Abstract
Volatile components from Rhizoma Alpiniae Officinarum were respectively extracted by three methods including hydrodistillation, headspace solid-phase microextraction (HS-SPME) and diethyl ether extraction. A total of 40 (hydrodistillation), 32 (HS-SPME) and 37 (diethyl ether extraction) compounds were respectively identified by gas chromatography–mass spectrometry (GC/MS) and 22 compounds were overlapped, including α-farnesene, γ-muurolene, 2,6-dimethyl-6-(4-methyl-3-pentenyl)bicyclo[3.1.1]hept-2-ene, eucalyptol and cadina-1(10), 4-diene and so forth, varying in relative contents. HS-SPME is fast, sample saving and solvent-free and it also can achieve similar profiles as those from hydrodistillation and solvent extraction. Therefore, it can be the priority for extracting volatile components from medicinal plants.
Keywords: Rhizoma Alpiniae Officinarum, Volatile components, Hydrodistillation, Headspace solid-phase microextraction, Diethyl ether extraction, Gas chromatography–mass spectrometry
1. Introdution
Rhizoma Alpiniae Officinarum (RAO), the dry root and rhizome of Alpinia officinarum Hance, is a traditional Chinese medicine (TCM) mainly distributed in southern China [1]. RAO has long been used in practice for its antioxidation, antidiabetic, anti-ulcer, anti-diarrhea, antiemetic, analgesia, anti-inflammatory and anticoagulation effects [2], [3]. Flavonoids, volatile components and diarylheptanoids are reported as the main constituents of RAO and volatile components contribute a lot to those bioactivities [4], [5]. Consequently, identifying and determining its volatile components make sense for quality control of the crude material.
Volatile components of TCMs can be extracted by many technologies including hydrodistillation, headspace solid-phase microextraction (HS-SPME) and solvent extraction [6]. Among of them, HS-SPME, a new sample pretreatment technique, was invented by Pawliszyn (University of Waterloo, Canada). Typically, the analytes are extracted from a sample adsorption on a thin polymer coating fiber inside an injection needle [7], [8], [9]. It is usually combined with gas chromatography–mass spectrometry (GC/MS) to analyze volatile components in natural products and foods [10].
In this paper, three sampling methods coupled with GC/MS, i.e., hydrodistillation, HS-SPME and diethyl ether extraction were compared and used for analysis of volatile components of RAO.
2. Experimental
2.1. Materials and chemicals
The dry RAO (Xuwen, Guangdong province, China) was purchased from Cai Zhi Lin pharmacy (Guangzhou, China) and authenticated by Dr. Xin-Jun Xu, Sun Yat-Sen University. It was ground to fine particles with the size about 40 mesh for follow-up pretreatments. Anhydrous sodium sulfate (Guangzhou Chemical Reagent Factory, Guangzhou, China), diethyl ether and n-hexane (Damao Chemical Reagents Works, Tianjin, China) were analytical pure.
2.2. Hydrodistillation procedure
About 15 g of the powder was weighed and suspended in 300 mL of water to collect the volatile oil by hydrodistillation for 4 h according to Appendix X D of Chinese Pharmacopoeia (2010, vol. 1) [11]. The volatile oil obtained was dried over anhydrous sodium sulfate and diluted with 4 mL of n-hexane. The solution was filtered through a 0.22 μm membrane filter and 1 μL was injected into the GC/MS port for analysis.
2.3. HS-SPME procedure
An HS-SPME holder and a coating fiber with a 50/30 μm layer of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) were purchased from Supelco (Bellefonte, Pennsylvania, USA). The fiber was conditioned prior to use by insertion to the GC injection port at 250 °C for 0.5 h. 0.5 g of the powder was weighed and introduced into a 20 mL flat bottom headspace vial which was then sealed with gray butyl headspace stopper and 20 mm unlined crimp cap using a crimper. The needle of SPME holder was injected to the vial and the fiber was pushed out and exposed to the headspace for the absorption of volatile components, with the vial heated and maintained at 80 °C for 40 min. Finally, the fiber was removed from the vial and analytes were desorbed by exposing the fiber to the GC/MS injection port at 250 °C for 2 min.
