Abstract
The qualitative and quantitative analysis of active constituents in Fructus Psoraleae is presented by high-performance liquid chromatography (HPLC) coupled with different detections. Extracts of Fructus Psoraleae were examined by HPLC with ion trap mass spectrometry (IT-MS) and 18 major compounds of coumarins, benzofuran glycosides, flavonoids, and meroterpene were identified. The determination of four major constituents including bavachin, isobavachalcone, bavachinin, and bakuchiol was accomplished by HPLC with UV, MS, and electrochemical detection (ECD). These methods were evaluated for a number of validation characteristics (repeatability, LOD, calibration range, and recovery). ECD obtained a high sensitivity for analysis of the four components; MS provided a high selectivity and sensitivity for determination of bavachin, isobavachalcone, and bavachinin in negative-ion mode. After optimization of the methods, separation, identification. and quantification of the four components in Fructus Psoraleae were comprehensively tested by HPLC with UV, MS, and ECD.
Keywords: HPLC-UV, HPLC-MS, HPLC-ECD, Fructus Psoraleae, Chinese medicine
1. Introduction
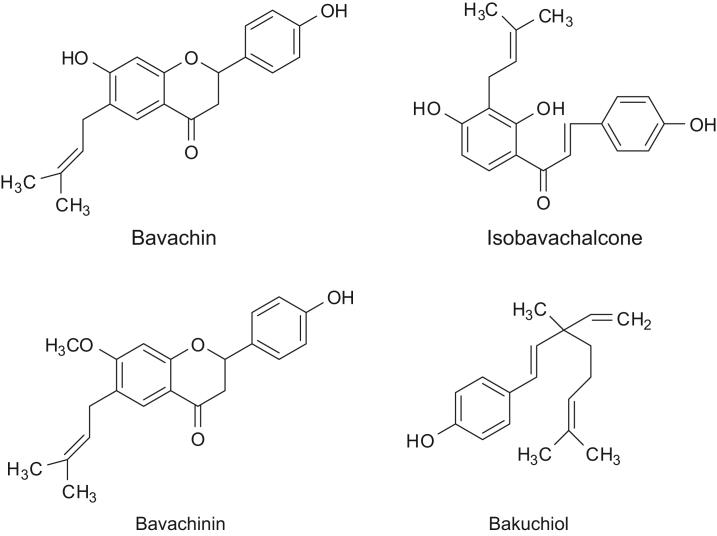
Fructus Psoraleae (Buguzhi in Chinese), the dried ripe fruit of Psoralea corylifolia L. (Fabaceae), is a well-known traditional Chinese medicine (TCM) with many beneficial effects [1]. It is traditionally used to alleviate asthma and diarrhea, and to treat vitiligo and alopecia areata in East Asian countries. The major active constituents of this herb contain coumarins, benzofuran glycosides, flavonoids, and meroterpene, such as psoralenoside, isopsoralenoside, psoralen, isopsoralen, neobavaisoflavone, bavachin, psoralidin, isobavachalcone, corylifol A, bavachinin, and bakuchiol, etc. [2], [3], [4]. Bavachin, isobavachalcone, and bavachinin are three typical flavonoid ingredients and bakuchiol is typical meroterpene in Fructus Psoraleae. Their chemical structures are shown in Fig. 1. They have functions in common such as inhibition to α-glucosidase activities [3] and anti-oxidation [5]. Clinical studies have shown that bavachin can stimulate bone formation [6] and that isobavachalcone exhibits a broad spectrum of biological activities, such as enhancing cardiac contractility, preventing cardiac fatigue due to lactic acid, and so on [7], bavachinin inhibits the accumulation of nitric oxide (NO) [8]. In addition, bakuchiol, a principal constituent, and its related compounds show a variety of bioactivities such as inhibition of monoamine transporters, immunosuppressive effect, caspase-3-depended apoptosis, hepatoprotective effect, antimicrobial activity, anti-inflammatory effect, inhibition of DNA polymerase, and topoisomerase II, cytotoxic effect, and anti-diabetic effects [9], [10].
Figure 1.
Chemical structures of bavachin, isobavachalcone, bavachinin and bakuchiol.
As we know, the active constituents in traditional Chinese medicine (TCM) are very complex so that the mechanism to cure disease is still unclear. In order to isolate and identify these active compounds in Fructus Psoraleae, many methods such as thin-layer chromatography (TLC) [11], high-performance liquid chromatography (HPLC) [12], [13], liquid chromatography-mass spectrometry (LC/MS) [14], [15], high-speed counter-current chromatography (HSCCC) [16], and micellar electrokinetic chromatography (MEKC) [17] were reported. Compared with CE, HPLC can afford better analytical precision and higher sample loading capacity. HPLC coupled to UV [12], [13] and MS detection [14], [15] is applied in the determination of the active compounds in Fructus Psoraleae. HPLC in combination with tandem mass spectrometry (MS) appears to be a more suitable technique for the identifying of the active compounds in plant samples in terms of sensitivity and selectivity.
