, accuracy, selectivity and robustness [17].
2.4.1. Precision
The intra-day precision of the assay method was evaluated by carrying out 9 independent assays of a test sample of Letrozole at three concentration levels (10, 20 and 50 μg/mL) (n=3) against a qualified reference standard. The % RSD of three obtained assay values at three different concentration levels was calculated.
The inter–day precision study was performed on three different days i.e. day 1, day 2 and day 3 at three different concentration levels (10, 20 and 50 μg/mL) and each value is the average of three determinations (n=3). The % RSD of three obtained assay values on three different days was calculated.
2.4.2. LOQ and LOD
The LOQ and LOD were based on the standard deviation of the response and the slope of the constructed calibration curve (n=3), as described in International Conference on Harmonization guidelines Q2 (R1) [17].
2.4.3. Linearity
Linearity test solutions for the assay method were prepared from a stock solution at eleven concentration levels of the assay analyte concentration (0.5, 1, 2, 5, 10, 20, 50, 80, 100, 120 and 150 μg/mL). 20 μL of each solution was injected into the HPLC system and the peak area of the chromatogram obtained was noted.
The solutions extracted from the marketed formulations were injected into the HPLC system and the peak area of the chromatograms was noted. The analytical curve was evaluated on three different days. The peak area vs. concentration data was analyzed with least squares linear regression. The slope and y-intercept of the calibration curve was reported.
2.4.4. Accuracy
The accuracy of the assay method was evaluated in triplicate at three concentration levels (80, 100 and 120%), and the percentage recoveries were calculated. Standard addition and recovery experiments were conducted to determine the accuracy of the method for the quantification of Letrozole in the drug product. The study was carried out in triplicate at 36, 40 and 44 μg/mL. The percentage recovery in each case was calculated.
2.4.5. Robustness
The robustness of the assay method was established by introducing small changes in the HPLC conditions which included wavelength (238 and 242 nm), percentage of methanol in the mobile phase (82% and 78%), flow rate (0.9 and 1.1 mL/min) and pH (3.3 and 3.5). Robustness of the method was studied using six replicates at a concentration level of 20 μg/mL of Letrozole.
2.4.6. Solution stability and mobile phase stability
The solution stability of Letrozole in the assay method was carried out by leaving both the sample and reference standard solutions in tightly capped volumetric flasks at room temperature for 48 h. The same sample solutions were assayed at 12 h intervals over the study period. The mobile phase stability was also assessed by assaying the freshly prepared sample solutions against freshly prepared reference standard solutions at 12 h intervals up to 48 h. The prepared mobile phase remained constant during the study period. The %RSD of the Letrozole assay was calculated for the mobile phase and solution stability experiments. An additional study was carried out using the stock solution by storing it in a tightly capped volumetric flask at 4 °C.
3. Results and discussion
The present proposed method is more simple, precise and accurate in comparison to the reported methods in the literature (Table 1). The linearity range for the methods reported in the literature was narrow and some of the methods were applicable only for bioanalytical determination of Letrozole. The present developed method is more sensitive and can be used in a wide concentration range for the determination of Letrozole in pharmaceutical formulations. The complete separation of the analytes was accomplished in less than 10 min and the method has been successfully used to perform long-term and accelerate stability studies of Letrozole formulations.
Table 1.
Comparison of the performance characteristics of the present method with the published methods.
| Serial no. | Method/reagent | λ (nm) | Linearity (μg/mL) | Remarks | Ref. |
|---|---|---|---|---|---|
| 1. | Spectrophotometry | 238 | 2–20 | Very narrow range | [4] |
| 2. | Spectrophotometry | 240 | 1–10 | Very narrow range | [5] |
| 3. | HPLC/ Water:acetonitrile:methanol (50:30:20, v/v/v) | 240 | 1–50 | Narrow linearity range and use of three solvents | [6] |
| 4. | HPLC (bioanalytical method) / Acetonitrile: phosphate buffer (pH 7.0) | 230 and 295 | - | Liquid–solid extraction with fluorescence detection | [7] |
| 5. | HPLC/ Acetonitrile and phosphate buffer (pH 7.8) (70:30, v/v) | 232 | 10–100 | Limited linearity range and PDA detector used | [8] |
| 6. | HPLC (bioanalytical method)/Acetonitrile and phosphate buffer (pH 5.5) (35:65, v/v) | 230 and 295 | - | Liquid–solid extraction with fluorescence detection | [9] |
| 7. | HPLC/ Methanol:tetra butyl ammonium hydrogen sulfate (10 mM) (80:20, v/v) | 240 | 0.5–150 | Wide linearity range. No extra reagents for pH adjustment. Simple, sensitive and stability indicating method | Present work |
The present method is a stability indicating RP-HPLC method which was not reported earlier. The present stability-indicating method for the determination of Letrozole in pharmaceutical formulations is specific because the drug peak was well separated even in the presence of degradation products. Overall, the data demonstrated that the excipients and the degradation products did not interfere with the Letrozole peak, indicating the selectivity of the method.
