Abstract
A simple, rapid and sensitive liquid chromatography-tandem mass spectrometric (LC–MS/MS) assay method has been developed and fully validated for the simultaneous quantification of atorvastatin, metformin and glimepiride in human plasma. Carbamazepine was used as internal standard (IS). The analytes were extracted from 200 μL aliquots of human plasma via protein precipitation using acetonitrile. The reconstituted samples were chromatographed on a Alltima HP C18 column by using a 60:40 (v/v) mixture of acetonitrile and 10 mM ammonium acetate (pH 3.0) as the mobile phase at a flow rate of 1.1 mL/min. The calibration curves obtained were linear (r2≥0.99) over the concentration range of 0.50–150.03 ng/mL for atorvastatin, 12.14–1207.50 ng/mL for metformin and 4.98–494.29 ng/mL for glimepiride. The API-4000 LC–MS/MS in multiple reaction monitoring (MRM) mode was used for detection. The results of the intra- and inter-day precision and accuracy studies were well within the acceptable limits. All the analytes were found to be stable in a battery of stability studies. The method is precise and sensitive enough for its intended purpose. A run time of 2.5 min for each sample made it possible to analyze more than 300 plasma samples per day. The developed assay method was successfully applied to a pharmacokinetic study in human male volunteers.
Keywords: Atorvastatin, Metformin, Glimepiride, LC–MS/MS, Human plasma, Pharmacokinetics
1. Introduction
Type 2 diabetes is a complex metabolic disorder with two major biochemical defects, namely impaired insulin secretion and impaired insulin action at the periphery. Chronic hyperglycemia results from these defects. Current American Diabetes Association guidelines suggest that all adults with diabetes should be managed to achieve a low density lipoprotein (LDL) cholesterol less than 100 mg/dl employing statins as first-line therapy [1].
Atorvastatin is a lipid-lowering agent that specifically, competitively, and reversibly inhibits 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, which catalyzes the conversion of HMG-CoA to mevalonic acid, the rate-limiting step in the cholesterol biosynthesis [2], [3]. US FDA (2006) has approved atorvastatin for use to reduce the risk of stroke and heart attack in people with type 2 diabetes without evidence of heart disease. Metformin is an orally administered biguanide that lowers glucose by reducing hepatic glucose production and gluconeogenesis and by enhancing peripheral insulin sensitivity [4], [5], [6]. Glimepiride is an oral sulfonylurea hypoglycemic agent indicated for the treatment of type 2 diabetes mellitus. The primary mechanism of action of glimepiride for lowering blood glucose appears to be dependent on stimulating the release of insulin from functioning pancreatic cells [7], [8].
Hence, the combination of atorvastatin, metformin and glimepiride extends release complement each other and provides reduction in plasma cholesterol along with glycemic control, thereby providing a comprehensive control of diabetes and associated dyslipidemia. TRIPILL (Cipla Limited, Mumbai, India) is a fixed dose combination of metformin hydrochloride (500 mg), atorvastatin (10 mg) and glimepiride (2 mg). For many patients with type 2 diabetes, monotherapy with an oral antidiabetic agent is not sufficient to reach target blood glucose levels and multiple drugs may be necessary to achieve adequate control [9], [10]. In such cases metformin has been coadministered with glimepiride [4], [9]. The combination of atorvastatin and metformin has greater benefit in improving liver injury in type 2 diabetes with hyperlipidemia [11].
As per the literature, several LC–MS/MS methods have been reported for the determination of atorvastatin [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], metformin [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33] and glimepiride [34], [35], [36], [37], [38] individually or with some other drugs in biological samples. To date, no LC–MS/MS method has been reported for the simultaneous determination of atorvastatin, metformin and glimepiride in human plasma. Simultaneous determination of atorvastatin, metformin and glimepiride remains difficult using single mode of separation and extraction due to their different physico-chemical properties and polarities. To address the pharmacokinetics of the new combined formulation, a sensitive and specific method that allows simultaneous measurement of atorvastatin, metformin and glimepiride in human plasma is needed. We felt that this simultaneous estimation method will help the researchers as the three drugs used in this method were available in market with fixed dose combination. The present work describes a simple, selective and sensitive method, which employs simple protein precipitation technique for sample preparation and liquid chromatography with electrospray ionization-tandem mass spectrometry for simultaneous quantitation of atorvastatin, metformin and glimepiride in human plasma. The application of this assay method to a clinical pharmacokinetic study in healthy male volunteers following oral administration of atorvastatin, metformin and glimepiride is described. The authenticity in the measurement of study data is demonstrated through incurred samples reanalysis.
2. Experimental
2.1. Chemicals and materials
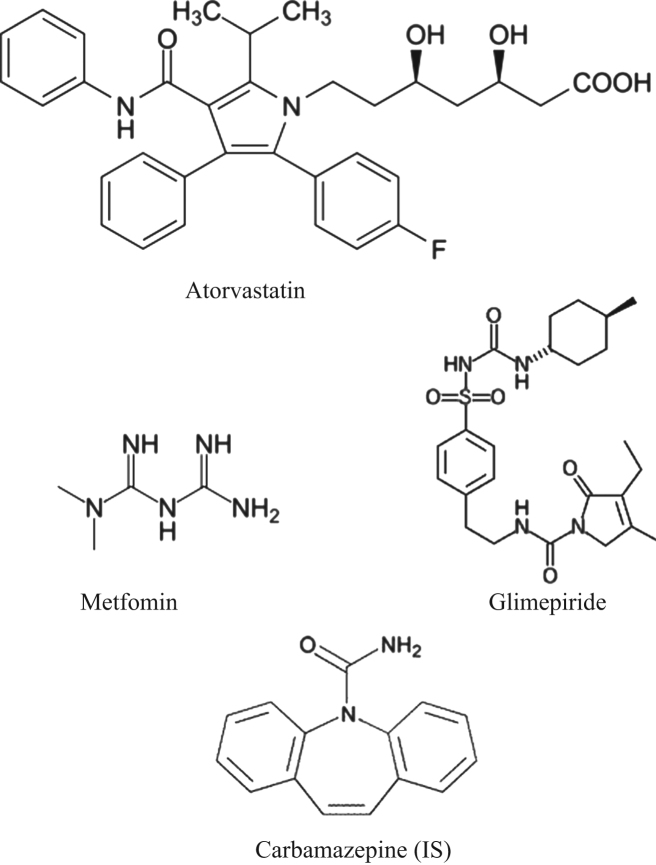
The reference samples of atorvastatin calcium (97.90%), metformin hydrochloride (99.60%), glimepiride (99.40%) and IS (98.71%) were procured form Neucon Pharma Pvt. Ltd., (Goa, India). Chemical structures of these compounds are presented in Fig. 1. Water used for the LC–MS/MS analysis was prepared from Milli-Q water purification system procured from Millipore (Bangalore, India). Acetonitrile and methanol were of HPLC grade and purchased from J.T. Baker (Phillipsburg, NJ, USA). Analytical grade ammonium acetate and acetic acid were purchased from Merck Ltd., (Mumbai, India). The control human plasma sample was procured from Deccan’s Pathological Labs (Hyderabad, India).
