Abstract
A rapid and sensitive method based on liquid chromatography–tandem mass spectrometry (LC–MS/MS) for the determination of a novel anticoagulant peptide bivalirudin in human plasma has been developed and validated. Plasma samples were precipitated protein with acetonitrile and re-extracted with dichloromethane, after which the analyte and triptorelin as an internal standard (IS) were separated on a 300SB-C18 column (150 mm×4.6 mm i.d., 5 μm particle size) using 0.1% formic acid:methanol (45:55, v/v) as mobile phase. The triple-quadrupole mass spectrometer, equipped with electrospray ionization (ESI) interface, was operated in the positive ion mode, and the multiple-reaction monitoring (MRM) transitions of bivalirudin and IS were at m/z 1091.0→650.4 and m/z 656.5→249.3, respectively. The lower limit of quantification (LLOQ) was 1 ng/mL for 100 μL plasma sample and the assay was linear over the concentration range 1–1000 ng/mL. The accuracy was within a range from −0.4% to 0.5% in terms of relative error (RE) and the intra- and inter-day precisions in terms of relative standard deviation (RSD) were ≤2.92 and ≤3.36, respectively. The method was successfully applied to a pharmacokinetic study involving intravenous administration of bivalirudin (0.5 mg/kg) to Chinese volunteers.
Keywords: Bivalirudin, LC–MS/MS, Pharmacokinetics, Human plasma, Anticoagulant
1. Introduction
In the past few decades, the anticoagulants heparin and warfarin have been crucial in the prevention and treatment of venous and arterial thromboembolism. However, both drugs have significant limitations, including adverse reactions such as immune-mediated thrombocytopenia with heparin and microvascular thrombosis with warfarin [1]. As a result, new anticoagulants have been sought with high affinity and specificity for thrombin. Among these, a number of analogs of hirudin have been synthesized, of which only bivalirudin is currently marketed.
Bivalirudin is a small polypeptide (2180 Da), comprising of 20 amino acids (d-Phe–Pro–Arg–Pro–Gly–Gly–Gly–Gly–Asn–Gly–Asp–Phe–Glu–Glu–Ile–Pro–Glu–Glu–Tyr–Leu). The N-terminal d-Phe–Pro–Arg–Pro region binds with high affinity to the active site of thrombin and the C-terminal Asn–Gly–Asp–Phe–Glu–Glu–Ile–Pro–Glu–Glu–Tyr–Leu dodecapeptide binds to the fibrinogen-binding region of thrombin. Through this dual mechanism of action, bivalirudin acts as a short acting reversible thrombin inhibitor which has been approved for percutaneous coronary intervention (PCI) and acute coronary syndromes (ACSs) [2].
Most previous studies of bivalirudin have focused on its pharmacodynamic properties in a variety of clinical settings [3], [4], [5], [6]. In contrast, reports of pharmacokinetic studies and the analytical methods required to support them are sparse. Farthing et al. [7] used liquid chromatography with fluorescence detection to quantitate bivalirudin in human plasma and urine but the method involved a time consuming derivatization step and with a lower limit of quantification (LLOQ) of 3 μg/mL, was relatively insensitive. Robson et al. [8] were the first to report a method based on liquid chromatography–tandem mass spectrometry (LC–MS/MS) with linear range of 0.5–25 μg/mL, also with relatively high LLOQ. Recently, Pan et al. [9] developed a sensitive LC–MS/MS method with LLOQ 1.25 ng/mL, but the gradient eluting chromatographic condition required a relatively long cycle time (7 min), and it also suffered from complicated and time-consuming (about 40 min) sample preparation, thus the total time for testing a sample was about 1 h from sample preparation to detection. In view of these limitations, we have developed a rapid and highly sensitive LC–MS/MS method for quantitation of bivalirudin in human plasma which combines simple sample preparation with high sensitivity (1 ng/mL), isocratic elution, a short run time (3.5 min). Moreover, this method only needs a small volume (100 μL) plasma, compared with relatively large volume (200 μL) reported by Pan et al. [9], which greatly reduces the distress and improves the compliance of volunteers in the process of blood samples collection. This rapid method shows high sample throughput (15 min for one sample preparation and 3.5 min for detection, indicating that more than 150 samples could be assayed daily) making it eminently suitable for large batches of biological samples in clinical pharmacokinetic studies. The assay was fully validated including an assessment of the adsorption of bivalirudin to polypropylene vials and was shown to be suitable for clinical pharmacokinetic studies through its successful application to a study involving intravenous administration of bivalirudin to healthy Chinese volunteers.
