Abstract
To enable reliable quantification of natamycin in rabbit and human plasma, a validated, sensitive and selective liquid chromatography–tandem mass spectrometry assay was developed. The chromatographic separation was achieved isocratically on a Cyano column using methanol: aqueous 3.5 mM ammonium acetate (pH 4) (90:10 v/v). The assay was validated over a concentration range of 6.25–400 ng/mL with lower limit of detection of 3.12 ng/mL. Quantification was performed using the transitions 664.5→137.2m/z for natamycin and 923.5→183.4m/z for the IS. The method was validated with respect to linearity, accuracy, precision, recovery and stability. This assay has been successfully applied to a pharmacokinetic study of natamycin in NZ rabbit and plasma protein binding in human plasma.
Keywords: Natamycin, LC–MS/MS, Pharmacokinetics, Protein binding
1. Introduction
Natamycin (NAT), known as pimaricin, is a naturally occurring product of Streptomyces species. NAT has been used to treat several human clinical fungal infections, such as candidiasis and trichomoniasis [1], [2], [3]. NAT medical utilization is currently confined to the topical treatment (commercially available as topical 5% suspension for ophthalmic use) of corneal fungal infections and prevention of such infections in contact lens users [2], [4]. As reported in British National Formulary, natamycin is sold under the trade name “Pimafucin” in oral suspension, suspension for inhalation, cream and vaginal tablet formulations. The parenteral formulation of NAT offers a best alternative as it could be used for systemic fungal infections [5]. NAT has been approved as food preservative in several countries and applied widely in cheese, meat products, wine and other foods [6], [7]. Besides this, NAT is also used as feed efficiency agent in feed of swine, poultry, dairy animals, rabbits, fish and other animals [8]. Based on its potential to be used in many ways such as food preparations and in various fungal infections (topically and systemic), it will be interesting to explore its pharmacokinetics characteristics. The in-vivo preclinical and clinical disposition characteristics of NAT have not been reported in details, probably due to lack of sensitive bioanalytical method.
Presently, there are few articles reported for the analytical method of NAT in wine and other food stuff [7], [9], [10]. However, only two bioanalytical methods have been reported. One describes the HPLC-UV bioanalytical method for estimation of NAT in canine plasma and the other LC–MS/MS method for ocular pharmacokinetics [5], [11]. The application of LC–MS/MS is currently considered the method of choice for supporting pharmacokinetic and toxicokinetic studies due to its selectivity, sensitivity and short run time [12], [13].
Therefore, the objective of this study was, in first instance the development of a rapid, selective, highly sensitive and robust method for the determination of NAT in human and rabbit plasma. The validated method has been successfully applied to the evaluation of NAT pharmacokinetics in NZ rabbits after i.v. administration and its plasma protein binding determination in human plasma. The present method can also be exploited in preclinical and clinical pharmacokinetic/toxicological evaluation of NAT.
2. Materials and methods
2.1. Chemicals and materials
Natamycin and amphotercin B (Internal standard; IS) of pharmaceutical grade were gifted by Cipla Ltd. (Mumbai, India). Methanol (HPLC grade) was purchased from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India). Oasis HLB 3 cc, 60 mg solid phase extraction cartridges were procured from Waters (India) Pvt. Ltd. Ultrapure water was obtained from a Milli-Q PLUS PF water purification system. All other reagents were of analytical grade.