Fig. 1.
Chemical structures of atorvastatin, metformin, glimepiride and carbamazepine (IS).
2.2. Instrumentation and chromatographic conditions
An HPLC system (Shimadzu, Kyoto, Japan) consisting of a Alltima HP C18 HL column (50 mm×4.6 mm, 3 μm; Grace Davison, Deerfield, Ireland), a binary LC-20 AD prominence pump, an auto sampler (SIL-HTc) and a solvent degasser (DGU-20 A3) were used for the study. Aliquots of the processed samples (25 μL) were injected into the column, which was kept at room temperature. The isocratic mobile phase, 60:40 (v/v) mixture of acetonitrile and 10 mM ammonium acetate (pH 3.00±0.05), was delivered at 1.1 mL/min into the electrospray ionization chamber of the mass spectrometer. Quantitation was achieved with MS-MS detection in positive ion mode for all the analytes and the internal standard using an MDS Sciex API-4000 mass spectrometer (Foster City, CA, USA) equipped with a Turboionspray ™ interface at 550 °C. The ion spray voltage was set at 4800 V. The source parameters viz. the nebulizer gas, curtain gas, auxiliary gas and collision gas were set at 33, 15, 35 and 7 psi, respectively. The compound parameters viz. the declustering potential (DP), collision energy (CE), entrance potential (EP) and collision cell exit potential (CXP) were 60, 20, 10, 7 V for atorvastatin, 45, 25, 10, 6 V for metformin, 40, 28, 10, 6 V for glimepiride and 75, 30, 10, 6 V for IS. Detection of the ions was carried out in the multiple reaction monitoring (MRM) mode by monitoring the transition pairs of m/z 559.5 precursor ion to the m/z 440.4 for atorvastatin, m/z 130.1 precursor ion to the m/z 60.1 for metformin, m/z 491.5 precursor ion to the m/z 352.6 for glimepiride and m/z 237.2 precursor ion to the m/z 194.1 product ion for the IS. Quadrupoles Q1 and Q3 were set on unit resolution. The analysis data obtained were processed by Analyst software™ (version 1.4.2).
2.3. Preparation of stock solutions of analytes and IS
Primary stock solutions of atorvastatin, metformin and glimepiride for preparation of standard and quality control (QC) samples were prepared from separate weighing. Primary stock solutions of atorvastatin, metformin, glimepiride and IS at 1000 μg/mL were prepared in acetonitrile and these stocks were stored at 2–8 °C; they were found to be stable for 15 days. The stock solutions were suitably diluted with a mixture of acetonitrile and water (60:40, v/v; diluent) to prepare working standard solutions for the purpose of plotting the calibration curve (CC). Another set of working solutions of atorvastatin, metformin and glimepiride were made in diluent (from primary stock) at appropriate dilutions for preparation of QC samples. A working IS solution (5 μg/mL) was also prepared in diluent. Working solutions of glimepiride and metformin were prepared in combination, whereas atorvastatin was prepared separately.
2.4. Preparation of calibration curve standards and quality control samples
Calibration samples were prepared by spiking 950 μL of control human plasma with the appropriate working standard solution of the each analyte (25 μL combined dilution of metformin, glimepiride and 25 μL of atorvastatin). Calibration curve (CC) standards of atorvastatin, metformin and glimepiride in blank plasma were prepared by spiking with an appropriate volume of the working solutions, giving final concentrations of 0.50, 1.00, 2.50, 10.05, 20.10, 40.21, 80.41, 120.02, and 150.03 ng/mL for atorvastatin, 12.14, 24.27, 60.08, 121.35, 242.71, 485.42, 724.50, 966.00, and 1207.50 ng/mL for metformin and 4.97, 9.94, 24.84, 49.68, 99.35, 198.71, 296.58, 395.44, and 494.29 ng/mL for glimepiride. The CC samples were analyzed along with the quality control (QC) samples for each batch of plasma samples. The QC samples were prepared at five different concentration levels of 0.50 (lower limit of quantification, LLOQ), 1.50 (low quality control, LQC), 25.05 (middle quality control, MQC-1), 90.09 (MQC-2) and 125.13 ng/mL (high quality control, HQC) for atorvastatin, 12.25 (LLOQ), 36.14 (LQC), 182.55 (MQC-1), 676.10 (MQC-2) and 1081.76 ng/mL (HQC) for metformin and 4.97 (LLOQ), 14.66 (LQC), 74.05 (MQC-1), 274.24 (MQC92) and 438.79 ng/mL (HQC) for glimepiride in blank plasma. All the prepared plasma samples were stored at−70 °C.
2.5. Sample preparation
All frozen subject samples, calibration standards and quality control samples were thawed and allowed to equilibrate at room temperature prior to analysis. The samples were vortexed to mix for 10 s prior to spiking. 200 μL aliquot of human plasma sample was mixed with 20 μL of the internal standard working solution (5 μg/mL of carbamazepine). To this, 50 μL of the ammonia solution (25%) and 1.0 mL of acetonitrile were added. After vortex-mixing for 30 s and centrifugating at 4000 rpm for 10 min, the supernatant was transferred to another clean test tube and evaporated to dryness at 45 °C under a gentle stream of nitrogen. The residue was reconstituted with 500 μL of the mobile phase and 25 μL was injected into LC–MS/MS system.
2.6. Method validation
The validation of the above method was carried out as per US FDA guidelines [40]. The parameters determined were selectivity, sensitivity, matrix effect, linearity, precision, accuracy, recovery, stability and dilution integrity. Selectivity was assessed by comparing the chromatograms of six different batches of blank plasma obtained from six different sources including one lipemic and one hemolyzed plasma. Potential interference from commonly used medications acetaminophen, diphenhydramine, pantoprazole, nicotine, ibuprofen, caffeine and pseudoephedrine was evaluated. Sensitivity was determined by analyzing six replicates of plasma samples spiked with the lowest level of the calibration curve concentrations. Matrix effect was checked with six different lots of K2-EDTA plasma. Three replicate samples each of LQC and HQC were prepared from different lots of plasma (36 QC samples in total). For checking the linearity standard calibration curves containing at least nine points (non-zero standards) was plotted (0.50–150.03 ng/mL for atorvastatin, 12.14–1207.50 ng/mL for metformin and 4.98–498.29 ng/mL for glimepiride). In addition, blank plasma samples were also analyzed to confirm the absence of direct interferences. Intra-day precision and accuracy were determined by analyzing six replicates at five different QC levels on two different days. Inter-day precision and accuracy were determined by analyzing six replicates at five different QC levels of five different runs. Recoveries of atorvastatin, metformin, glimepiride and IS were determined by comparing the peak area of extracted analyte standard with the peak area of non-extracted standard. Recoveries of atorvastatin, metformin and glimepiride were determined at concentrations of 1.50, 36.14, 14.66 (LQC), 90.09, 676.10, 274.24 (MQC-2) and 125.13, 1081.76, 438.79 ng/mL (HQC), respectively, whereas for internal standards were determined at a concentration of 5 μg/mL. Dilution integrity was performed to extend the upper concentration limit with acceptable precision and accuracy. Six replicates each at a concentration of about 1.6 times of the uppermost calibration standard were diluted 2- and 4-fold with blank plasma. The diluted samples were processed and analyzed.