2. Experimental
2.1. Chemicals and reagents
Bivalirudin (purity>99% by HPLC) was kindly provided by Shenzhen Wanle Drug Research and Development Co. Ltd. (Shenzhen, PR China). The internal standard (IS), triptorelin (purity>99% by HPLC), was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, PR China). HPLC grade methanol and acetonitrile were purchased from Fisher Scientific (Fair Lawn, NJ, USA). All other chemicals were commercially available analytical grade materials used as received. Distilled water prepared from demineralized water was used throughout the study.
2.2. Apparatus
LC–MS/MS was performed on an Agilent 1100 Series HPLC system (Agilent Technologies, Palo Alto, CA, USA) connected to an API 4000 triple-quadrupole mass spectrometer (Applied Biosystems MDS Sciex, Ontario, Canada) equipped with an electrospray ionization (ESI) source. Data acquisition was controlled by the Analyst Software 1.3 (Applied Biosystems Sciex, Ontario, Canada).
2.3. Chromatography
Chromatography was performed on a Zorbax 300SB-C18 column (150 mm×4.6 mm i.d., 5 μm particle size, Agilent Technologies) maintained at 35 °C preceded by a Security Guard C18 guard column (4 mm×3.0 mm i.d., Phenomenex, USA). The mobile phase consisted of 0.1% formic acid:methanol (45:55, v/v) delivered at 0.8 mL/min.
2.4. Mass spectrometry
Tandem mass spectrometry was carried out in the positive ionization mode using multiple-reaction monitoring (MRM) of the precursor to product ion transitions at m/z 1091.0→650.4 for bivalirudin and m/z 656.5→249.3 for the IS. Optimized mass spectrometer conditions were as follows: curtain gas, gas 1 and gas 2 (all nitrogen) 15, 40 and 50 psi, respectively; dwell time 200 ms; ion spray voltage 5200 V; source temperature 450 °C; declustering potential (DP) 63 V for bivalirudin and 70 V for triptorelin; collision energy (CE) 57 eV for bivalirudin and 73 eV for triptorelin. Low resolution was set for both Q1 and Q3 mass detection.
2.5. Calibration standards and quality control samples
All solutions were prepared in methanol/water (50:50, v/v). A stock solution (1 mg/mL) of bivalirudin was diluted to give standard solutions with concentrations of 1000, 300, 100, 30, 10, 3 and 1 ng/mL. The QC solutions with concentrations of 240, 30 and 3 ng/mL were prepared independently in the same way. An IS stock solution (1 mg/mL) was used to prepare an IS working solution (500 ng/mL). All stock and working solutions were stored at 4 °C when not in use.
2.6. Sample preparation
Frozen plasma samples were thawed at room temperature prior to analysis. An aliquot of 100 μL plasma, 100 μL methanol/water (50:50, v/v) (or a standard or QC solution of bivalirudin), 50 μL 2% aqueous ammonia and 100 μL IS working solution were added to a 1.5 mL Eppendorf tube, and then 300 μL acetonitrile was added to precipitate the proteins. Tubes were vortex-mixed for 30 s and centrifuged at 15,000 rpm for 10 min. The supernatant was transferred to a 2 mL Eppendorf tube followed by 800 μL dichloromethane. After vortex-mixing for 30 s and centrifugation at 15,000 rpm for 3 min, an aliquot of 30 μL of the upper aqueous layer was injected into the LC–MS/MS system. The samples with concentrations exceeding the ULOQ (1000 ng/mL) were quantified by dilution of these samples with blank human plasma.
2.7. Adsorption to plastic
Because peptides are known to adsorb on solid-surfaces, the adsorption of bivalirudin to polypropylene vials was evaluated using a previously developed protocol [10]. The experiment involved 7 consecutive transfers of QC solutions and samples from one polypropylene vial (1.5 mL) to another. Vials were maintained at 4 °C and every 30 min 500 μL was transferred from one vial to the other and another 100 μL was removed for analysis.