2.2. LC–MS/MS conditions
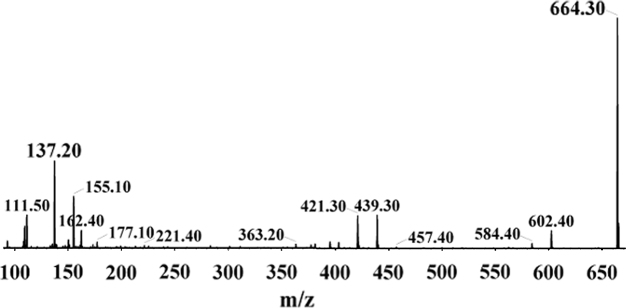
The system consisted of a Shimadzu UFLC pump (LC-20AD) with degasser (DGU-20A3) and autosampler (SIL-HTc), with an API-4000Q trap mass spectrometer (Applied Biosystems-SCIEX, Canada), in negative ionization mode. In this study, chromatographic separation was achieved on a Phenomenex, Luna 3 μm Cyano column (3 μm, 100 mm×2 mm). The mobile phase was composed of ammonium acetate buffer (3.5 mM, pH 4):methanol (10:90, v/v), and eluted at a flow rate of 0.3 mL/min. Injection volume was set at 20 μL. The spray needle voltage was −4500 V and the source temperature was 300 °C. The curtain gas was 10 psi and entrance potential was −10 V. The collision energy was −35 eV for both NAT and IS. The detection and quantification of analytes were performed using the multiple reaction monitoring (MRM) mode using ion precursor→product ion combinations of 664.5→137.2m/z for NAT (Fig. 1) and 923.5→183.4m/z for the IS. Data acquisition and processing were performed with Analyst software 1.4.1.
Fig. 1.
MS/MS ion spectra of natamycin.
2.3. Preparation of standard and quality control samples
The standard stock solutions of 1 mg/mL for NAT and amphotericin B (IS) were prepared in methanol and DMSO respectively. Further working standards of NAT and IS were prepared in methanol. Calibration standard and quality control (QC) samples were prepared by adding appropriate volumes of working standard solutions to 200 μL blank human plasma. The final calibration standard concentrations were in the range of 6.25–400 ng/mL. Final QC samples concentrations were 7.5, 12.5, 125 and 375 ng/mL. In all calibration and QC samples 20 μL IS (20 μg/mL) was added.
2.4. Sample preparation
The calibration standards, QC and plasma samples were extracted by solid phase extraction (SPE) using Oasis HLB cartridge. Prior to SPE, a 20 μL aliquot of IS working solution was added to all plasma samples except blank plasma sample, to which a 750 μL aliquot of 0.1% ammonia solution was added. The SPE cartridge was conditioned sequentially with 2.0 mL of methanol and 1.0 mL of ultrapure water (UPW). Then plasma sample mixture and 250 μL of 0.1% aqueous ammonia solution were loaded. The cartridge was sequentially washed with 1.0 mL UPW and 1 mL of 5% aqueous methanol. Finally NAT was eluted from cartridge with 2.0 mL methanol. The eluted samples were dried under vacuum and residue was reconstituted in 200 μL of methanol and 20 μL was injected into LC–MS/MS.
2.5. Method validation
The method was validated for selectivity, accuracy, precision, recovery, matrix effect and stability according to the FDA guideline for validation of bioanalytical methods [14]. The lower limit of detection (LLOD) was evaluated by spiking the analyte into six replicates in pooled rabbit and human plasma followed by extraction and quantification. The specificity was investigated for potential matrix interferences in six lots of blank rabbit and human plasma by extraction and inspection of the resulting chromatograms for interfering peaks at the retention times of the analyte and IS. Linearity was tested at six different concentrations, covering a range of 6.25–400 ng/mL. The calibration curves were established by plotting the peak area ratio of analyte and IS versus concentration. To evaluate the precision and accuracy of the method, QC samples at four concentration levels (7.5, 12.5, 125 and 375 ng/mL of NAT) were analyzed in five replicates on five different days. The recovery of NAT and the IS from human plasma during extraction was determined at 12.5 and 400 ng/mL by comparing the response ratio in rabbit and human plasma samples spiked with the analyte prior to extraction with those spiked post-extraction. The matrix effect was assessed at concentrations of 12.5 and 400 ng/mL for NAT and IS by comparing the corresponding peak areas of the post-extraction spiked samples to those of the standard solutions evaporated directly and reconstituted in mobile phase [15]. All stability studies were carried out at 12.5 and 400 ng/mL.