2.4. Diethyl ether extraction
About 5 g of the powder was weighed and extracted with diethyl ether (1:8, w/v) for three times (15 min each time) using ultrasonic-assisted technology. The obtained turbid solution was filtrated and then the solvents in the filtrate were removed to obtain extractum by rotary evaporation under reduced pressure at 30 °C. Afterward, the extractum was diluted in n-hexane and filtered through a 0.22 μm membrane filter. One microliter of subsequent filtrate was analyzed by GC/MS.
2.5. GC/MS conditions
GC/MS (Thermo Electron Corporation, USA) instrument was Finnigan Trace DSQ with an electron impact (EI) ion source and an Xcalibur 2.0 workstation. The analytes were separated on a DB-5MS capillary column (30 m×0.25 mm×0.25 μm, Agilent, USA) coated with phenyl arylene polymer. Oven temperature program was as follow: initial 50 °C was increased to 100 °C at 5 °C/min and maintained for 3 min, then increased to 140 °C at 3 °C/min, maintained for 3 min. 140 °C was again increased to 200 °C at 2.5 °C/min and finally ramped to 250 °C at 10 °C/min, lasting for 5 min. High pure Helium (99.999%) was used as carrier gas at a constant flow rate of 1 mL/min. The injection port, transfer line and ion source temperatures were all 250 °C. Electronic energy was set at 70 eV and the mass scanning range was set from 50 to 650 amu in full scan. The injection was performed by split mode with a split ratio of 30: 1. Solvent delay time was 3 min for sample generated by hydrodistillation and diethyl ether extraction, while 0 min for sample by HS-SPME.
Identification of volatile compounds was based on the comparison of their mass spectra with those supplied by the National Institute of Standards and Technology (NIST) database and some literature [12], [13], [14]. Peak areas of all components were calculated by Xcalibur 2.0 and relative contents of volatile compounds were calculated on the basis of peak area ratios.
3. Results and discussion
3.1. Analysis of volatile oil extracted by hydrodistillation
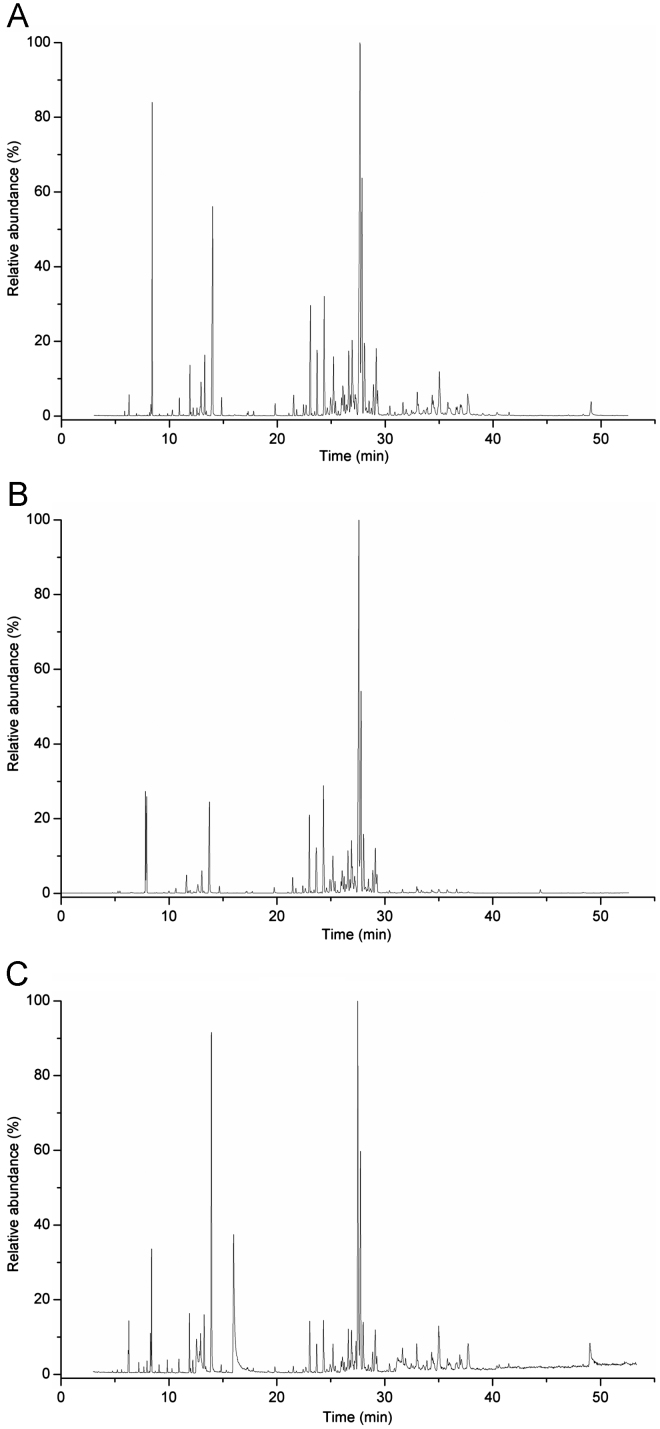
The typical total ion chromatogram of the volatile oil extracted by hydrodistillation is shown in Fig. 1(A). Forty compounds were identified by GC/MS (Table 1). Main compounds were presented as follows: α-farnesene (19.68%), γ-muurolene (13.33%), p-menth-1-en-8-ol (10.16%), eucalyptol (6.00%), 2,6-dimethyl-6-(4-methyl-3-pentenyl)bicyclo[3.1.1]hept-2-ene (5.01%), isocaryophillene (3.97%), cadinol (3.23%), cadina-1(10),4-diene (3.21%) and caryophyllene (2.76%).