2.8. Assay validation
To check the reliability and robust of this method and meet the guidelines required by the Food and Drug Administration (FDA) [11], the method was validated in terms of specificity, linearity, LLOQ, extraction recovery, matrix effect, accuracy, precision and stability studies.
2.9. Pharmacokinetic study
The clinical pharmacokinetic study protocol was approved by the local Ethics Committee and carried out at Peking University First Hospital, Beijing (China) according to the guidelines of the International Conference on Harmonization (ICH) [12]. Formal informed consent form was signed by each volunteer to participate in the study according to the principles of the Declaration of Helsinki. A total of 9 healthy Chinese male volunteers were enrolled in this study based on their results of clinical examinations and medical history. They were not allowed to consume alcohol or take any other medication during the study. After a 12 h fast, a group of healthy male volunteers (n=9) was administered single i.v. doses (0.5 mg/kg) of bivalirudin. Venous blood samples (approximately 1 mL) were collected into heparinized vacuum tubes prior to dosing and at 5, 10, 20, 30, 45, 60, 80, 100, 120 min after the dose. Blood samples were centrifuged at 15,000 rpm for 5 min at 4 °C and plasma samples were stored at −80 °C prior to analysis. Pharmacokinetic parameters were calculated with non-compartmental model using a TopFit 2.0 program [13], [14].
3. Results and discussion
3.1. The physicochemical characteristics of bivalirudin
The amphoteric behavior is one of the most important physicochemical properties of peptides. The isoelectric point (pI) of them is not only affected by N-terminal amino and C-terminal carboxyl groups, but also by the number of basic AAs (Lys, Arg and His) and acidic AAs (Asp and Glu) in structures. Bivalirudin comprises 20 amino acids (Phe–Pro–Arg–Pro–Gly–Gly–Gly–Gly–Asn–Gly–Asp–Phe–Glu–Glu–Ile–Pro–Glu–Glu–Tyr–Leu), which includes 5 acidic AAs (Glu and Asp) and 1 basic AA (Arg). The above explanation implies that the pI of bivalirudin is below 7, though there is no relative report about bivalirudin's amphoteric behavior. This result is significant in evaluating the solubility and the ionization properties for mass spectrometric detection of bivalirudin. Besides, in the structure there are no methionine, cysteine or tryptophan residues, which are potentially sensitive to oxidation, indicating that bivalirudin was stable in chemical structure and did not exhibit chemical changes when exposed to the air.
3.2. Chromatography
To optimize the chromatographic conditions in terms of robust, sensitivity, run time and peak shape, the following factors were considered:
3.2.1. Column selection
The 300SB-C18 column used in this study has a large pore size (300 Å) which makes it suitable for chromatography of high molecular weight peptides (>2000 Da) such as bivalirudin (MW 2180.3 Da). Pan et al. [9] used a small pore size (80 Å) column with less retention, poorer peak shape and a shorter column life. In our hands the 300SB-C18 column provided adequate retention and good peak shape.
3.2.2. Choice of mobile phase
Methanol was used as an organic modifier since it provided higher sensitivity and better peak shape for bivalirudin compared to acetonitrile. In positive ion mode, the presence of a low amount of acid in the mobile phase could improve the sensitivity of MS signal. Consequently, it was also found that a low concentration of formic acid (0.1%) in the mobile phase enhanced sensitivity and improved peak shape of bivalirudin. However, larger concentrations of formic acid or the use of ammonium acetate was found to suppress the MS signal.
3.2.3. Choice of internal standard
Stable, isotopically labeled analogs of peptides are commonly used as the IS in the LC–MS/MS assay of peptides. However, due to the lack of such commercially available analogs of bivalirudin, some structurally similar compounds (e.g. octreotide and triptorelin) were evaluated as the IS. Subsequently triptorelin was selected as an IS because it gave similar extraction recovery and LC retention time to bivalirudin.
3.3. Mass spectrometry
During the early stage of mass spectrometry method development, both the electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) sources were evaluated. Results showed that ESI could offer higher sensitivity for the bivalirudin than APCI under both positive and negative ion modes. Therefore, ESI was chosen as the ion source for further study.