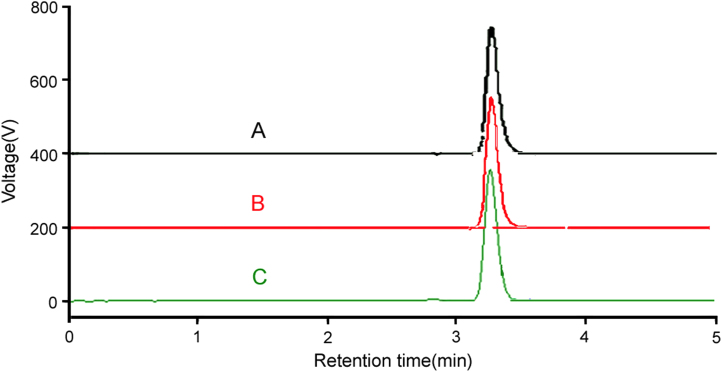
Quantification was achieved with UV detection at 240 nm based on peak area (Fig. 2A). The main objective of the chromatographic method development was to separate the degradation products which were obtained from the stress studies from the Letrozole peak. The proposed method separates Letrozole from all its degradation products. It is also possible to perform a stability study for the degradation kinetics of the drug and it permits the quantitation of Letrozole in commercial tablets.
Figure 2.
Representative chromatograms of Letrozole (20 μg/mL) [A], LETOCOR® (2.5 mg) [B], and LETORIPE® (2.5 mg) [C].
3.1. HPLC method development and optimization
Initially the stressed samples were analyzed using a mobile phase consisting of methanol:TBAHS (70:30, v/v) at a flow rate of 0.8 mL/min. Under these conditions, the resolution and peak symmetry were not satisfactory, so the mobile phase was changed to methanol: TBAHS (80:20, v/v) at a flow rate of 1 mL/min. Using these experimental conditions all peaks were well resolved with good peak shape. Therefore, a mobile phase of methanol: TBAHS (80:20, v/v) provided the best chromatographic response and was used for further studies.
3.2. Method validation
3.2.1. System suitability
The system suitability test was performed to ensure that the complete testing system was suitable for the intended application. The parameters measured were peak area, retention time, tailing factor, capacity factor and theoretical plates. In all measurements the peak area varied lesser than 2.0%, the average retention time was 3.25 min relative standard deviation (%RSD)=0.15%), the capacity factor was more than 2, theoretical plates were 4876 (more than 2000) and tailing factor was 1.24 (less than 2) for the Letrozole peak. The proposed method offers high sensitivity and Letrozole can be detected accurately. In all the cases, the Letrozole peak was well separated from the degradation products.
3.2.2. Precision
The precision of the method was determined by repeatability (intra-day precision) and intermediate precision (inter-day precision) of the Letrozole standard solutions. Repeatability was calculated by assaying three samples of each at three different concentration levels (10, 20 and 50 μg/mL) on the same day. The inter-day precision was calculated by assaying three samples of each at three different concentration levels (10, 20 and 50 μg/mL) on three different days. The % RSD range was obtained as 0.78–0.97 and 0.86–0.96 for intra-day and inter-day precision studies, respectively (Table 2).
Table 2.
Precision study of Letrozole.
| Sample no. | Conc. (μg/mL) | Intra-day precision |
Inter-day precision |
||
|---|---|---|---|---|---|
| Mean*±SD | RSD (%) | Mean*±SD | RSD (%) | ||
| 1 | 10 | 1174432±10804 | 0.92 | 1166569±10599 | 0.91 |
| 2 | 20 | 2394187±18674 | 0.78 | 2449910±21109 | 0.86 |
| 3 | 50 | 5646156±54672 | 0.97 | 5746353±55171 | 0.96 |
Mean of three replicates.