2.6. Application of the method
2.6.1. In-vitro plasma protein binding
NAT plasma protein binding in human plasma was performed at three different concentrations (6.25, 50 and 400 ng/mL). The bound and unbound fractions of NAT were separated from human plasma samples by ultra-filtration through the centrifuge micro partition system (Amicon, Centrifree device Inc., MA, USA) [16], [17]. Samples in duplicates (0.5 mL) were placed in Centrifree devices and centrifuged at 1500g for 10 min to collect approximate 10% (100 μL) of the original volume of plasma as ultrafiltrate. Non-specific binding of NAT was determined by spiking test concentration into 0.01 M phosphate buffer (pH 7.4) and applied same procedure as plasma. The in-vitro samples and their respective ultra-filtrates were analyzed by LC–MS/MS. The non-specific binding and plasma protein binding were determined using the following equations:
Non-specific binding (%)=100−[(conc. in buffer ultrafiltrate)×100/conc. in buffer]
Plasma protein binding (%)=100−[(conc. in plasma ultrafiltrate)×100/conc. in plasma].
2.6.2. Pharmacokinetic study
The animal studies were carried out as per the guidelines of the local ethical committee on animal experimentation. To assess the applicability of the assay, intravenous pharmacokinetic at 1 mg/kg dose was carried out in NZ rabbit (n=3). NAT was administered i.v. at a dose of 1.0 mg/kg. Blood samples (∼1.0 mL) were collected into heparinized tubes via the marginal ear vein at 0.083, 0.16, 0.33, 0.5, 0.75, 1, 2, 3, 4, 6, and 8 h post-dose. Plasma was separated by centrifugation (2200 rpm, 5 min) and stored at −80 °C until analysis. The plasma concentration versus time data for NAT was analyzed with a non-compartmental method using the WinNonlin software (ver. 5.1; Pharsight, Mountain View, CA, USA).
3. Results and discussion
3.1. Method development and validation
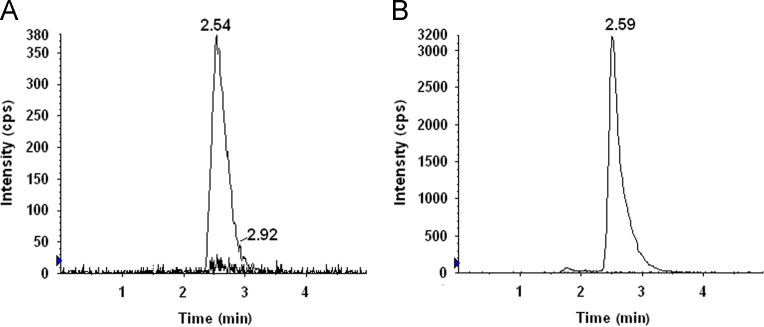
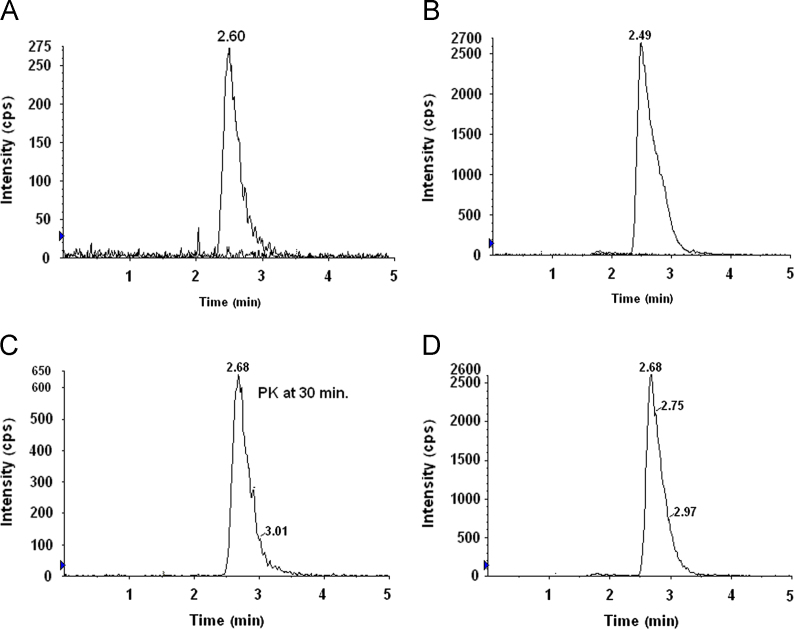