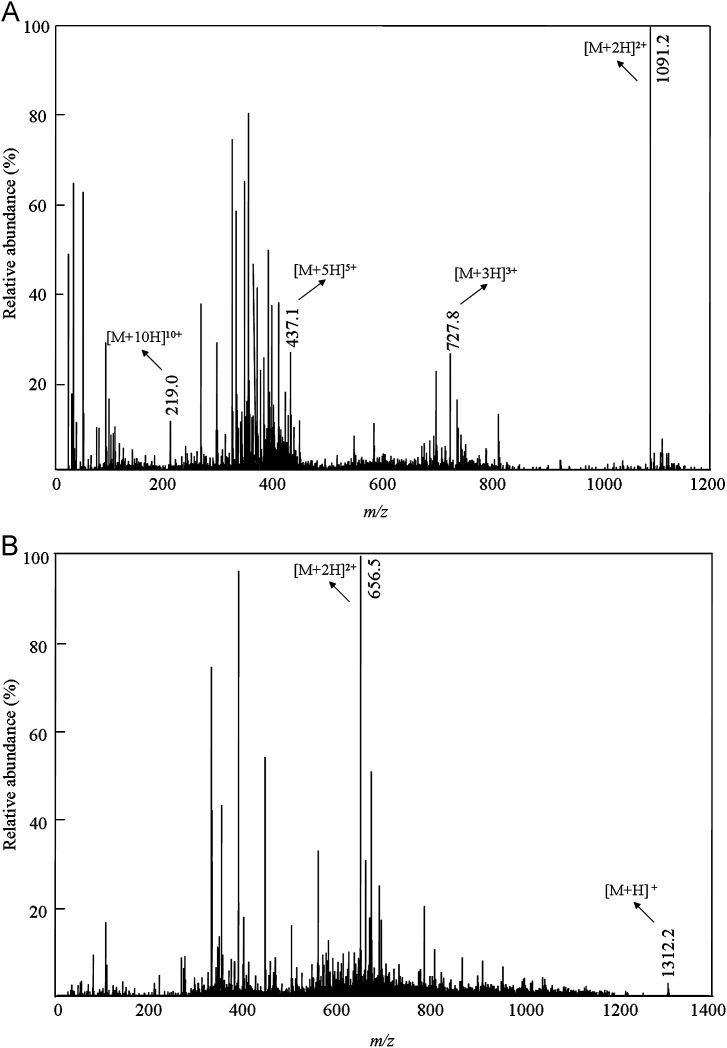
The optimization of mass spectrometer conditions was performed by direct infusion of solutions of bivalirudin and IS into the ESI source at a flow rate of 20 μL/min. The positive ion mode was selected since it produced higher intensity signals than the negative ion mode. Q1 MS full-scan spectra for bivalirudin (Fig. 1A) contained [M+2H]2+, [M+3H]3+, [M+5H]5+ and [M+10H]10+ protonated precursor ions at m/z 1091.2, 727.8, 437.1 and 219.0, respectively. The full-scan spectrum was dominated by molecule [M+2H]2+ (m/z 1091.2). Bivalirudin product ions at m/z 650.4, 356.2 and 227.1 shown in Fig. 2A correspond to the fragment ions m/z 650.9[y5]+, m/z 356.2[a3-NH3]+ and m/z 227.1[a4-NH3]2+, respectively. The transition at m/z 1091.2→650.4 was selected for the quantitation of bivalirudin because it provided the highest sensitivity using the optimized MS/MS parameters listed in Section 2.4. Full-scan spectra for IS (Fig. 1B) contained [M+H]+and [M+2H]2+ protonated precursor ions at m/z 1312.2 and 656.5, respectively. The IS product ions at m/z 249.3 and 221.3 shown in Fig. 2B correspond to the fragment ions m/z 249.3[b2]+ and m/z 221.3 [y4]2+, respectively. The higher sensitivity of m/z 249.3[b2]+ clearly indicates the basis of the choice of the transition at m/z 656.5→249.3 for determination of the IS.
Fig. 1.
Q1 MS full-scan spectra of bivalirudin (A) and triptorelin (B).
Fig. 2.
Full-scan product ion spectra of [M+2H]2+ ions for bivalirudin (A) and triptorelin (B).