Fig. 1.
GC/MS total ion chromatograms of Rhizoma Alpiniae Officinarum by (A) hydrodistillation, (B) HS-SPME and (C) diethyl ether extraction.
Table 1.
Volatile compounds of Rhizoma Alpiniae Officinarum identified by GC/MS.
| No. | Retention time (min) | CAS no. | Compounds | Relative content (%) |
||
|---|---|---|---|---|---|---|
| Hydrodistillation | HS-SPME | Diethyl ether extraction | ||||
| 1 | 5.85 | 13466-78-9 | 3-Carene | 0.09 | – | – |
| 2 | 6.26 | 79–92-5 | Camphene | 0.42 | 0.21 | 1.45 |
| 3 | 6.95 | 80-56-8 | α-Pinene | 0.06 | – | 0.19 |
| 4 | 7.69 | 555-10-2 | β-Phellandrene | – | – | 0.14 |
| 5 | 7.97 | 99-85-4 | γ-Terpinene | – | – | 0.28 |
| 6 | 8.18 | 535-77-3 | m-Cymene | 0.08 | – | – |
| 7 | 8.30 | 5989-27-5 | Limonene | 0.16 | – | 0.70 |
| 8 | 8.40 | 470-82-6 | Eucalyptol | 6.00 | 7.59 | 2.95 |
| 9 | 9.09 | 586-62-9 | Terpinene | – | – | 0.52 |
| 10 | 9.85 | 69073-38-7 | p-Mentha-1,4(8)-diene | – | – | 0.33 |
| 11 | 10.27 | 78-70-6 | Linalool | 0.20 | 0.17 | – |
| 12 | 10.93 | 2217-02-9 | (1R)-endo-(+)-Fenchyl alcohol | 0.52 | 0.34 | 0.38 |
| 13 | 11.90 | 76-22-2 | Camphor | 1.45 | 1.21 | 1.83 |
| 14 | 12.00 | 72402-00-7 | (1R,2S,3R)-3-Isopropenyl-1,2-dimethyl-cyclopentan-1-ol | – | 0.21 | – |
| 15 | 12.23 | 465-31-6 | Camphenehydrate | – | – | 0.47 |
| 16 | 12.55 | 104-53-0 | Benzenepropanal | – | – | 2.20 |
| 17 | 12.93 | 507-70-0 | Borneol | 1.88 | 0.94 | 1.08 |
| 18 | 13.29 | 20126-76-5 | (+)-Terpinen-4-ol | 1.98 | 1.44 | 1.88 |
| 19 | 14.00 | 98-55-5 | p-Menth-1-en-8-ol | 10.16 | 6.68 | 13.88 |
| 20 | 14.86 | 13851-11-1 | Fenchyl acetate | 0.59 | 0.42 | – |
| 21 | 16.00 | 2550-26-7 | Benzyl acetone | – | – | 13.50 |
| 22 | 17.81 | 76-49-3 | Bornyl acetate | 0.16 | – | – |
| 23 | 19.81 | 120-50-3 | Benzoic acid, 2-methyl propylester | 0.50 | 0.39 | 0.23 |
| 24 | 21.52 | 14912-44-8 | Ylangene | 0.77 | 0.92 | 0.28 |
| 25 | 21.80 | 3856-25-5 | Copaene | 0.22 | 0.25 | – |
| 26 | 22.44 | 515-13-9 | (-)-β-Elemene | 0.36 | 0.41 | – |
| 27 | 22.66 | 103-52-6 | Phenylethyl butyrate | 0.54 | 0.21 | – |
| 28 | 23.02 | 13877-93-5 | Isocaryophillene | 3.97 | 4.35 | 1.88 |
| 29 | 23.68 | 87-44-5 | Caryophyllene | 2.76 | 3.05 | 1.36 |
| 30 | 24.34 | 17699-05-7 | 2,6-Dimethyl-6-(4-methyl-3-pentenyl)bicyclo[3.1.1]hept-2-ene | 5.01 | 7.05 | 2.44 |
| 31 | 24.64 | 6831-16-9 | Aristolene | 0.35 | 0.28 | – |