Our previously developed LC–MS/MS assay was reported for estimation of NAT in tear for ocular pharmacokinetic studies [11]. In view of need for the sensitive and selective LC–MS/MS method for estimation of NAT in plasma (human and rabbit) was developed. No significant interference was detected at the retention times of the analyte and IS, in the six different blank plasma chromatograms (Fig. 2, Fig. 3). Calibration curves were linear over the range 6.25–400.0 ng/mL for NAT. Correlation coefficients (r2) were >0.9981 for NAT with a weighted factor 1/x2. The limit of detection (LOD) was 3.12 ng/mL. The obtained data for intra-run and inter-run precision and accuracy were within the acceptable limit as per FDA guideline (Table 1). The mean recovery of NAT (n=5), determined at 12.5 and 400 ng/mL, was 72.37% and 84.10%, respectively. NAT was stable during three freeze–thaw cycles, long-term (30 days) and auto-sampler stability (24 h) at 4 °C (Table 2). The matrix effect for NAT at 12.5, and 400 ng/mL concentration levels in plasma was less than ±15 %. Thus no significant matrix effect was observed.
Fig. 2.
Typical MRM chromatograms of (A) overlay of blank plasma and NAT spiked in human plasma (25 ng/mL), (B) overlay of blank plasma and IS spiked in plasma for IS (2 μg/mL).
Fig. 3.
MRM chromatograms of (A) overlay of blank plasma and NAT spiked (25 ng/mL) in rabbit plasma, (B) overlay of blank plasma and IS spiked in plasma for IS (2 μg/mL), (C) pharmacokinetic sample at 30 min of NAT and (D) pharmacokinetic sample at 30 min of IS.
Table 1.
Accuracy (% bias) and precision (% RSD) of NAT.
| Conc. | Theoretical conc. (ng/mL) | Rabbit plasma |
Human plasma |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Intra-assay |
Inter-assay |
Intra-assay |
Inter-assay |
||||||
| % Bias | RSD (%) | % Bias | RSD (%) | % Bias | RSD (%) | % Bias | RSD (%) | ||
| LLOQ | 6.5 | −1.4 | 5.1 | −4.8 | 7.9 | −0.2 | 3.5 | 1.2 | 4.2 |
| LQC | 7.5 | 8.9 | 8.9 | 9.8 | 11.8 | −5.4 | 5.4 | −4.3 | 3.1 |
| MQC | 125.0 | 0.8 | 4.9 | 2.8 | 5.8 | 0.8 | 4.1 | 1.4 | 7.2 |
| HQC | 375.0 | −1.1 | 4.6 | −2.2 | 6.3 | −2.7 | 3.9 | −3.1 | 8.0 |
Table 2.
Stability data of NAT in NZ rabbit and human plasma.