3.4. Sample preparation
Sample preparation by liquid–liquid extraction (LLE), protein precipitation (PPT) and re-extraction the supernatant of PPT was evaluated in the present study. LLE with ethyl acetate gave poor recovery (<30%). In contrast, PPT using ice-cold methanol was superior to acetonitrile and gave high recovery and good peak shapes. However, the LLOQ was high (15 ng/mL) due to the dilution of analyte in PPT procedure. To improve the sensitivity, the deproteinated samples were re-extracted with dichloromethane according to previous studies [15], [16], [17], after centrifugalization bivalirudin and IS in upper aqueous layer were concentrated. As for extracting a peptide, the pH is vital for the recovery. In the present study, the re-extraction recovery was 3 times higher when 2% aqueous ammonia (pH=9) was added (Recovery 90%) compared to spiked 1% formic acid (pH=2.8). The LLOQ reached 1 ng/mL when 2% aqueous ammonia was added at re-extraction step, so this method was used to sample pre-treatment in the present study. The above sample preparation methods for bivalirudin extraction and their extraction effects are listed in Table 1.
Table 1.
Sample preparation methods for bivalirudin extraction from human plasma and their extraction effects.
| Sample preparation method | Extraction effect |
|---|---|
| LLE with ethyl acetate | Low recovery |
| PPT with ice-cold methanol | High recovery (>90%) and good peak shape |
| PPT with acetonitrile | High recovery (>90%) but poor peak shape |
| Re-extracted the supernatant of PPT with dichloromethane (added 1% formic acid) | Recovery about 30% |
| Re-extracted the supernatant of PPT with dichloromethane (added 2% aqueous ammonia) | Recovery about 90%, LLOQ can reach 1 ng/mL |
3.5. Adsorption to plastic
The adsorption of peptides to solid-surfaces is a significant issue in the analysis of peptides although few studies have investigated the phenomenon in detail. In this study the adsorption of bivalirudin to polypropylene vials was investigated during a series of transfers from one vial to another. No evidence of adsorption was detected.
3.6. Assay validation
The validation was carried out by analyzing drug free plasma samples after sample preparation in which standard or QC solutions replaced the 100 μL methanol/water (50:50, v/v).
3.6.1. Specificity and selectivity
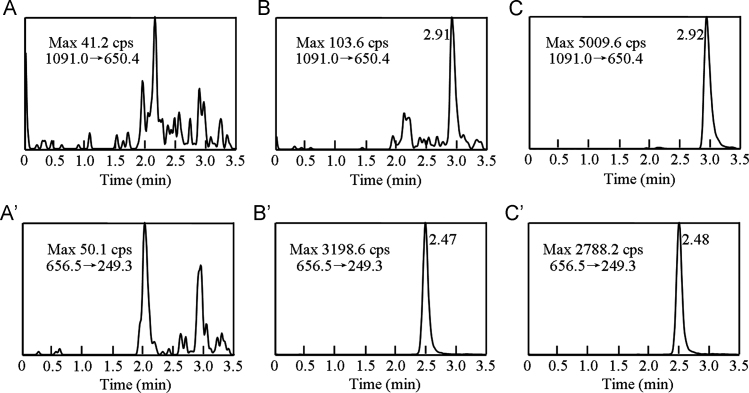
Typical MRM chromatograms of blank plasma, blank plasma spiked with bivalirudin at the LLOQ and a study sample 1 h after i.v. administration of bivalirudin are shown in Fig. 3. The retention times of bivalirudin and IS were at 2.91 min and 2.47 min, respectively. No significant interference from endogenous substances was observed in the retention time windows of bivalirudin and IS.
Fig. 3.
MRM chromatograms for bivalirudin[(A), (B), (C)] and triptorelin(IS)[(A′), (B′), (C′)] in human plasma samples. (A), (A′): a blank plasma sample; (B), (B′): a blank sample spiked with bivalirudin at the LLOQ (1 ng/mL) and IS (500 ng/mL); (C), (C′): a plasma sample from a human volunteer 1 h after intravenous administration of bivalirudin (0.5 mg/kg). Max is the maximum of the relative intensity.