In recent years analytical techniques have made great advances. LC-MS and LC-tandem MS (LC-MS/MS) such as quadrupole time of flight (Q-TOF) [18] and quadrupole-linear ion trap (Q-LIT) [19] have played a crucial role in plant analysis. The application of ion trap (IT) mass spectrometers in the structure elucidation and in the identification of unknowns in complex matrices has been well established [20]. In previous work, we developed an LC-IT-MS for identification and quantification of oleanolic acid and ursolic acid in nine Chinese herbs [21].
Electrochemical detection (ECD) is a powerful detection technique for HPLC separation. ECD has many advantages such as simple, rapid, inexpensive, and sensitive. HPLC coupled with ECD has been reported to analyze phenolic and flavonid compounds in natural products. Analysis of amino acid biomarkers and active constituents in Chinese medicine by HPLC-ECD has been reported in our group [22], [23].
The aim of the present work was the identification of the main active compounds in Fructus Psoraleae by HPLC-ESI-IT-MS and development of HPLC-UV, HPLC-MS, and HPLC-ECD methods for simultaneous determination and quantification of four active components (bavachin, isobavachalcone, bavachinin, and bakuchiol). The analytical characteristics of HPLC coupled with different detections have been compared and discussed.
2. Experimental
2.1. Chemicals and materials
The ripe seed of Psoralea corylifolia L. was purchased from Liuyouyu Drugstore, Wuhan (Hubei, China). Bavachin (purity≥99%), isobavachalcone (purity≥99%), bavachinin (purity≥99%), and bakuchiol (purity≥99%) were obtained from Shanghai Shunbo Bio-engineering Technology (Shanghai, China). Methanol and acetonitrile of HPLC grade were purchased from Tedia (USA) and VBS (USA), respectively. Deionized water was purified using a Milli-Q system (Millipore, Bedford, MA, USA); Helium (purity, 99.999%), and liquid nitrogen were obtained from Wuhan Analytical Instrument Factory (Wuhan, China); other reagents used in the experiments were of analytical grade and from commercial sources.
2.2. Standard solutions
The standard stock solutions of bavachin, isobavachalcone, bavachinin, and bakuchiol were prepared by dissolving 20.0 mg of each in 5 mL methanol to yield a concentration of 4.00 mg/mL, and kept at 4 °C. The bavachin, isobavachalcone, bavachinin, and bakuchiol stock solutions were diluted with methanol to obtain calibration solutions ranging from 10–1000, 20–2000, 20–2000 to 40–4000 μg/mL, respectively.
2.3. Preparation of samples
Extraction of Fructus Psoraleae was performed by using hydrochloric acid in methanol as an extraction solvent under ultrasonication. The fruit of Psoralea corylifolia L was pulverized into appropriate powder. The powder sample (1.00 g) was weighed and mixed with 10 mL extraction solvent of methanol/concentrated hydrochloric acid (5/1, v/v). The sample was subjected to ultrasound treatment at 20 °C for 45 min. Then, the sample was taken out of the ultrasonic bath and left at room temperature for 30 min. The extract was centrifuged at 3 000 g for 20 min, the supernatant was collected and diluted to 10 mL with extraction solvent and stored in a refrigerator at 4 °C.
2.4. Chromatography conditions and instrumentation
HPLC analyses were performed on a 1100 HPLC instrument (Agilent Technologies, California, USA) equipped with a binary pump, a UV detector, an autosampler, and a column thermostat. Chromatographic separations were carried out on a DL-Cl8 column (5.0 μm, 250 mm×4.6 mm, Japan) at 30 °C. Elution was performed with a flow rate of 0.5 mL/min. Acetonitrile (A) and 0.01 M formic acid (B) were used as a mobile phase. Gradient elution was used as follows: 15%–40% B (10 min), 40%–55% B (30 min), 55%–75% B (40 min), 75%–80% B (60 min), 80%–95% B (70 min). After finishing the run, the gradient was set back to 15% A and the system was allowed to equilibrate. The injection volume was 10 μL and the detection wavelength was 246 nm.
For LC-MS analysis, the Agilent 1100 HPLC system was coupled on-line to an LC/MSD Trap SL Plus spectrometer (Agilent Corp, Waldbronn, Germany) equipped with electrospray ionization (ESI) source. The Auto MS operation parameters are described as follows: negative-ion mode (ESI−); nitrogen drying gas, 10 L/min; nebulizer, 50 psi; gas temperature, 350 °C; compound stability, 80%; mass range, 50–1000 m/z. Detection of bavachin, isobavachalcone, bavachinin and bakuchiol was performed in selected ion monitoring (SIM) mode with (m/z)− 323, 323, 337 and 255, respectively.