| Storage conditions | Nominal conc. (ng/mL) | Rabbit plasma |
Human plasma |
||||
|---|---|---|---|---|---|---|---|
| Measured mean conc. (ng/mL) | CV (%) | Accuracy (%) | Measured mean conc. (ng/mL) | CV (%) | Accuracy (%) | ||
| Freeze–thaw stability (−80 °C) | 12.5 | 13.2±0.7 | 5.5 | 98.9 | 13.1±0.8 | 6.1 | 101.7 |
| 400 | 429.6±14.2 | 3.3 | 99.4 | 422.4±19.3 | 4.6 | 95.2 | |
| Long-term stability (−80 °C, 30 days) | 12.5 | 12.9±0.1 | 3.6 | 101.2 | 12.8±0.4 | 1.6 | 103.7 |
| 400 | 408.3±3.6 | 3.1 | 102.5 | 402.6±11.6 | 2.9 | 96.4 | |
| Auto-sampler stability (4 °C, 24 h) | 12.5 | 12.4±0.9 | 6.8 | 94.2 | 12.2±0.5 | 4.2 | 91.5 |
| 400 | 406.7 ±7.3 | 4.8 | 97.1 | 407.0±15.1 | 3.9 | 99 | |
| Dry residue stability (−4 °C, 48 h) | 12.5 | 12.2±0.4 | 2.9 | 93.1 | 12.1±0.6 | 5.0 | 91.2 |
| 400 | 411.3±5.0 | 1.2 | 98.2 | 417.3±12.8 | 3.1 | 101.5 | |
| Bench-top stability (at ambient temperature, 6h) | 12.5 | 13.4±0.8 | 6.2 | 101.8 | 12.4±0.5 | 4.0 | 93.5 |
| 400 | 434.1±12.7 | 6.0 | 103.7 | 444.0±11.3 | 2.6 | 108.0 | |
3.2. Application of the method
3.2.1. In-vitro plasma protein binding studies
The present in-vitro study was carried out to determine the extent of plasma protein binding of NAT. Bound and unbound drug from spiked/treated plasma was separated by the ultra-filtration technique. Plasma protein binding of NAT was found 76.23%, 73.40% and 71.37% at 6.25, 50 and 400 ng/mL respectively. Concentration dependent human plasma protein binding was not observed within the selected concentration range (6.25–400 ng/mL). Non-specific binding of NAT was found<5%.
3.2.2. Pharmacokinetics study
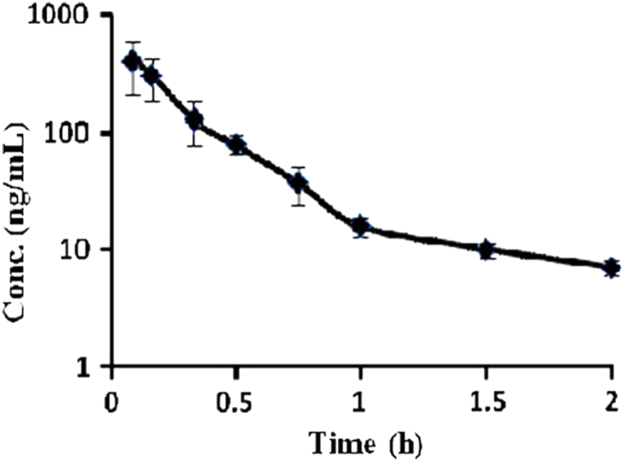
The validated method performed well during sample analysis of preclinical pharmacokinetics samples. The concentration–time curve was smooth enough to estimate pharmacokinetic parameters and the elimination phase was well fitted to the first-order one compartment pharmacokinetic model (Fig. 4 and Table 3). The sensitivity and specificity of the assay were sufficient for accurately characterizing the pharmacokinetics profile of NAT in NZ rabbits.
Fig. 4.
Plasma concentration–time profile of NAT after intravenous administration (n=3).
Table 3.
Pharmacokinetic profile of NAT after intravenous administration in NZ rabbit.
| Parameters | Estimates |
|---|---|
| C0 (ng/mL) | 537±322 |
| AUC0−∞ (h*ng/mL) | 162±55 |
| t1/2 (h) | 1.4±0.4 |
| Vd (L/kg) | 8.3±2.8 |
| Cl (L/h/kg) | 6.6±1.9 |
Abbreviation: C0: concentration at time zero, AUC: area under the curve from 0 to ∞, Vd: volume of distribution, Cl: clearance, t1/2: terminal half-life, n=3.
4. Conclusion
The current validated LC–MS/MS method for NAT offers significant advantages in terms of sensitivity and selectivity, sample preparation, short run time (5 min) and lower volume of sample requirements (∼200 μL). From the results of all the validation parameters and applicability of the assay, we can conclude that the present method can be satisfactorily used for pharmacokinetic characterization (both preclinical and clinical) of NAT. The present method can also be exploited in pharmacokinetic/toxicokinetic evaluation of NAT with desired precision and accuracy along with high-throughput.