3.6.2. Linearity of calibration curves and LLOQ
Two independent calibration curves based on analyte:IS peak area ratios were prepared on three separate days and analyzed by weighted linear regression (1/x2) to validate the linearity of the method. The LLOQ was the lowest concentration of analyte that could be determined with precision≤20% and accuracy±20%. The limit of detection (LOD) was determined as the concentration with signal-to-noise ratio of 3.
The assay was linear in the concentration range 1–1000 ng/mL (r>0.997) with an LOD of 0.3 ng/mL. A typical standard curve of a validation intra-day assay (after weighted linear regression 1/x2) is y=0.00561x+0.000698, r=0.9971, where x represents the plasma concentration (ng/mL) of bivalirudin, and y is the peak area ratio of bivalirudin to IS.
3.6.3. Recovery and matrix effect
Recovery of bivalirudin was determined by comparing peak areas of QC samples with those of blank plasma samples spiked with standard solutions at corresponding concentrations. The recoveries of bivalirudin at concentrations of 3, 30, and 240 ng/mL were 98.6±3.6%, 99.1±4.3%, and 97.8±2.9%, respectively. The recovery of the IS was 97.3±5.3%.
Matrix effects were evaluated by comparing peak areas of post-extraction blank plasma spiked with QC solutions with peak areas of post-extraction aqueous blank samples spiked with QC solutions. In the study of matrix effects, concentrations of bivalirudin and IS were 94.2%, 95.8% and 97.0% of nominal concentrations respectively, indicating that no significant signal suppression or enhancement occurred in the ionization of bivalirudin or IS.
3.6.4. Precision and accuracy
Intra- and inter-day precision (as relative standard deviation, RSD) and accuracy (as relative error, RE) were assessed by assay of QC samples (n=6) on three separate days.
Table 2 summarizes the accuracy and precision data based on analysis of QC samples. Intra- and inter-day precisions were 0.62–2.92% and 1.84–3.36%, respectively with accuracy of 99.6–100.5%.
Table 2.
Precision and accuracy for the determination of bivalirudin in human plasmaa.
| Nominal concentration (ng/mL) | Calculated concentration (ng/mL) | Intra-day precision RSD (%) | Inter-day precision RSD (%) | Accuracy RE (%) |
|---|---|---|---|---|
| 3.0 | 2.99±0.07 | 2.92 | 2.35 | −0.3 |
| 30.0 | 29.9±0.5 | 0.62 | 1.84 | −0.4 |
| 240.0 | 241.1±9.0 | 2.63 | 3.36 | 0.5 |
Data are based on assay of 6 replicates on 3 different days.
3.6.5. Stability
Stability of bivalirudin was tested by analysis of QC samples under the following conditions: long-term stability in plasma samples maintained at −80 °C for 75 days; short-term stability in plasma samples maintained at 25 °C for 4 h; in processed samples in autosampler vials for 7 h; and after three freeze/thaw cycles (−20 to 25 °C). Stability of stock solutions stored at 4 °C for one week was also assessed.
In the present study, bivalirudin was shown to be stable under all the storage conditions evaluated with mean recoveries of 93.7–101.4% of the nominal concentrations (Table 3). Stock solutions of bivalirudin and IS stored for one week at 4 °C were comparable to freshly prepared ones.
Table 3.
Stability of bivalirudin under various storage conditions (n=3).