Because the stability of standard solutions can also affect the robustness of analytical methods, the stability of standard solutions of the drug substance used in this method was tested over a long period of time. One portion of a standard solution was kept at room temperature and the other portion was stored under refrigeration at approximately 4 °C and the content of these solutions was regularly compared with that of freshly prepared solutions. No change in drug concentrations was observed for solutions stored under refrigeration. But it is recommended that the sample and standard solutions must therefore, be freshly prepared in amber colored flasks to protect from light.
3.2.3. LOQ and LOD
The LOQ and LOD were determined based on the 10 and 3.3 times the standard deviation of the response, respectively, divided by the slope of the calibration curve. The LOQ was found to be 0.043 μg/mL and the LOD was found to be 0.012 μg/mL.
3.2.4. Linearity
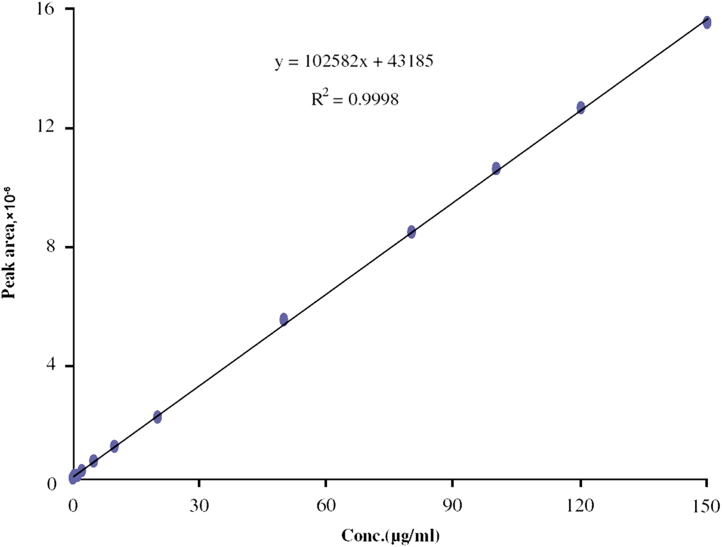
The representative chromatogram for Letrozole is shown in Fig. 2A and the chromatograms obtained from the extracted marketed formulations are shown in Fig. 2B and C. The calibration curve for Letrozole was linear over the concentration range of 0.5–150 μg/mL. The data for the peak area of Letrozole versus Letrozole concentration were treated by linear regression analysis and the correlation coefficient (R2) of 0.9998 was obtained. The regression equation for the calibration curve (Fig. 3) was found to be y=102582x+43185.
Figure 3.
Calibration curve of Letrozole.
3.2.5. Accuracy
The method accuracy was proven by the recovery test. A known amount of Letrozole standard (20 μg/mL) was added to aliquots of sample solutions (16, 20 and 24 μg/mL), and then diluted to yield total concentrations as 36, 40 and 44 μg/mL as described in Table 3. The assay was repeated (n=9) over 3 consecutive days. The resultant % RSD for this study was found to be 0.142% with a corresponding percentage recovery value of 99.51%.
Table 3.
Accuracy-recovery study of Letrozole by standard-addition method.
| Sample no. | Amount of standard Letrozole added (μg/mL) | Total found (μg/mL) | Mean recovery* (%) | Mean* RSD (%) |
|---|---|---|---|---|
| 1 | 16 | 15.93 | 99.56 | 0.1424 |
| 2 | 20 | 19.87 | 99.35 | |
| 3 | 24 | 23.91 | 99.62 |
Mean of three replicates.
3.2.6. Robustness
The robustness of an analytical procedure refers to its ability to remain unaffected by small and deliberate variations in method parameters and provides an indication of its reliability for routine analysis [16]. The robustness of the method was evaluated by assaying the same sample under different analytical conditions deliberately changing from the original condition. The detection wavelength was set at 238 and 242 nm (±2 nm), the ratio of percentage of methanol: TBAHS in the mobile phase was applied as 78:22 and 82:18 (v/v) (±2%), the flow rate was set at 0.9 and 1.1 mL/min (±0.1 mL/min) and the pH was 3.3 and 3.5 (±0.1). The results obtained from assay of the test solutions were not affected by varying the conditions and were in accordance with the results for original conditions. The % RSD value of assay determined for the same sample under original conditions and robustness conditions was less than 2.0%, indicating that the developed method was robust.
3.2.7. Selectivity/specificity
The specificity of the developed method was determined by injecting sample solutions (20 μg/mL) which were prepared by forcibly degrading under such stress conditions as heat, light, oxidative agent, acid and base under the proposed chromatographic conditions.