Acknowledgments
Authors Yashpal Singh Chhonker and Hardik Chandasana are also thankful to the ICMR and CSIR for fellowship, respectively. The CSIR-CDRI communication number is 8332.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Asbell P., Stenson S. Ulcerative keratitis: survey of 30 years' laboratory experience. Arch. Ophthalmol. 1982;100:77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan M. Fungal keratitis. Curr. Opin. Ophthalmol. 2004;15:321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Shukla P.K., Kumar M., Keshava G.B. Mycotic keratitis: an overview of diagnosis and therapy. Mycoses. 2008;51:183–199. doi: 10.1111/j.1439-0507.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaur I.P., Rana C., Singh H. Development of effective ocular preparations of antifungal agents. J. Ocul. Pharmacol. Ther. 2008;24:481–493. doi: 10.1089/jop.2008.0031. [DOI] [PubMed] [Google Scholar]
- 5.S.B. Andersson, E.J. Anaissie, Parenteral Pimaricin as Treatment of Systemic Infection, US Patent no. 6045815, 2000.
- 6.Reps A., Drychowski L., Tomasik J. Natamycin in ripening cheeses. Pak. J. Nutr. 2002;1:243–247. [Google Scholar]
- 7.Alberts P., Stander M.A., de Villiers A. Development of a fast, sensitive and robust LC-MS/MS method for analysis of nataycin in wine. S. Afr. J. Enol. Vitic. 2010;32:51–59. [Google Scholar]
- 8.A.F. Carter, Animal Feed Method Employing Natamycin, US Patent no. 4,536,494, 1985.
- 9.Repizo L.M., Martinez L.D., Olsina R.A. A novel and rapid method for determination of natamycin in wines based on ultrahigh-performance liquid chromatography coupled to tandem mass spectrometry: validation according to the 2002/657/EC European decision. Anal. Bioanal. Chem. 2012;402:965–973. doi: 10.1007/s00216-011-5481-6. [DOI] [PubMed] [Google Scholar]
- 10.Capitán-Vallvey L.F., Checa-Moreno R., Navas N. Rapid ultraviolet spectrophotometric and liquid chromatographic methods for the determination of natamycin in lactoserum matrix. J. AOAC Int. 2000;83:802–808. [PubMed] [Google Scholar]
- 11.Bhatta R.S., Chandasana H., Rathi C. Bioanalytical method development and validation of natamycin in rabbit tears and its application to ocular pharmacokinetic studies. J. Pharm. Biomed. Anal. 2010;54:1096–1100. doi: 10.1016/j.jpba.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Ponnuru V.S., Challa B.R., Nadendla R. Quantification of sibutramine and its two metabolites in human plasma by LC–ESI-MS/MS and its application in a bioequivalence study. J. Pharm. Anal. 2012;2:249–257. doi: 10.1016/j.jpha.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatta R.S., Kumar D., Chhonker Y.S. Simultaneous estimation of E- and Z-isomers of guggulsterone in rabbit plasma using liquid chromatography tandem mass spectrometry and its application to pharmacokinetic study. Biomed. Chromatogr. 2011;25:1054–1060. doi: 10.1002/bmc.1574. [DOI] [PubMed] [Google Scholar]
- 14.Guidelines for Industry: Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research Center (CDER), 2001.
- 15.Matuszewski B.K., Constanzer M.L., Chavez-Eng C.M. Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal. Chem. 1998;70:882–889. doi: 10.1021/ac971078+. [DOI] [PubMed] [Google Scholar]
- 16.Bekersky I., Fielding R.M., Dressler D.E. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 2002;46:834–840. doi: 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Y.H., Wang J.Y., Hu H.H. Analysis of species-dependent hydrolysis and protein binding of esmolol enantiomers. J. Pharm. Anal. 2012;2:220–225. doi: 10.1016/j.jpha.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]