| Storage conditions | Nominal concentration (ng/mL) | Calculated concentration (ng/mL) | RSD (%) | RE (%) |
|---|---|---|---|---|
| Room temperature for 4 h | 3.0 | 3.04±0.10 | 4.72 | 1.44 |
| 30.0 | 29.8±0.5 | 1.51 | −0.78 | |
| 240.0 | 243.0±8.1 | 3.35 | 1.40 | |
| Processed QC samples under autosampler conditions | 3.0 | 2.95±0.10 | 2.76 | −1.56 |
| 30.0 | 29.9±0.9 | 2.85 | −0.44 | |
| 240.0 | 241.0±11.7 | 4.85 | 0.30 | |
| Three freeze/thaw cycles | 3.0 | 2.99±0.10 | 1.84 | −0.22 |
| 30.0 | 29.7±1.3 | 4.38 | −0.89 | |
| 240.0 | 239.0±4.5 | 1.89 | −6.30 | |
| Storage at −80 °C for 75 days | 3.0 | 2.97±0.10 | 2.99 | −0.22 |
| 30.0 | 29.8±0.3 | 1.01 | −0.67 | |
| 240.0 | 239.0±9.8 | 4.12 | −0.40 | |
3.7. Pharmacokinetic study
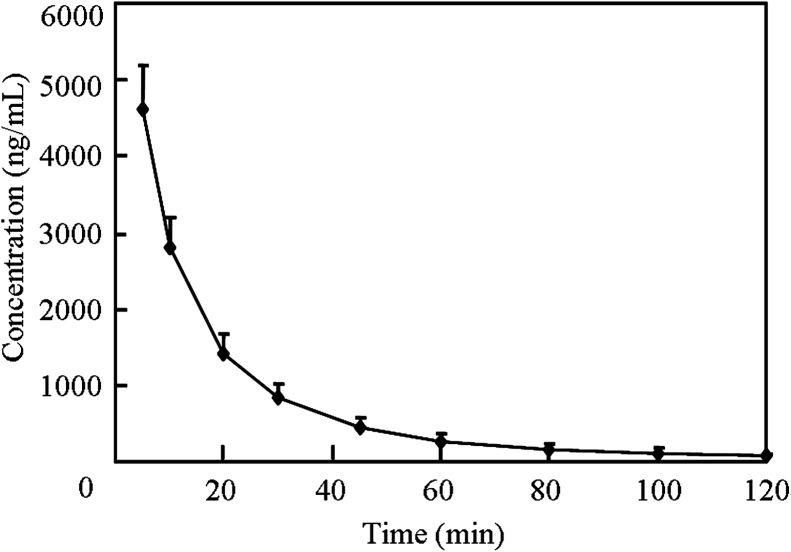
The plasma concentration–time profile of bivalirudin after a single i.v. dose (0.5 mg/kg) in 9 healthy volunteers is illustrated in Fig. 4. Corresponding pharmacokinetic parameters are listed in Table 4. After i.v. administration, the concentration of bivalirudin was eliminated with a half-life (t1/2) of 20.2±1.7 min and the clearance 5.8±0.9 mL/min/kg. The area under the plasma concentration–time curve (AUC0−t) was 88,630±16,690 ng min/mL and the volume of distribution (Vd) of bivalirudin was 0.17±0.02 L/kg. These pharmacokinetic parameters are similar to those reported by Pan et al. [9] and in other previous studies [6], [8], [18].
Fig. 4.
Mean plasma concentration–time profile of bivalirudin after a single intravenous dose of 0.5 mg/kg (Data are mean±SD, n=9.).
Table 4.
Pharmacokinetic parameters for bivalirudin in healthy volunteers after a single intravenous dose (0.5 mg/kg).
| Parameters | Mean±SDa |
|---|---|
| AUC0−t (ng min/mL) | 88,630±16,690 |
| AUC0−∞ (ng min/mL) | 90,700±18,670 |
| t1/2 (min) | 20.2±1.7 |
| MRT0−t (min) | 24.0±2.0 |
| CL (mL/min/kg) | 5.8±0.9 |
| Vd (L/kg) | 0.17±0.02 |
Data are mean±SD, n=9.
4. Conclusion
The present LC–MS/MS method is a more sensitive, versatile and rapid assay for the determination of bivalirudin in human plasma with a run time of only 3.5 min. The method only needs a simple re-extraction sample pre-treatment, no interference caused by endogenous compounds was observed. More than 150 samples could be assayed daily, including sample extraction, data collection and processing. The LLOQ was 1 ng/mL and LOD was 0.3 ng/mL, with only 100 μL of plasma sample in this method, which greatly reduces the distress and improves the compliance of the volunteers. The assay was successfully applied to a clinical pharmacokinetic study of bivalirudin in healthy volunteers, which indicate that it is suitable for analysis of large batches of bivalirudin biological samples.
Acknowledgments
The authors are grateful to the National Natural Science Foundation (30973587), the China Postdoctoral Science Foundation (20110491328), and the National Natural Science Funds for Young Scholar (81102383) for financial support.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Yi-Min Cui, Email: cuiymzy@126.com.