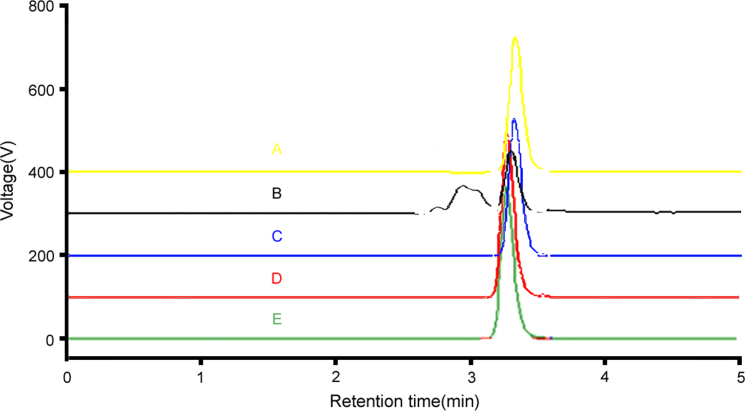
The stability indicating capability of the method was established from the separation of Letrozole peak from the degraded samples derived from the Class Vp software. The degradation of Letrozole was found to be very similar for both the tablets and standard. Typical chromatograms obtained following the assay of stressed samples are shown in Fig. 4A–E.
Figure 4.
Representative chromatograms of Letrozole (20 μg/mL) on acidic [A], alkaline [B], oxidative [C], thermal [D] and photolytic [E] degradations.
3.2.8. Solution stability and mobile phase stability
The %RSD of the assay of Letrozole from the solution stability and mobile phase stability experiments was within 2%. The results of the solution and mobile phase stability experiments confirm that the sample solutions and mobile phase used during the assays were stable up to 48 h at room temperature and up to 3 months at 4 °C.
3.2.9. Analysis of commercial formulations (tablets)
The proposed method was applied to the determination of Letrozole tablets (LETOCOR® and LETORIPE®) and the result of these assays yielded 99.84% and 99.72%, respectively, (RSD is <2.0%). The result of the assay (Table 4) indicates that the method is selective for the assay of Letrozole without interference from the excipients used in these tablets (Fig. 2B and C).
Table 4.
Analysis of Letrozole commercial formulation (tablets).
| Sample no. | Formulation | Labeled claim (mg) | Amount found* (mg) | Label claim*±SD (%) |
|---|---|---|---|---|
| 1 | LETOCOR® | 2.5 | 2.496 | 99.840±0.021 |
| 2 | LETORIPE® | 2.5 | 2.493 | 99.720±0.014 |
Mean of three values.
3.2.10. Forced degradation studies
Letrozole standard and tablet powder was found to be quite stable under dry heat conditions. A slight decomposition was seen on exposure of Letrozole drug solution to acid, oxidation and heat. On the other hand, the basic solution underwent degradation. The drug decomposition under alkaline degradation was found to be 57.9%, indicating that the drug is more sensitive under alkaline conditions. The cyano phenyl group present in the Letrozole chemical structure may be responsible for the reported alkaline degradation. Letrozole has undergone oxidative and thermal degradation slightly, i.e. 0.8% and 5.0%, respectively. The drug decomposition under acidic degradation was found to be 3.6% indicating that the drug is resistant towards acidic conditions and quite stable towards photolysis. It can be concluded that Letrozole is more resistant towards acidic, oxidative, thermal and photolytic conditions in comparison to alkaline conditions (Table 5).
Table 5.
Forced degradation studies of Letrozole.
| Stress conditions | Drug recovered* (%) | Drug decomposed* (%) |
|---|---|---|
| Standard drug | 100.0 | - |
| Acidic hydrolysis | 96.4 | 3.6 |
| Alkaline hydrolysis | 42.1 | 57.9 |
| Oxidative degradation | 99.2 | 0.8 |
| Thermal degradation | 95.0 | 5.0 |
| Photolytic degradation | 100.0 | - |
Mean of three replicates.
4. Conclusion
A stability-indicating HPLC method was developed, validated and applied for the determination of Letrozole in pharmaceutical dosage forms. The developed method was validated as per ICH guidelines and was found to be accurate, precise, robust and specific. The chromatographic elution step is undertaken in a short time (<4 min). No interference from any components of pharmaceutical dosage form or degradation products was observed and the method has been successfully used to perform long-term and accelerate stability studies of Letrozole formulations.