For LC-ECD analysis, An LC-20AT system (Shimadzu, Japan) was equipped an injector with a 20-μL loop, a Sepax amethyst C18-P column (5.0 μm, 4.6 mm×250 mm; Sepax Technologies, USA), and a CHI 842B electrochemical analyzer (Shanghai Chenhua Instrument, Shanghai, China) with a thin layer radial flow cell (Shanghai Chenhua Instrument). In the ECD cell, a diameter of 1-mm glassy carbon electrode (GCE) was used as a working electrode, the stainless steel tube was used as counter electrode and an Ag/AgCl (saturated KCl) electrode was used as a reference electrode. The data acquisition and treatment is controlled from a CHI 842B electrochemical analyzer equipped with CHI software package (Shanghai Chenhua Instrument). The pH values of buffers were measured with a PHSJ-3F pH meter (Shanghai Jingmi Scientific Instrument, Shanghai, China). Acetonitrile (A) and 0.03 M acetate buffer solution with pH 5.17 (B) were used as the mobile phases. Gradient elution was employed as follows: mobile phase A started at 55% and was held for 1.0 min, mobile phase A was increased linearly to 58% A from 1.0 min to 5.0 min, and increased linearly to 62% A from 5.0 min to 10.0 min. Then, mobile phase A was raised to 68%, spent for 5 min, and increased to 72% A from 15–20 min. Finally, the gradient was up to 82% A in 30 min. After finishing the run, the gradient was set back to 55% A and the system was allowed to equilibrate. The injection volume was 20 μL. The flow rate was adjusted to 0.80 mL/min. The working electrode was set at a potential of 0.80 V versus the Ag/AgCl reference electrode [22].
The mobile phases were filtered through a 0.22 μm nylon filter membrane (Shanghai Xingya Jinghua Materials Factory, Shanghai, China) and degassed in ultrasonic bath before use.
3. Results and discussion
3.1. Identification of Fructus Psoraleae
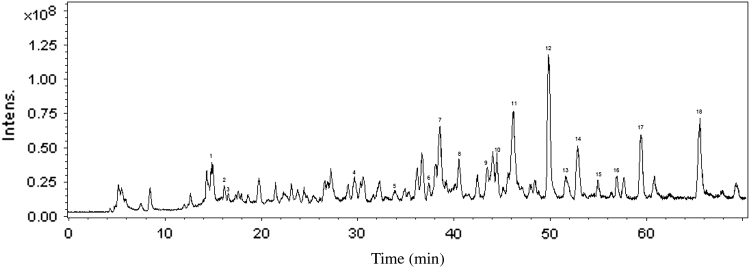
As ESI-IT-MS has been proved to be suitable tool for the identification of the active compounds, the separation of constituents in the extract of Fructus Psoraleae was performed by HPLC-UV and ESI-IT-MS in negative-ion mode. A typical total ion chromatogram (TIC) of the identified compounds with MS detection is displayed in Fig. 2, and the typical chromatograms with UV detection are shown in Fig. 3. It was observed that several peaks with MS and UV detections were composed of two or three components, but most peaks had symmetric peak shape and good resolution. The identification of components in the extracts was obtained by mass spectra, retention times, molecular weights (MW), and relative contents reported in literatures [9], [10], [11], [12], [13], [14], [15]. The peak identification of major active compounds in the extracts of Fructus Psoraleae in Fig. 2 is summarized in Table 1. In general, the identified components corresponded to about 53.9% of the total peak area in TIC chromatograms under negative-ion mode.
Figure 2.
Total ion chromatogram (TIC) of the active compounds of extract of Fructus Psoraleae by LC-MS.
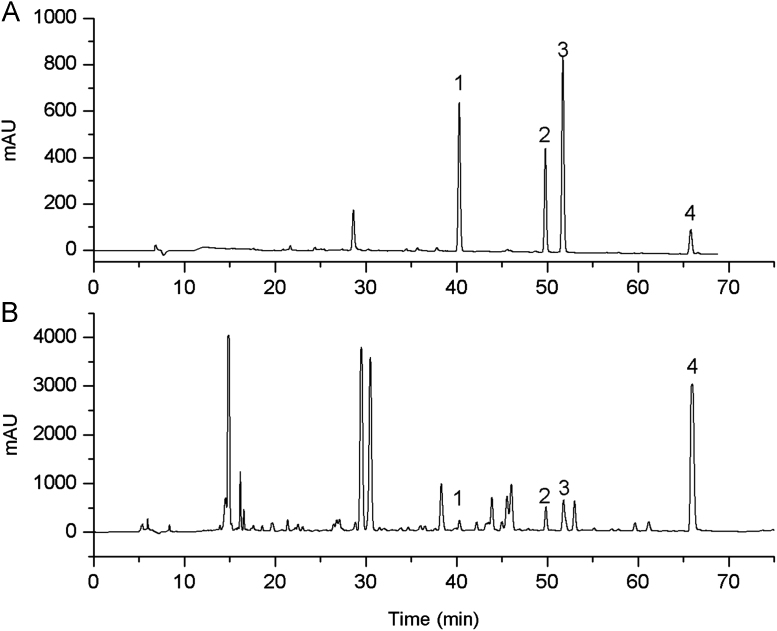
Figure 3.