Stability tests were conducted to evaluate the analyte stability in stock solutions and in plasma samples under different conditions. The stock solution stability at room temperature and refrigerated conditions (2–8 °C) was performed by comparing the area response of the analytes (stability samples) with the response of the sample prepared from fresh stock solution. Bench top stability (12 h), processed samples stability (autosampler stability for 51 h, wet extract stability for 27 h and reinjection stability for 44 h), freeze-thaw stability (5 cycles), long-term stability (68 days) were performed at LQC and HQC levels using six replicates at each level. Samples were considered to be stable if assay values were within the acceptable limits of accuracy (85–115%) and precision (≤15% RSD).
2.7. Pharmacokinetic study design
A pharmacokinetic study was performed in healthy male subjects (n=6). The ethics committee approved the protocol and the volunteers were provided with informed written consent. Blood samples were collected following oral administration of atorvastatin (40 mg), metformin (500 mg) and glimepiride (2 mg) at pre-dose and 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 10, 12, 16, 24 and 48 h, in K2-EDTA vacutainer collection tubes (BD, Franklin, NJ, USA). The tubes were centrifuged at 3200 rpm for 10 min and the plasma was collected. The collected plasma samples were stored at −70 °C till their use. Plasma samples were spiked with the IS and processed as per the extraction procedure described earlier. Along with the clinical samples, the QC samples at low, middle 1, middle 2 and high concentration levels were also assayed in triplicate. Plasma concentration–time profile of atorvastatin, metformin and glimepiride was analyzed by non-compartmental method using WinNonlin Version 5.1. An incurred sample re-analysis (ISR) was also conducted by selecting the 12 subject samples (two samples from each subject) near Cmax and the elimination phase. The percent change in the value should not be more than±20% [41].
3. Results and discussion
3.1. Mass spectrometry
MS parameters were optimized by infusing the standard analyte solution of 100 ng/mL into the mass spectrometer using electrospray as the ionization source and operating in the MRM mode. The signal intensities obtained in positive mode were much higher than those in negative ion mode since the analytes and IS have the ability to accept protons. Protonated form of each analyte and IS, [M+H]+ ion was the parent ion in the Q1 spectrum and was used as the precursor ion to obtain Q3 product ion spectra. The most sensitive mass transition was monitored from m/z 559.5 to 440.4 for atorvastatin, from m/z 130.1 to 60.1 for metformin, from m/z 491.5 to 352.6 for glimepiride and from m/z 237.2 to 194.1 for the IS. As earlier publications have discussed the details of fragmentation patterns of atorvastatin [12], metformin [28], glimepiride [35] and IS [39], we are not presenting the data pertaining to this. LC-MRM is a very powerful technique for pharmacokinetic studies since it provides sensitivity and selectivity requirements for analytical methods [42]. Thus, the MRM technique was chosen for the assay development.
3.2. Method development
Atorvastatin, metformin and glimepiride have different physicochemical properties; it was difficult to set chromatographic conditions that produced sharp peak shape and adequate response. The method development includes mobile phase selection, flow rate, column type and injection volume. Methanol and acetonitrile were tried in different ratio with buffers like ammonium acetate, ammonium formate as well as acid additives like formic acid and acetic acid in varying strength. It was observed that acetonitrile and 10 mM ammonium acetate (pH 3.0±0.05) (60:40, v/v) as the mobile phase was most appropriate to give best sensitivity, efficiency and peak shape. Acidic buffer helped to improve the peak shape and spectral response. 40% aqueous part was adequate to retain the polar compound metformin. The use of a short chromatography column Alltima HP C18 HL (50 mm×4.6 mm, 3 μm) helped in the separation and elution of all three compounds in a very short time. The total chromatographic run time was 2.5 min for each run.
Extraction of all analytes from plasma was difficult as metformin is highly polar, while atorvastatin and glimepiride are comparatively less polar compounds. Initially both the extraction methodologies solid phase extraction (SPE) and liquid–liquid extraction (LLE) were tried using Oasis HLB cartridges for SPE and different organic solvents like ethyl acetate, hexane, dichloromethane, diethyl ether and methyl tert-butyl ether (MTBE) for LLE. The recovery results obtained were consistent for atorvastatin with negligible matrix effect but not for metformin. But as the purpose was to develop a simple, quick and inexpensive method, protein precipitation (PP) was tested. Also, atorvastatin, metformin, and glimepiride had more protein binding nature and were precipitated easily with the single protein precipitant. Thus, in the present study PP was carried out using ethanol, methanol and acetonitrile solvents. The extracts were clear but the recovery was in the range of 70–90% for all the solvents but not reproducible. Addition of ammonia solution to the plasma samples in different volume ratios helped in obtaining consistent and reproducible response. Precipitation with acetonitrile containing ammonia solution caused the lowest matrix effect with better peak shape compared to other organic solvents. When direct residue of the protein precipitant was injected, the peak shape of atorvastatin was unacceptable at lower concentration levels and also matrix effect was high. Hence supernatant was evaporated and the residue was reconstituted with the mobile phase. The method gave clear extracts with minimum matrix effect and quantitative extraction was possible for all the analytes and IS. The mean recoveries for atorvastatin, metformin, glimepiride and IS were good and reproducible. Moreover, the validation results and subject sample analysis study support this extraction methodology and hence it was accepted in the present study.
It is necessary to use an internal standard to obtain high accuracy when HPLC is equipped with MS as the detector. For LC-MS/MS analysis, use of stable isotope-labeled drugs as internal standards proves to be helpful when a significant matrix effect is possible [43]. Isotope-labeled analyte was not available to serve as IS, so, in the initial stages of this work, many compounds were investigated in order to find suitable IS, Finally carbamazepine was selected, based on the chromatographic elution, ionization and extraction efficiency.