Acknowledgments
Authors are grateful to M/S Roland Institute of Pharmaceutical Sciences for providing research facilities and to Shantha Biotech (India) for providing gift samples of Letrozole.
References
- 1.O'Neil M.J. Whitehouse Station; NJ: 2006. The Merck Index, Merck Research Laboratories. [Google Scholar]
- 2.Lamb H.M., Adkins J.C. Letrozole a review of its use in post menopausal women with advanced breast cancer. Drugs. 1998;56(6):1125–1140. doi: 10.2165/00003495-199856060-00020. [DOI] [PubMed] [Google Scholar]
- 3.Iveson T.J., Smith I.E., Ahern J. Phase 1 study of the oral non steroidal aromatase inhibitor CGS 20267 in Healthy post menopausal women. J. Clin. Endocrinol. Metab. 1993;77:324–331. doi: 10.1210/jcem.77.2.8345035. [DOI] [PubMed] [Google Scholar]
- 4.Mondal N., Pal T.K., Ghosal S.K. Development and validation of a spectrophotometric method for estimation of Letrozole in bulk and pharmaceutical formulation. Die Pharm. 2007;62(8):597–598. [PubMed] [Google Scholar]
- 5.Ganesh M., Kamalakannan K., Patil R. A validated UV spectrophotometric method for the determination of Letrozole in bulk and solid dosage form. Rasyan J. Chem. 2008;1(1):55–58. [Google Scholar]
- 6.Mondal N., Pal T.K., Ghosal S.K. Development and validation of RP- HPLC method to determine Letrozole in different pharmaceutical formulations and its application to studies of drug release from nanoparticles. Acta Pol. Pharm. 2009;66(1):11–17. [PubMed] [Google Scholar]
- 7.Marfil F., Pineau V., Sioufi A. High-performance liquid chromatography of the aromatase inhibitor, Letrozole, and its metabolite in biological fluids with automated liquid–solid extraction and fluorescence detection. J. Chromatogr. B:Biomed. Sci. Appl. 1996;683(2):251–258. doi: 10.1016/0378-4347(96)00118-1. [DOI] [PubMed] [Google Scholar]
- 8.Laha T.K., Patnaik R.K., Sen S. Reverse phase high performance liquid chromatographic method for the analysis of Letrozole in pharmaceutical dosage forms. Indian J. Pharm. Sci. 2008;70(3):401–403. doi: 10.4103/0250-474X.43019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarghi A., Foroutan S.M., Shafaati A. HPLC determination of Letrozole in plasma using fluorescence detection: Application to pharmacokinetic studies. Chromatographia. 2007;66:747–750. [Google Scholar]
- 10.Sekar V., Jayaseelan S., Subash N. Bioanalytical method development and validation of Letrozole by RP-HPLC method. Int. J. Pharm. Res. Develop. 2009;1:1–7. [Google Scholar]
- 11.Pfister C.U., Duval M., Godbillon J. Development, application and comparison of an enzyme immunoassay and a high-performance liquid chromatography method for the determination of the aromatase inhibitor CGS 20,267 in biological fluids. J. Pharm. Sci. 1994;83:520–524. doi: 10.1002/jps.2600830415. [DOI] [PubMed] [Google Scholar]
- 12.Itoh T., Karlsberg K., Kijima I. Letrozole: A microarray Approach. Mol. Cancer Res. 2005;3:203–218. doi: 10.1158/1541-7786.MCR-04-0122. [DOI] [PubMed] [Google Scholar]
- 13.Berzas J.J., Rodriguez J., Contento A.M. Determination of drugs used in advanced breast cancer by capillary gas chromatography of pharmaceutical formulations. J. Sep. Sci. 2003;26:908–914. [Google Scholar]
- 14.Mareck U., Sigmund G., Opfermann G. Identification of the aromatase inhibitor Letrozole in urine by gas chromatography/ mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19(24):3689–3693. doi: 10.1002/rcm.2239. [DOI] [PubMed] [Google Scholar]
- 15.Ping G.U., Yuru L.I., Yuxia W.U. Determination of related sub stances in Letrozole and its tablets by TLC and HPLC. Chin. J. Pharm. 2001;7:317–318. [Google Scholar]
- 16.ICH Stability Testing of New Drug Substances and Products Q1A (R2), International Conference on Harmonization, 2003.
- 17.ICH Validation of analytical procedures: text and methodology Q2(R1), International Conference on Harmonization, 2005.