Typical chromatograms of four standard analytes (A) and sample (B) by HPLC-UV. Peak identification: 1 bavachin, 2 isobavachalcone, 3 bavachinin, 4 bakuchiol.
Table 1.
The constituents and relative compound contents in Fructus Psoraleae.
| Compound | Ret. time (min) | Name | Deprotonated molecule ion [M–H]− | Fragment ions (m/z) | Formula | Relative content (%) |
|---|---|---|---|---|---|---|
| 1 | 15.0 | Psoralenoside | 365 | 337, 285, 255, 237, 191, 137, 97 | C22H38O4 | 3.0 |
| 2 | 16.2 | Isopsoralenoside | 365 | 329, 285, 189, 176, 127, 97 | C22H38O4 | 0.7 |
| 3 | 16.6 | Psoralester | 365 | 347, 269, 203, 171, 153, 97 | C22H38O4 | 0.3 |
| 4 | 29.7 | 2E-1-[2-hydroxy-4-methoxy-5(3-methyl-2-buten)phenyl]-3-(4-hydroxyphenyl)-2-prophen-1-one | 337 | 329, 314, 295, 261, 195, 176, 121 | C21H22O4 | 2.3 |
| 5 | 33.9 | Psoralen | 185 | C11H6O3 | 0.2 | |
| 6 | 37.3 | Isopsorale | 185 | C11H6O3 | 0.8 | |
| 7 | 38.6 | Neobavaisoflavone | 321 | 195, 165, 97 | C20H18O4 | 6.0 |
| 8 | 40.6 | Bavachin | 323 | 297, 287, 274, 265,177,136 | C20H20O4 | 2.4 |
| 9 | 43.9 | Corylin | 319 | 301, 285, 97,79 | C20H16O4 | 0.5 |
| 10 | 45.1 | 18-prenyldaidzein | 321 | 303, 285, 217, 194, 164, 97 | C20H18O4 | 0.2 |
| 11 | 46.1 | Psoralidin | 335 | 323, 287, 269, 194, 134, 97 | C20H16O5 | 8.3 |
| 12 | 49.8 | Isobavachalcone | 323 | 285, 279, 265, 158, 97 | C20H20O4 | 11.7 |
| 13 | 51.7 | Bavachinin | 337 | 325, 315, 295, 287, 114, 79 | C21H22O4 | 1.5 |
| 14 | 52.8 | Corylifol A | 389 | 353, 311, 295, 176, 97 | C25H26O4 | 4.6 |
| 15 | 54.9 | Prorachromene | 321 | 303, 293, 261, 177,158, 97 | C20H18O4 | 1.0 |
| 16 | 56.9 | Isobavachromene | 321 | 293, 285, 177, 97 | C20H18O4 | 0.4 |
| 17 | 59.4 | 4-O-methylbavachalcone | 337 | 315, 261, 177, 97 | C21H22O4 | 5.6 |
| 18 | 65.5 | Bakuchiol | 255 | 239, 225, 187, 165, 97 | C18H24O | 4.4 |
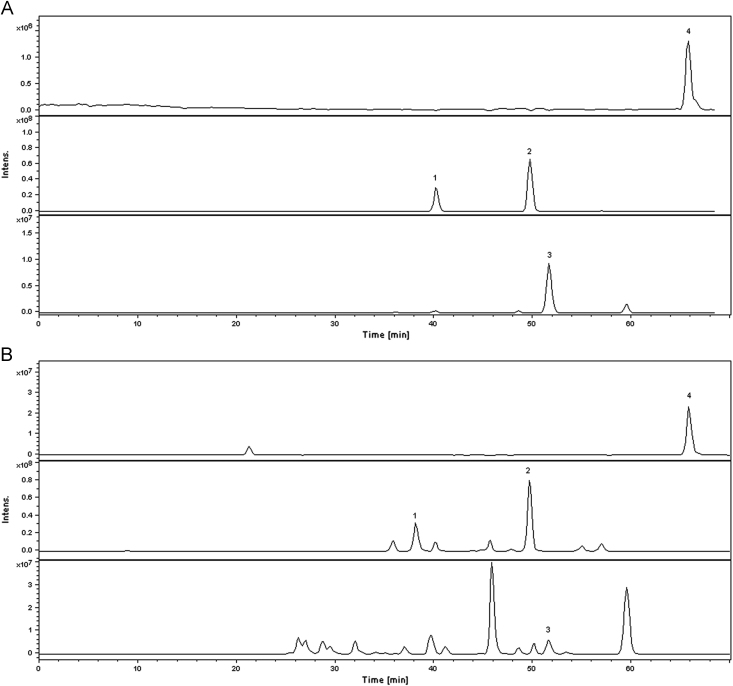
It was observed that there were many isomers in the extracts of Fructus Psoraleae, for example, three compounds (1–3) with identical [M–H]− at 365, four compounds (7,10,15,16) with identical [M–H]− at 321, and two compounds (8, 13) with identical [M–H]− at 323. In order to indentify these compounds, the property, retention time, deprotonated molecule ion, main fragment ion and relative content reported in literatures [9], [10], [11], [12], [13], [14], [15] can be taken into account. For m/z 365, compounds (1–2) have identical fragment of m/z 285 ([M–Glu–H]−), which is the characteristic fragment ion of psoralenoside and isopsoralenoside. Guo et al. had reported that the polarity of psoralenoside was higher than isopsoralenoside [24], so the peak of psoralenoside was quicker than isopsoralenoside in RP-HPLC. The typical chromatograms of bavachin, isobavachalcone, bavachinin, and bakuchiol with HPLC-UV and HPLC-MS are shown in Figure 3, Figure 4. The deprotonated molecule ions ([M–H]−) are m/z 323, 323, 337, and 255 and the retention time is 40.5, 49.8, 51.7, and 65.5 min, respectively. Compounds (8, 12, 13, 18) are bavachin, isobavachalcone, bavachinin, and