3.3. Selectivity and chromatography
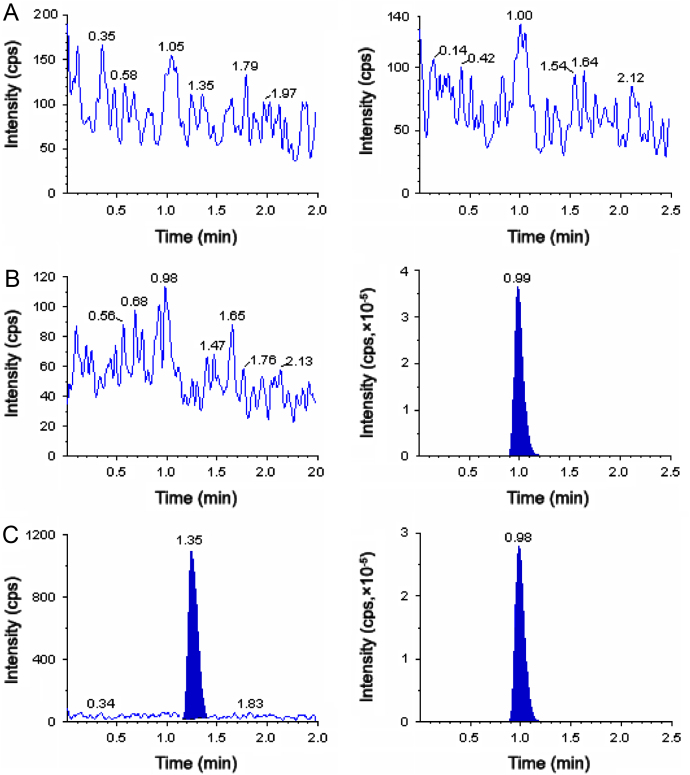
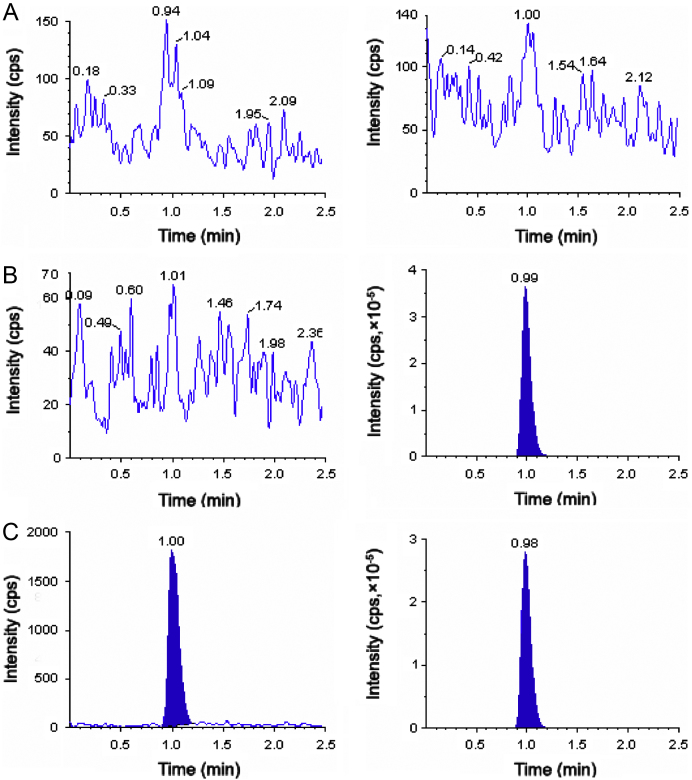
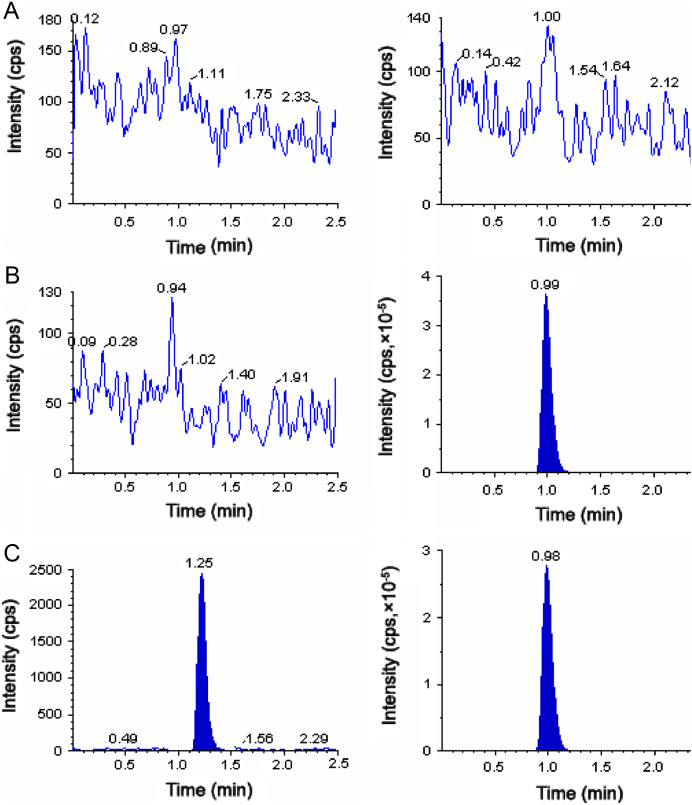
The degree of interference by endogenous plasma constituents with the analytes and IS was assessed by inspection of chromatograms derived from processed blank plasma sample. As shown in Fig. 2, Fig. 3, Fig. 4, no significant direct interference in the blank plasma traces was observed from endogenous substances in drug-free plasma at the retention time of the analytes. Similarly, no interference was observed from commonly used medications such as acetaminophen, diphenhydramine, pantoprazole, nicotine, ibuprofen, caffeine and pseudoephedrine.
Fig. 2.
Typical MRM chromatograms of atorvastatin (left panel) and IS (right panel) in human blank plasma (A), and human plasma spiked with IS (B), a LLOQ sample along with IS (C).
Fig. 3.
Typical MRM chromatograms of metformin (left panel) and IS (right panel) in human blank plasma (A), and human plasma spiked with IS (B), a LLOQ sample along with IS (C).
Fig. 4.
Typical MRM chromatograms of glimepiride (left panel) and IS (right panel) in human blank plasma (A), and human plasma spiked with IS (B), a LLOQ sample along with IS (C).
3.4. Sensitivity
The lowest limit of reliable quantification for the analytes was set at the concentration of the LLOQ. The precision and accuracy at LLOQ concentration were found to be 1.12% and 101.21%; 3.06% and 102.61%; 7.11% and 96.98% for atorvastatin, metformin and glimepiride, respectively.
3.5. Extraction efficiency
A simple protein precipitation with acetonitrile proved to be robust and provided cleanest samples. The recoveries of the analytes and the IS were good and reproducible. The mean overall recoveries (with the precision range) of atorvastatin, metformin and glimepiride were 96.72±0.88% (1.62–4.47%), 72.88±2.18% (1.16–2.47%) and 74.16±2.26% (0.88–2.72%), respectively. The recovery of the IS was 67.16% with a precision range of 0.72–0.90%.
3.6. Matrix effect
No significant matrix effect was observed in all the six batches of human plasma for the analytes at LQC and HQC concentrations. The precision and accuracy for atorvastatin, metformin and glimepiride at LQC concentration were found to be 0.59% and 100.57%; 2.43% and 96.63%; 1.53% and 96.43%, respectively. Similarly, the precision and accuracy for atorvastatin, metformin and glimepiride at HQC concentration were found to be 1.83% and 93.93%; 3.15% and 96.42%; 1.74% and 96.67%, respectively.
3.7. Linearity
Nine-point calibration curve was found to be linear over the concentration range of 0.50–150.03 ng/mL for atorvastatin, 12.14–1207.50 ng/mL for metformin and 4.97–494.29 ng/mL for glimepiride. After comparing the two weighting models (1/x and 1/x2), a regression equation with a weighting factor of 1/x2 of the drug to the IS concentration was found to produce the best fit for the concentration–detector response relationship for both the analytes in human plasma. The mean correlation coefficient of the weighted calibration curves generated during the validation was≥0.99.