Jing-Kai Gu, Email: gujk@jlu.edu.cn.
References
- 1.Warkentin T.E. Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract. Res. Clin. Haematol. 2004;17:105–125. doi: 10.1016/j.beha.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Lehman S.J., Chew D.P. Bivalirudin in percutaneous coronary intervention. J. Vasc. Health Risk Manage. 2006;2:357–363. doi: 10.2147/vhrm.2006.2.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rha S.W., Kuchulakanti P.K., Pakala R. Bivalirudin versus heparin as an antithrombotic agent in patients treated with a sirolimus-eluting stent. Am. J. Cardiol. 2004;94:1047–1050. doi: 10.1016/j.amjcard.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 4.Gladwell T.D. Bivalirudin: a direct thrombin inhibitor. Clin. Ther. 2002;24:38–58. doi: 10.1016/s0149-2918(02)85004-4. [DOI] [PubMed] [Google Scholar]
- 5.Gurm H.S., Rajagopal V., Fathi R. Effectiveness and safety of bivalirudin during percutaneous coronary intervention in a single medical center. Am. J. Cardiol. 2005;95:716–721. doi: 10.1016/j.amjcard.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Koster A., Spiess B., Chew D.P. Effectiveness of bivalirudin as a replacement for heparin during cardiopulmonary bypass in patients undergoing coronary artery bypass grafting. Am. J. Cardiol. 2004;93:356–359. doi: 10.1016/j.amjcard.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Farthing D., Larus T., Fakhry I. Liquid chromatography method for determination of bivalirudin in human plasma and urine using automated ortho-phthalaldehyde derivatization and fluorescence detection. J. Chromatogr. B. 2004;802:355–359. doi: 10.1016/j.jchromb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Robson R., White H., Aylward P. Bivalirudin pharmacokinetics and pharmacodynamics: effect of renal function, dose, and gender. Clin. Pharmacol. Ther. 2002;71:433–439. doi: 10.1067/mcp.2002.124522. [DOI] [PubMed] [Google Scholar]
- 9.Pan G.X., Wang X.M., Huang Y.H. Development and validation of a LC–MS/MS method for determination of bivalirudin in human plasma: application to a clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010;52:105–109. doi: 10.1016/j.jpba.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 10.John H., Walden M., Schafer S. Analytical procedures for quantification of peptides in pharmaceutical research by liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 2004;378:883–897. doi: 10.1007/s00216-003-2298-y. [DOI] [PubMed] [Google Scholar]
- 11.Guidance for Industry: Bioanalytical Method Validation, Food and Drug Administration, USA, 2001.
- 12.ICH Harmonised Tripartite Guideline, Guideline for Good Clinical Practice, 1996.
- 13.Tanswell P., Koup J. TopFit: a PC-based pharmacokinetic/pharmacodynamic data analysis program. Int. J. Clin. Pharmacol. Ther. Toxicol. 1993;31:514–520. [PubMed] [Google Scholar]
- 14.Ponnurua V.S., Challab B.R., Nadendla R. Quantification of desloratadine in humanplasma by LC–ESI-MS/MS and application to a pharmacokinetic study. J. Pharm. Anal. 2012;2:180–187. doi: 10.1016/j.jpha.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng C.L., Chou C.H. Determination of metformin in human plasma by high-performance liquid chromatography with spectrophotometric detection. J. Chromatogr. B Biomed. Sci. Appl. 2001;762:51–58. doi: 10.1016/s0378-4347(01)00342-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y.W., Tang Y.B., Gu J.K. Rapid and sensitive liquid chromatography–tandem mass spectrometric method for the quantitation of metformin in human plasma. J. Chromatogr. B. 2004;808:215–219. doi: 10.1016/j.jchromb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Li X., Wang Y.W., Wang J. Determination of miglitol in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:247–251. doi: 10.1002/rcm.2822. [DOI] [PubMed] [Google Scholar]
- 18.Koster A., Spiess B., Jurmann M. Bivalirudin provides rapid, effective, and reliable anticoagulation during off pump coronary revascularization: results of the “EVOLUTION OFF” trial. Anesth. Analg. 2006;103:540–544. doi: 10.1213/01.ane.0000226098.95698.0f. [DOI] [PubMed] [Google Scholar]