bakuchiol, respectively. Compounds (7, 10, 15, 16) with the same deprotonated molecule ions ([M–H]−) are m/z 321. Compounds (10, 15) have the characteristic fragment ion m/z 303(M–H–H2O]−), and compound (10) further loses H2O form the fragment ion m/z 285. It suggests the compound (10) is 18-prenyldaidzein and compound (15) is prorachromene. According to relative polarity of compounds and the mass spectra, we can draw a conclusion that the compounds (7, 16) are neobavaisoflavone and isobavachromene, respectively. It is agreement with the analysis in the literature [25]. The deprotonated molecule ion ([M–H]−) was m/z 185, which was fragment ions of the Coumadin's isomeric compounds (psoralen and isopsorale). At last, we validated the compounds (5, 6) were psoralen and isopsorale, respectively [26]. For the other compounds (9, 11, 14, 17), their deprotonated molecule ions ([M–H]−) were m/z 319, 335, 389 and 337, respectively. According to the retention times, deprotonated molecule ion, main fragment ions and relative content reported in literatures [14], [15], [25], [26], we can make a conclusion that compounds (9, 11, 14, 17) are corylin, psoralidin, corylifol A, and 4–O–methylbavachalcone, respectively.
Figure 4.
Typical total ion chromatograms of four standard analytes (A) and sample (B) by HPLC-MS in the negative-ion ion mode by selected ion monitoring. Peak identification: 1 bavachin, 2 isobavachalcone, 3 bavachinin, 4 bakuchiol.
The main compositions of compounds in the extracts of Fructus Psoraleae were identified as (i) flavonoids and isoflavonoids (37.6%); (ii) coumarins (3.3%); (iii) benzofuran glycosides (4.0%); and (iv) meroterpene (bakuchiol, 4.4%). The flavonoids and isoflavonoids are the major active components, including neobavaisoflavone, bavachin, corylin, 18-prenyldaidzein, psoralidin, isobavachalcone, bavachinin, corylifol A, prorachromene, and isobavachromene. However, by HPLC-MS, it is impossible to identify all of the compounds in the extracts of Fructus Psoraleae. Isolation and identification of chemical components from Fructus Psoraleae need further work.
3.2. Quantitative analysis by HPLC-UV, HPLC-MS, and HPLC-ECD
3.2.1. Linearity and the detection limits (LODs)
Detector response (relative peak area) was linearly dependent on sample concentration over the range 10–1000 μg/mL for bavachin, 20–2000 μg/mL for isobavachalcone and bavachinin, and 40–4000 μg/mL for bakuchiol. The typical chromatograms of analytes with different detection methods are shown in Figure 3, Figure 4, Figure 5. Linear calibration curves were obtained for standard analytes at different concentration levels. The characteristics of the calibration plots are summarized in Table 2. As it can be seen, the four compounds show excellent correlation coefficients and sensitivity in all analytical methods.
Figure 5.
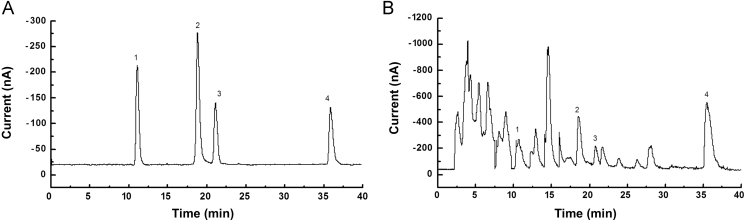
Typical chromatograms of four standard analytes (A) and sample (B) by HPLC-ECD. Peak identification: 1 bavachin, 2 isobavachalcone, 3 bavachinin, 4 bakuchiol.
Table 2.