3.8. Precision and accuracy
The results for intra-day and inter-day precision and accuracy in plasma quality control samples are summarized in Table 1. The intra-day and inter-day precision deviation values were all within 15% of the relative standard deviation (RSD) at low, middle 1, middle 2 and high quality control levels, whereas within 20% at LLOQ QCs level. The intra-day and inter-day accuracy deviation values were all within 100±15% of the actual values at low, middle 1, middle 2 and high quality control level, whereas within 100±20% at LLOQ QCs level. The results revealed good precision and accuracy.
Table 1.
Intra-day and inter-day precision and accuracy for atorvastatin, metformin and glimepiride.
| QC | QC (spiked concentration ng/mL) | Intra-day (n=12) |
Inter-day (n=30) |
||||
|---|---|---|---|---|---|---|---|
| Mean concentration found (ng/mL) | Accuracy(%) | CV(%) | Mean concentration found (ng/mL) | Accuracy(%) | CV(%) | ||
| Atorvastatin | |||||||
| LLOQ | 0.50 | 0.50 | 99.28 | 1.22 | 0.50 | 99.03 | 1.48 |
| LQC | 1.50 | 1.38 | 92.07 | 7.51 | 1.37 | 91.24 | 4.98 |
| MQC1 | 25.05 | 25.17 | 100.52 | 3.12 | 24.11 | 96.28 | 4.45 |
| MQC2 | 90.09 | 87.66 | 97.30 | 4.41 | 85.57 | 94.98 | 3.82 |
| HQC | 125.13 | 111.05 | 88.75 | 3.36 | 122.34 | 97.77 | 7.75 |
| Metformin | |||||||
| LLOQ | 12.25 | 12.18 | 99.42 | 6.03 | 11.99 | 97.89 | 6.16 |
| LQC | 36.14 | 35.15 | 97.25 | 1.70 | 36.08 | 99.82 | 3.37 |
| MQC1 | 182.55 | 173.73 | 95.17 | 1.66 | 177.35 | 97.15 | 4.58 |
| MQC2 | 676.10 | 676.37 | 100.04 | 1.79 | 671.99 | 99.39 | 1.73 |
| HQC | 1081.76 | 1091.69 | 100.92 | 1.56 | 1068.40 | 98.77 | 2.32 |
| Glimepiride | |||||||
| LLOQ | 4.97 | 4.59 | 92.36 | 5.56 | 4.81 | 96.81 | 10.75 |
| LQC | 14.66 | 14.21 | 96.96 | 3.98 | 15.09 | 102.91 | 7.27 |
| MQC1 | 74.05 | 73.76 | 99.62 | 3.51 | 74.99 | 101.27 | 5.76 |
| MQC2 | 274.24 | 287.68 | 104.90 | 3.02 | 285.16 | 103.98 | 2.16 |
| HQC | 438.79 | 455.70 | 103.85 | 3.39 | 445.99 | 101.64 | 2.96 |
3.9. Dilution integrity
The upper concentration limits can be extended to 256.51 ng/mL for atorvastatin, 2048.95 ng/mL for metformin, and 805.70 ng/mL for glimepiride by 1/2 or 1/4 dilution with screened human blank plasma. The mean back calculated concentrations for 1/2 and 1/4 dilution samples were within 85–115% of their nominal. The coefficients of variation (%CV) for 1/2 and 1/4 dilution samples were less than 5% for all the analytes.
3.10. Stability studies
In the different stability experiments carried out viz. bench top stability (12 h), autosampler stability (51 h), repeated freeze-thaw cycles (six cycles), reinjection stability (44 h), wet extract stability (27 h at 2–8 °C) and long-term stability at−70 °C for 68 days the mean % nominal values of the analytes were found to be within±15% of the predicted concentrations for the analytes at their LQC and HQC levels (Table 2). Thus, the results were found to be within the acceptable limits during the entire validation.
Table 2.
Stability tests for atorvastatin, metformin and glimepiride (n=6).
| Stability test | Atorvastatin |
Metformin |
Glimepiride |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QC (spiked concentration ng/mL) | Mean (ng/mL) | Accuracy/Stability (%) | Precision(%) | QC (spiked concentration ng/mL) | Mean (ng/mL) | Accuracy/ Stability (%) | Precision (%) | QC (spiked concentration ng/mL) | Mean (ng/mL) | Accuracy/ Stability (%) | Precision (%) | |