Calibration curve and limits of detection of bavachin, isobavachalcone, bavachinin and bakuchiol in Fructus Psoraleae by HPLC-UV, HPLC-MS, and HPLC-ECD.
| Method | Compound | Calibration curve | R2 | Line arrange (μg/mL) | limits of detection (LOD) (ng/mL) |
|---|---|---|---|---|---|
| UV | Bavachin | y=2.961x+75.30 | 0.998 | 10–1,000 | 50 |
| Isobavachalcone | y=1.699x+34.16 | 0.993 | 20–2,000 | 50 | |
| Bavachinin | y=2.437x+46.27 | 0.995 | 20–2,000 | 100 | |
| Bakuchiol | y=1.436x+22.95 | 0.997 | 40–4,000 | 100 | |
| MS | Bavachin | y=68315x+2×107 | 0.990 | 10–1,000 | 0.50 |
| Isobavachalcone | y=74497x+3×107 | 0.997 | 20–2,000 | 0.50 | |
| Bavachinin | y=11,925x+5×106 | 0.991 | 20–2,000 | 10 | |
| Bakuchiol | y=33,031x+26,342 | 0.992 | 40–4,000 | 500 | |
| ECD | Bavachin | y=0.345x+0.889y | 0.996 | 10–1,000 | 2.86 |
| Isobavachalcone | y=1.051x+0.507 | 0.998 | 20–2,000 | 0.30 | |
| Bavachinin | y=0.210x+1.039 | 0.997 | 20–2,000 | 1.50 | |
| Bakuchiol | y=0.921x+0.476 | 0.998 | 40–4,000 | 18.15 | |
The limits of detection (LODs) were determined by the signal-to-noise (S/N) ratio of 3. The LODs of bavachin, isobavachalcone, bavachinin, and bakuchiol were determined to be 50, 50, 100,100 ng/mL for HPLC-UV, 0.5, 0.5, 10, 500 ng/mL for HPLC-MS, and 2.86, 0.3, 1.50, 18.15 ng/mL for HPLC-ECD, respectively. In general, LODs of bavachin, isobavachalcone, and bavachinin by HPLC-MS and HPLC-ECD were higher than those by HPLC-UV. However, LOD of bakuchiol by HPLC-MS was lower in negative-ion mode than that in positive-ion mode.
3.2.2. Precision
To carry out this precision study, different solutions containing bavachin (100 μg/mL), isobavachalcone (200 μg/mL), bavachinin (200 μg/mL), and bakuchiol (400 μg/mL) were prepared and analyzed on three different days by using current method.
Repeatability was investigated by sequentially injecting a series of six standards in solutions. Precision was studied by injecting six freshly prepared mixtures, RSD values for relative peak areas are shown in Table 3 using three analytical methods. As shown in Table 3, intra-day precisions were from 1.03% to1.86%, 3.65% to 5.05%, and 2.64% to 3.53% for analytes by HPLC-UV, HPLC-MS and HPLC-ECD, respectively. And inter-day precisions were from 1.56% to 2.03%, from 5.11% to 7.08%, and from 2.73% to 4.17% for analytes by HPLC-UV, HPLC-MS, and HPLC-ECD, respectively. Although there was better repeatability by HPLC-UV than that by HPLC-MS and HPLC-ECD, the precision of the three methods did not show significant difference.
Table 3.
Precisions and recoveries of bavachin, isobavachalcone, bavachinin and bakuchiol in Fructus Psoraleae by HPLC-UV, HPLC-MS, and HPLC-ECD.
| Method | Compound | Precision |
Recovery |
||
|---|---|---|---|---|---|
| Intra-day (RSD, %) | Inter-day (RSD, %) | Mean (%) | RSD (%) | ||
| UV | Bavachin | 1.82 | 2.03 | 99.7 | 1.34 |
| Isobavachalcone | 1.86 | 1.95 | 102.3 | 1.98 | |
| Bavachinin | 1.55 | 1.87 | 98.5 | 2.01 | |
| Bakuchiol | 1.03 | 1.56 | 96.3 | 2.54 | |
| MS | Bavachin | 4.39 | 6.75 | 95.3 | 7.85 |
| Isobavachalcone | 4.63 | 5.21 | 97.4 | 6.34 | |
| Bavachinin | 5.05 | 7.08 | 105.3 | 3.29 | |
| Bakuchiol | 3.65 | 5.11 | 108.2 | 7.01 | |
| ECD | Bavachin | 3.53 | 4.17 | 97.9 | 3.55 |
| Isobavachalcone | 3.47 | 3.68 | 98.5 | 2.52 | |
| Bavachinin | 3.18 | 3.44 | 98.7 | 3.42 | |
| Bakuchiol | 2.64 | 2.73 | 98.6 | 2.43 | |
3.2.3. Accuracy
In order to evaluate the accuracy of proposed method, recovery was tested. Accurate amounts of mixed standards were added to powder sample, and then extracted as described in the preparation of sample and analyzed. The recovery values were obtained by their peak areas from the calibration curves under the same conditions by HPLC-UV, HPLC-MS and HPLC-ECD. As shown in Table 3, the recoveries of analytes were from 96.3% to 102.3% with RSD from 1.34% to 2.54%, from 95.3% to 108.2% with RSD from 3.29% to 7.85%, and from 97.9% to 98.7% by HPLC-UV, HPLC-MS, and HPLC-ECD, respectively. The results indicate that the three methods are suitable for the sample analysis.
3.2.4. Applications
The content of bavachin, isobavachalcone, bavachinin, and bakuchiol in Fructus Psoraleae was determined by triplicate injections of three samples. Typical HPLC-UV, HPLC-MS, and HPLC-ECD chromatograms are shown in Figure 3, Figure 4, Figure 5. The contents are listed in Table 4. The results obtained by the three techniques were comparable as it was estimated by the Student's t-test at 95% confidence level which indicated no significant difference among three methods. Thus, the developed HPLC-UV, HPLC-MS, and HPLC-ECD methods are suitable for the determination of four active compounds in Fructus Psoraleae.
Table 4.
The contents of bavachin, isobavachalcone, bavachinin and bakuchiol in Fructus Psoraleae by HPLC-UV, HPLC-MS, and HPLC-ECD.
| Method | Content (mg/g) |
|||
|---|---|---|---|---|
| Bavachin | Isobavachalcone | Bavachinin | Bakuchiol | |
| HPLC-UV | 0.148 | 0.809 | 0.746 | 28.18 |
| HPLC-MS | 0.156 | 1.020 | 0.704 | 21.52 |
| HPLC-ECD | 0.092 | 2.100 | 0.870 | 17.60 |
4. Conclusions
The analysis of active compounds in Fructus Psoraleae was carried out by HPLC-IT-MS. A total of 18 compounds of extracts in Fructus Psoraleae were identified. The availability of a suitable HPLC/MS method optimized for identification of active compounds prompted us to evaluate its feasibility in quantitative analysis. Three analytical methods (HPLC-UV, HPLC-MS, and HPLC-ECD) have been developed for the determination of bavachin, isobavachalcone, bavachinin, and bakuchiol in Fructus Psoraleae. Compared with the three methods, ECD can provide a high sensitivity for analysis of the four analytes, MS can supply a high selectivity and sensitivity for determination of bavachin, isobavachalcone, and bavachinin in negative-ion mode and UV can obtain an excellent repeatability for analysis of the four compounds in Fructus Psoraleae. In conclusion, the three methods have a good linear, reproducibility, precision, accuracy, and recovery, and could be used for quantitative analysis of the four active compounds in Fructus Psoraleae.
Acknowledgments
This work was supported by the National Mega Project on Major Drug Development (No. 2009ZX09301-014-1), the National Scientific Foundation of China (Nos. 90817103, 20775055, 30973672), the start-up funds for ZC's Luojia chair professorship of Wuhan University (Nos. 306276216, 306271159) and the Fundamental Research Funds for the Central Universities.
References
- 1.The State Pharmacopoeia Commission of PR China . 2005 Edition. vol. 1. Chemical Industry Press; Beijing: 2005. (Pharmacopoeia of the People's Republic of China). p. 129. [Google Scholar]
- 2.Wong R.W.K., Rabie A.B.M. Effect of Buguzhi (Psoralea corylifolia fruit) extract on bone formation. Phytother. Res. 2010;24:155–160. doi: 10.1002/ptr.3049. [DOI] [PubMed] [Google Scholar]
- 3.Oh K.Y., Lee J.H., Curtis-Long M.J. Glycosidase inhibitory phenolic compounds from the seed of Psoralea corylifolia. Food Chem. 2010;121:940–945. [Google Scholar]
- 4.Xu Q., Pan Y., Yi L.T. Antidepressant-like effects of psoralen isolated from the seeds of Psoralea corylifolia in the mouse forced swimming test. Biol. Pharm. Bull. 2008;31:1109–1114. doi: 10.1248/bpb.31.1109. [DOI] [PubMed] [Google Scholar]
- 5.Haraguchi H., Inoue J., Tamura Y. Antioxidative components of Psoralea coryliflia(Leguminosae) Phytother. Res. 2002;16:539–544. doi: 10.1002/ptr.972. [DOI] [PubMed] [Google Scholar]
- 6.Wang D.W., Li F.M., Jiang Z.M. Osteoblastic proliferation-stimulating activity of Psoralea corylifolia extracts and two of its flavonoids. Planta Med. 2001;67:748–749. doi: 10.1055/s-2001-18343. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura R., Tabata K., Arakawa M. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007;30:1878–1883. doi: 10.1248/bpb.30.1878. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda H., Kiyohara S., Sugimoto S. Constituents from Chinese natural medicines. XXXIII. Inhibitors from the seeds of Psoralea corylifolia on production of nitric oxide in lipopolysaccharide-activated macrophages. Biol. Pharm. Bull. 2009;32(1):147–149. doi: 10.1248/bpb.32.147. [DOI] [PubMed] [Google Scholar]