| Autosampler stability (at 5 °C for 51 h) | 1.50 | 1.33 | 88.72 | 5.63 | 36.14 | 33.96 | 93.94 | 3.38 | 14.66 | 14.03 | 95.67 | 7.41 |
| 125.13 | 134.75 | 107.69 | 4.36 | 1081.76 | 1019.67 | 94.26 | 0.94 | 438.79 | 419.55 | 95.62 | 1.83 | |
| Wet extract stability (at 2–8 °C for 27 h) | 1.50 | 1.52 | 101.41 | 9.43 | 36.14 | 33.46 | 92.58 | 6.18 | 14.66 | 14.65 | 99.91 | 5.90 |
| 125.13 | 137.69 | 110.05 | 12.03 | 1081.76 | 1037.62 | 95.92 | 1.15 | 438.79 | 429.79 | 97.95 | 1.74 | |
| Bench top stability (12 h in ice water bath) | 1.50 | 1.38 | 91.61 | 1.41 | 36.14 | 33.74 | 93.35 | 2.04 | 14.66 | 13.69 | 93.37 | 5.82 |
| 125.13 | 128.02 | 102.32 | 6.71 | 1081.76 | 1011.83 | 93.54 | 1.26 | 438.79 | 419.35 | 95.57 | 0.95 | |
| Freeze-thaw stability (six cycles) | 1.50 | 1.49 | 99.19 | 7.55 | 36.14 | 33.74 | 93.35 | 3.43 | 14.66 | 13.67 | 93.24 | 4.52 |
| 125.13 | 139.65 | 111.61 | 2.18 | 1081.76 | 1021.70 | 94.45 | 1.20 | 438.79 | 411.92 | 93.88 | 1.79 | |
| Reinjection stability (44 h) | 1.50 | 1.52 | 110.89 | 0.85 | 36.14 | 34.53 | 93.43 | 3.45 | 14.66 | 15.00 | 93.23 | 3.42 |
| 125.13 | 126.55 | 98.14 | 1.18 | 1081.76 | 1014.03 | 96.49 | 1.00 | 438.79 | 450.71 | 101.82 | 2.54 | |
| Long-term stability (at −70 °C for 68 days) | 1.50 | 1.50 | 112.29 | 3.30 | 36.14 | 36.42 | 103.81 | 3.57 | 14.66 | 15.15 | 108.99 | 3.29 |
| 125.13 | 127.71 | 101.79 | 4.96 | 1081.76 | 1069.43 | 98.59 | 1.41 | 438.79 | 449.94 | 101.30 | 2.23 | |
3.11. Pharmacokinetic study results
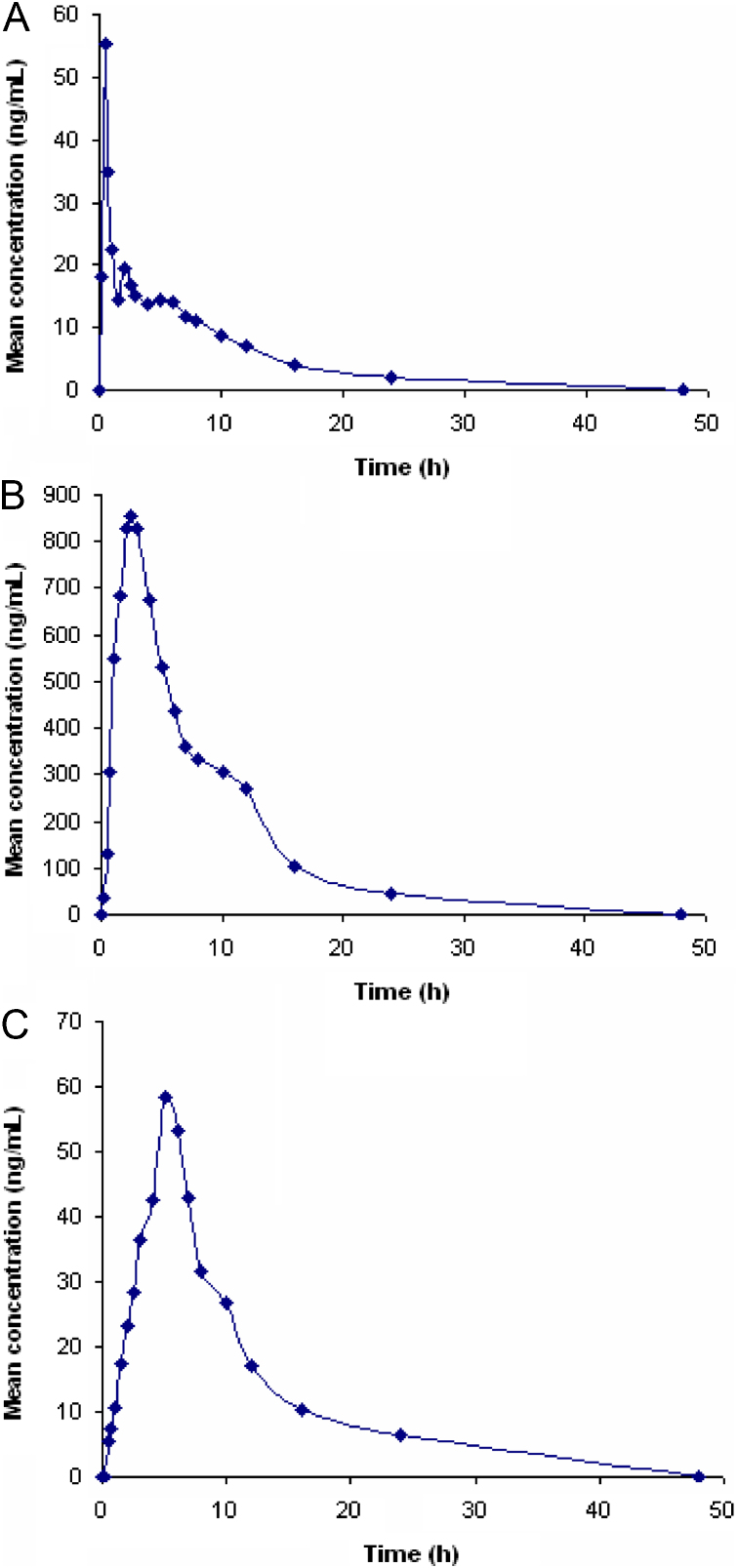
In order to verify the sensitivity and selectivity of this method in a real-time situation, the present method was used to test for atorvastatin, metformin and glimepiride concentrations in human plasma samples collected from healthy male volunteers (n=6). The mean plasma concentrations vs time profile of atorvastatin, metformin and glimepiride are shown in Fig. 5. The pharmacokinetic parameters estimated are shown in Table 3. These values were in close proximity when compared with earlier reported values [16], [28], [36].
Fig. 5.
Mean plasma concentration–time profile of atorvastatin (A), metformin (B), and glimepiride (C) in human plasma following oral dosing of atorvastatin (40 mg), metformin (500 mg ) and glimepiride (2 mg) tablet to healthy volunteers (n=6).
Table 3.
Pharmacokinetic parameters for atorvastatin (40 mg), metformin (500 mg) and glimepiride (2 mg) (n=6, Mean±SD).
| PK parameter | Atorvastatin | Metformin | Glimepiride |
|---|---|---|---|
| tmax (h) | 0.75±0.61 | 2.42±0.38 | 5.33±0.52 |
| Cmax (ng/mL) | 59.8±11.5 | 877.5±162.2 | 62.8±7.9 |
| AUC0–t (ng h/mL) | 213±86 | 6852±1312 | 508±52 |
| AUC0–inf (ng h/mL) | 236±83 | 7191±1465 | 589±75 |
| t1/2 (h) | 7.92±2.72 | 4.95±0.93 | 8.55±1.87 |
| Kel (h–1) | 0.10±0.04 | 0.14±0.03 | 0.08±0.02 |
3.12. Incurred sample reanalysis
Since the FDA has introduced the necessity of incurred sample reanalysis evaluation at the Crystal City III meeting [44] it is necessary to demonstrate assay reproducibility by using dosed subject samples. Incurred sample reanalysis (ISR) was performed using two plasma samples from each subject and re-assayed in a separate batch run. The differences in concentrations between the ISR and the initial values for all the tested samples were less than 20% (Table 4), indicating good reproducibility of the present method.
Table 4.
Incurred samples re-analysis data of atorvastatin, metformin and glimepiride.
| Subject no. | Atorvastatin |
Metformin |
Glimepiride |
||||||||||||
| Sampling point (h) | Original conc. (ng/mL) | Incurred sample conc. (ng/mL) | Mean of original and ISR conc. (ng/mL) | Difference (%) | Sampling point (h) | Original conc. (ng/mL) | Incurred sample conc. (ng/mL) | Mean of original and ISR conc. (ng/mL) | Difference (%) | Sampling point (h) | Original conc. (ng/mL) | Incurred sample conc. (ng/mL) | Mean of original and ISR conc. (ng/mL) | Difference (%) | |
| 1 | 0.75 | 50.59 | 57.37 | 53.98 | 12.55 | 3 | 1084.03 | 1051.37 | 1067.70 | 3.06 | 6 | 46.56 | 43.67 | 45.11 | 6.41 |
| 1 | 24 | 2.52 | 2.37 | 2.45 | 6.34 | 24 | 30.37 | 32.67 | 31.52 | 7.30 | 16 | 11.10 | 12.67 | 11.88 | 13.18 |
| 2 | 0.75 | 47.01 | 43.67 | 45.34 | 7.38 | 3 | 864.61 | 789.65 | 827.13 | 9.06 | 6 | 53.62 | 51.37 | 52.49 | 4.30 |
| 2 | 24 | 1.86 | 1.97 | 1.91 | 5.85 | 24 | 51.93 | 61.67 | 56.80 | 17.14 | 10 | 19.99 | 18.97 | 19.48 | 5.22 |
| 3 | 0.75 | 21.25 | 22.67 | 21.96 | 6.49 | 4 | 575.98 | 594.66 | 585.32 | 3.19 | 7 | 50.31 | 54.66 | 52.48 | 8.28 |
| 3 | 24 | 2.05 | 2.10 | 2.07 | 2.24 | 16 | 50.66 | 42.63 | 46.64 | 17.22 | 12 | 23.46 | 25.64 | 24.55 | 8.89 |
| 4 | 0.75 | 26.91 | 24.67 | 25.79 | 8.70 | 3 | 651.59 | 692.37 | 671.98 | 6.07 | 6 | 48.72 | 46.37 | 47.55 | 4.96 |
| 4 | 24 | 1.54 | 1.85 | 1.69 | 18.37 | 16 | 50.16 | 52.66 | 51.41 | 4.88 | 12 | 12.33 | 13.65 | 12.99 | 10.19 |
| 5 | 0.75 | 30.45 | 33.24 | 31.85 | 8.73 | 3 | 999.19 | 1051.66 | 1025.43 | 5.12 | 6 | 54.91 | 58.69 | 56.80 | 6.65 |
| 5 | 24 | 1.60 | 1.85 | 1.73 | 14.48 | 24 | 75.46 | 77.99 | 76.72 | 3.29 | 12 | 12.68 | 15.00 | 13.84 | 16.77 |
| 6 | 0.75 | 33.72 | 32.37 | 33.04 | 4.09 | 3 | 711.45 | 806.64 | 759.04 | 12.54 | 7 | 40.99 | 42.69 | 41.84 | 4.07 |
| 6 | 24 | 1.98 | 2.07 | 2.02 | 4.30 | 24 | 65.66 | 72.68 | 69.17 | 10.15 | 16 | 14.56 | 15.70 | 15.13 | 7.50 |
4. Conclusion
In summary, we have developed and validated a rapid, specific, reproducible and high-throughput LC-MS/MS method to quantify atorvastatin, metformin and glimepiride simultaneously using single IS. So far no published methods are available for the simultaneous quantification of these three drugs in human plasma. Validated methods are essential for the determination of atorvastatin, metformin and glimepiride concentrations in human plasma simultaneously for bioequivalence studies. To the best of knowledge, this is the first time that all three analytes were estimated simultaneously in any of the biological matrix. The cost-effectiveness, simplicity of the assay and usage of protein precipitation extraction and sample turnover rate of less than 2.5 min per sample, make it an attractive procedure in high-throughput bioanalysis of atorvastatin, metformin and glimepiride. From the results of all the validation parameters, we can conclude that the developed method can be useful for bioavailability/bioequivalence studies and routine therapeutic drug monitoring with desired precision and accuracy.
Acknowledgments
The authors gratefully acknowledge Wellquest Clinical Research Laboratories, Hyderabad for providing necessary facilities for carrying out this study.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Srinivasa Rao Polagani, Email: srinu_polagani@yahoo.co.in.
Venkateswarlu Gandu, Email: venkateshwarlugoud@yahoo.com.
References
- 1.American Diabetes Association Standards of medical care for patients with diabetes mellitus (Position Statement) Diabetes Care. 2003;26(Suppl. 1):S33–S50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 2.Bakker-Arkema R.G., Davidson M.H., Goldstein R.J. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. J. Am. Med. Assoc. 1996;275:128–133. [PubMed] [Google Scholar]
- 3.Nawrocki J.W., Weiss S.R., Davidson M.H. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler. Thromb. Vasc. Biol. 1995;15:678–682. doi: 10.1161/01.atv.15.5.678. [DOI] [PubMed] [Google Scholar]
- 4.Krentz A.J., Bailey C.J. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo R.A., Barzilai N., Simonson D.C. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J. Clin. Endocrinol. Metab. 1991;73:1294–1301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- 6.Bailey C.J., Turner R.C. Metformin. N. Engl. J. Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 7.Rosskamp R., Wernicke–Panten K., Draeger E. Clinical profile of the novel sulphonylurea glimepiride. Diabetes Res. Clin. Pract. 1996;31:S33–S42. doi: 10.1016/0168-8227(96)01228-4. [DOI] [PubMed] [Google Scholar]
- 8.Massi-Benedetti M. Glimepiride in type 2 diabetes mellitus: a review of the worldwide therapeutic experience. Clin. Ther. 2003;25:799–816. doi: 10.1016/s0149-2918(03)80109-1. [DOI] [PubMed] [Google Scholar]
- 9.Bell D.S. Type 2 diabetes mellitus: what is the optimal treatment regimen? Am. J. Med. 2004;116:23S–29S. doi: 10.1016/j.amjmed.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Padwal R., Majumdar S.R., Johnson J.A. A systematic review of drug therapy to delay or prevent type 2 diabetes. Diabetes Care. 2005;28:736–744. doi: 10.2337/diacare.28.3.736. [DOI] [PubMed] [Google Scholar]
- 11.Matafome P., Louro T., Rodrigues L. Metformin and atorvastatin combination further protect the liver in type 2 diabetes with hyperlipidaemia. Diabetes Metab. Res. Rev. 2011;27:54–62. doi: 10.1002/dmrr.1157. [DOI] [PubMed] [Google Scholar]
- 12.Nirogi R.V.S., Kandikere V.N., Shukla M. Simultaneous quantification of atorvastatin and active metabolites in human plasma by liquid chromatography-tandem mass spectrometry using rosuvastatin as internal standard. Biomed. Chromatogr. 2006;20:924–936. doi: 10.1002/bmc.622. [DOI] [PubMed] [Google Scholar]
- 13.Borek-Dohalsky V., Huclova J., Barrett B. Validated HPLC-MS-MS method for simultaneous determination of atorvastatin and 2-hydroxyatorvastatin in human plasma-pharmacokinetic study. Anal. Bioanal. Chem. 2006;386:275–285. doi: 10.1007/s00216-006-0655-3. [DOI] [PubMed] [Google Scholar]
- 14.Bullen W.W., Miller R.A., Hayes R.N. Development and validation of a high-performance liquid chromatography tandem mass spectrometry assay for atorvastatin, ortho-hydroxy atorvastatin, and para-hydroxy atorvastatin in human, dog, and rat plasma. J. Am. Soc. Mass. Spectrom. 1999;10:55–66. doi: 10.1016/S1044-0305(98)00118-4. [DOI] [PubMed] [Google Scholar]
- 15.Ma L., Dong J., Chen X.J. Development and validation of atorvastatin by LC-ESI-MS and application in bioequivalence research in healthy Chinese volunteers. Chromatographia. 2007;65:737–741. [Google Scholar]
- 16.Pilli N.R., Inamadugu J.K., Mullangi R. Simultaneous determination of atorvastatin, amlodipine, ramipril and benazepril in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. Biomed. Chromatogr. 2011;25:439–449. doi: 10.1002/bmc.1462. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh C., Jain I., Gaur S. Simultaneous estimation of atorvastatin and its two metabolites from human plasma by ESI-LC-MS/MS. Drug Test. Anal. 2011;3:352–362. doi: 10.1002/dta.228. [DOI] [PubMed] [Google Scholar]