- 9.Adhikari S., Joshi R., Patro B.S. Antioxidant activity of bakuchiol: experimental evidences and theoretical treatments on the possible involvement of the terpenoid chain. Chem. Res. Toxicol. 2003;16:1062–1069. doi: 10.1021/tx034082r. [DOI] [PubMed] [Google Scholar]
- 10.Katsura H., Tsukiyama R., Suzuki A. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001;45:3009–3013. doi: 10.1128/AAC.45.11.3009-3013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali J., Akhtar N., Sultana Y. Thin-layer chromatographic analysis of psoralen in babchi (psoralea corylifolia) oil. Acta Chromatogr. 2008;20:277–282. [Google Scholar]
- 12.Wang Y.F., Wu B., Yang J. A rapid method for the analysis of ten compounds in psoralea corylifolia L. by UPLC. Chromatographia. 2009;70:199–204. [Google Scholar]
- 13.Qiao C.F., Han Q.B., Song J.Z. Chemical fingerprint and quantitative analysis of Fructus Psoraleae by high-performance liquid chromatography. J. Sep. Sci. 2007;30:813–818. doi: 10.1002/jssc.200600339. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L.H., Huang C.Y., Shan Z. Fingerprint analysis of Psoralea corylifolia L. by HPLC and LC–MS. J. Chromatogr. B. 2005;821:67–74. doi: 10.1016/j.jchromb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Chen X.G., Kong L., Su X.Y. Integration of ion-exchange chromatography fractionation with reversed-phase liquid chromatography-atmospheric pressure chemical ionization mass spectrometer and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for isolation and identification of compounds in Psoralea corylifolia. J. Chromatogr. A. 2005;1089:87–100. doi: 10.1016/j.chroma.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 16.Xiao G.D., Li G.W., Chen L.A. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J. Chromatogr. A. 2010;1217:5470–5476. doi: 10.1016/j.chroma.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Wang D.X., Yang G.L., Engelhardt H. Micellar electrokinetic capillary chromatography of psoralen and isopsoralen. Electrophoresis. 1999;20:1895–1899. doi: 10.1002/(SICI)1522-2683(19990701)20:9<1895::AID-ELPS1895>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Klausen K., Mortensen A.G., Laursen B. Phenolic compounds in different barley varieties: identification by tandem mass spectrometry (QStar) and NMR; quantification by liquid chromatography triple quadrupole-linear ion trap mass spectrometry (q-trap) Nat. Prod. Commun. 2010;5:407–414. [PubMed] [Google Scholar]
- 19.Ferreres F., Pereira D.M., Valentao P. First report of non-coloured flavonoids in echium plantagineum bee pollen: differentiation of isomers by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass. Spectrom. 2010;24:801–806. doi: 10.1002/rcm.4454. [DOI] [PubMed] [Google Scholar]
- 20.Izumi Y., Okazawa A., Bamba T. Development of a method for comprehensive and quantitative analysis of plant hormones by highly sensitive nanoflow liquid chromatography-electrospray ionization-ion trap mass spectrometry. Anal. Chim. Acta. 2009;648:215–225. doi: 10.1016/j.aca.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Q. Chen, Y. Zhang, W. Zhang, et al., Identification and quantification of oleanolic acid and ursolic acid in Chinese herbs by liquid chromatography-ion trap mass spectrometry, Biomed. Chromatogr. doi:10.1002/bmc.1614. [DOI] [PubMed]
- 22.Li Y., Wang F., Chen Z. Determination of bavachin and iosobavachalcone in fructus psoraleae by high-performance liquid chromatography with electrochemical detection. J. Sep. Sci. 2011;34:514–519. doi: 10.1002/jssc.201000801. [DOI] [PubMed] [Google Scholar]
- 23.Liu L., Chen Y., Zhang Y. Determination of tryptophan and kynurenine in human plasma by liquid chromatography–electrochemical detection with multi-wall carbon nanotube-modified glassy carbon electrode. Biomed. Chromatogr. 2011;25:938–942. doi: 10.1002/bmc.1550. [DOI] [PubMed] [Google Scholar]
- 24.Guo J., Liu Y., Wang Y. Preparation of psoralenoside and isopsoralenoside by preparative high performance liquid chromatography from psoralen. J. Tianjin Univ. Tradition. Chin. Med. 2009;28:136–137. [Google Scholar]
- 25.Liu Y., Wang Y., Han L. Identif ication of compounds in fruits of Psora lea corylifolia by HPLC-MS. China J. Chin. Materia Medica. 2009;34:2898–2902. [PubMed] [Google Scholar]
- 26.Zhao L., Huang C., Tu Y. Determination of six constituents in fructus psoraleae by HPLC. Chin. J. Nat. Med. 2005;3:242–244. [Google Scholar]