- 18.Jani A.J., Dasandi B., Rathnam S. Liquid chromatographic-MS/MS determination of atorvastatin and metabolites in human plasma. Eurasian J. Anal. Chem. 2010;5:46–52. [Google Scholar]
- 19.Liu D., Jiang J., Zhou H. Quantitative determination of atorvastatin and para-hydroxy atorvastatin in human plasma by LC-MS-MS. J. Chromatogr. Sci. 2008;46:862–866. doi: 10.1093/chromsci/46.10.862. [DOI] [PubMed] [Google Scholar]
- 20.Macwan J.S., Ionita I.A., Dostalek M. Development and validation of a sensitive simple and rapid method for simultaneous quantitation of atorvastatin and its acid and lactone metabolites by liquid chromatography-tandem mass spectrometry LC-MS/MS. Anal. Bioanal. Chem. 2011;400:423–433. doi: 10.1007/s00216-011-4804-y. [DOI] [PubMed] [Google Scholar]
- 21.Nováková L., Vlcková H., Satínský D. Ultra high performance liquid chromatography tandem mass spectrometric detection in clinical analysis of simvastatin and atorvastatin. J. Chromatogr. B:Analyt. Technol. Biomed. Life Sci. 2009;877:2093–2103. doi: 10.1016/j.jchromb.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 22.V.B. Ravi, R. Mullangi, J.K. Inamadugu, et al., Simultaneous determination of atorvastatin and niacin in human plasma by LC-MS/MS and its application to a human pharmacokinetic study, Biomed. Chromatogr. 2012 101002bmc2715. [DOI] [PubMed]
- 23.Mistri H.N., Jangid A.G., Shrivastav P.S. Liquid chromatography tandem mass spectrometry method for simultaneous determination of antidiabetic drugs metformin and glyburide in human plasma. J. Pharm. Biomed. Anal. 2007;45:97–106. doi: 10.1016/j.jpba.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhong G., Bi H., Zhou S. Simultaneous determination of metformin and gliclazide in human plasma by liquid chromatography-tandem mass spectrometry: application to a bioequivalence study of two formulations in healthy volunteers. J. Mass Spectrom. 2005;40:1462–1471. doi: 10.1002/jms.907. [DOI] [PubMed] [Google Scholar]
- 25.Ding C., Zhou Z., Ge Q. Simultaneous determination of metformin and glipizide in human plasma by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2007;21:132–138. doi: 10.1002/bmc.723. [DOI] [PubMed] [Google Scholar]
- 26.Georgita C., Albu F., David V. Simultaneous assay of metformin and glibenclamide in human plasma based on extraction-less sample preparation procedure and LC/(APCI)MS. J. Chromatogr. B. 2007;854:211–218. doi: 10.1016/j.jchromb.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Liu A., Coleman S.P. Determination of metformin in human plasma using hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2009;877:3695–3700. doi: 10.1016/j.jchromb.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Tang Y., Gu J. Rapid and sensitive liquid chromatography-tandem mass spectrometric method for the quantitation of metformin in human plasma. J. Chromatogr. B. 2004;808:215–219. doi: 10.1016/j.jchromb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L., Tian Y., Zhang Z. Simultaneous determination of metformin and rosiglitazone in human plasma by liquid chromatography/tandem mass spectrometry with electrospray ionization: application to a pharmacokinetic study. J. Chromatogr. B. 2007;854:91–98. doi: 10.1016/j.jchromb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Marques M.A., Soares A.S., Pinto O.W. Simple and rapid method determination for metformin in human plasma using high performance liquid chromatography tandem mass spectrometry: application to pharmacokinetic studies. J. Chromatogr. B. 2007;852:308–316. doi: 10.1016/j.jchromb.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Gu Q., Qiu F. Rapid determination of metformin in human plasma by liquid chromatography-tandem mass spectrometry method. J. Chromatogr. B. 2004;802:377–381. doi: 10.1016/j.jchromb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta P., Bhaumik U., Ghosh A. LC-MS-MS development and validation for simultaneous quantitation of metformin, glimepiride and pioglitazone in human plasma and its application to a bioequivalence study. Chromatographia. 2009;69:1243–1250. [Google Scholar]
- 33.AbuRuz S., Millership J., McElnay J. The development and validation of liquid chromatography method for the simultaneous determination of metformin and glipizide, gliclazide, glibenclamide or glimperide in plasma. J. Chromatogr. B. 2005;817:277–286. doi: 10.1016/j.jchromb.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Ho E.N.M., Yiu K.C.H., Wan T.S.M. Detection of anti-diabetics in equine plasma and urine by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. 2004;811:65–73. doi: 10.1016/j.jchromb.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 35.Kim H., Chang K.Y., Lee H.J. Determination of glimepiride in human plasma by liquid chromatography. Bull. Korean Chem. Soc. 2004;25:109–114. [Google Scholar]
- 36.Liu Y., Zhang M., Zhu J. Bioequivalence and pharmacokinetic evaluation of two formulations of glimepiride 2 mg: a single-dose, randomized-sequence, open-label, two-way crossover study in healthy Chinese male volunteers. Clin. Ther. 2010;32:986–995. doi: 10.1016/j.clinthera.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Salem I.I., Idrees J., Tamimi J.I.A. Determination of glimepiride in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. B. 2004;799:103–109. doi: 10.1016/j.jchromb.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Chakradhar L., Kallem R., Karthik A. A rapid and highly sensitive method for the determination of glimepiride in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry: application to a pre-clinical pharmacokinetic study. Biomed. Chromatogr. 2008;22:58–63. doi: 10.1002/bmc.896. [DOI] [PubMed] [Google Scholar]
- 39.Van Rooyen G.F., Badenhorst D., Swart K.J. Determination of carbamazepine and carbamazepine 10,11-epoxide in human plasma by tandem liquid chromatography-mass spectrometry with electrospray ionisation. J. Chromatogr. B:Analyt. Technol. Biomed. Life Sci. 2002;769:1–7. doi: 10.1016/s1570-0232(01)00590-6. [DOI] [PubMed] [Google Scholar]
- 40.Guidance for Industry: Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM). May 2001.
- 41.Fast DM., Kelley M., Viswanathan CT. Workshop report and follow-up – AAPS workshop on current topics in GLP bioanalysis: assay reproducibility for incurred samples – implications of crystal city recommendations. AAPS J. 2009;11:238–241. doi: 10.1208/s12248-009-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karra V.K., Pilli N.R., Inamadugu J.K. Simultaneous determination of pioglitazone and candesartan in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. J. Pharm. Anal. 2012;2:167–173. doi: 10.1016/j.jpha.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polagani S.R., Pilli N.R., Gandu V. High performance liquid chromatography mass spectrometric method for the simultaneous quantification of pravastatin and aspirin in human plasma: pharmacokinetic application. J. Pharm. Anal. 2012;2:206–213. doi: 10.1016/j.jpha.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Boer T., Wieling J. Incurred sample accuracy assessment: design of experiments based on standard addition. Bioanalysis. 2011;3:983–992. doi: 10.4155/bio.11.36. [DOI] [PubMed] [Google Scholar]