| 32 | 24.94 | 20085-93-2 | Seychellene | 0.98 | 1.08 | – |
| 33 | 25.21 | Not available | (Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene | 2.62 | 2.73 | 1.45 |
| 34 | 25.96 | Not available | 2-Isopropenyl-4α,8-dimethyl-1,2,3,4,4α,5,6,7-octahydronaphthalene | 0.30 | 0.37 | 0.23 |
| 35 | 26.22 | 10208-80-7 | α-Muurolene | 0.53 | 0.68 | 0.33 |
| 36 | 26.59 | 17066-67-0 | Eudesma-4(14),11-diene | 2.67 | 2.52 | 1.50 |
| 37 | 27.34 | 128-37-0 | Butylated hydroxytoluene | – | – | 0.94 |
| 38 | 27.60 | 502-61-4 | α-Farnesene | 19.68 | 25.37 | 15.80 |
| 39 | 27.85 | 30021-74-0 | γ-Muurolene | 13.33 | 14.02 | 8.91 |
| 40 | 28.08 | 483-76-1 | Cadina-1(10),4-diene | 3.21 | 3.89 | 2.25 |
| 41 | 28.51 | 560-32-7 | α-Patchoulene | 0.51 | – | – |
| 42 | 29.16 | 21391-99-1 | α-Calacorene | 2.70 | 2.62 | 1.73 |
| 43 | 29.30 | 33880-83-0 | Elemene | 0.54 | 0.68 | – |
| 44 | 32.97 | 21284-22-0 | Cubenol | 0.60 | 0.23 | 1.08 |
| 45 | 34.35 | 5937-11-1 | τ-Cadinol | 0.52 | 0.25 | 0.47 |
| 46 | 34.45 | 19912-62-0 | τ-Muurolol | 0.15 | – | – |
| 47 | 35.02 | 481-34-5 | Cadinol | 3.23 | 0.46 | 4.08 |
| 48 | 36.96 | 473-04-1 | Eudesm-7(11)-en-p-ol | 0.17 | – | 0.52 |
| 49 | 37.12 | 11031-45-1 | Santalol | – | – | 0.23 |
| 50 | 37.65 | 88034-74-6 | Z-α-Trans-bergamotol | 1.56 | – | 2.25 |
| 51 | 49.02 | 66321-94-6 | Hexadecanoic acid | – | – | 1.88 |
3.2. Analysis of volatile components extracted by HS-SPME
The selection of coating fiber was very important to HS-SPME procedure because different kinds of coats have their unique properties about analytes absorption and desorption. Although different types of fibers possess different affinities, they are particularly sensitive to nonpolar compounds [15]. In order to achieve absorption of volatile components as full as possible from RAO for qualitative analysis, DVB/CAR/PDMS fiber, which was suitable for analytes with a broad range of polarities (suitable for C2–C20 range), was chosen to perform the extraction. To ensure its full volatilization, the absorption condition was set at 80 °C for 40 min.
Thirty-two volatile compounds from RAO were identified by GC/MS (Fig. 1(B) and Table 1). Their peak areas occupied 91.02% of the total and the major components included α-farnesene (25.37%), γ-muurolene (14.02%), eucalyptol (7.59%), 2,6-dimethyl-6-(4-methyl-3-pentenyl)bicy- clo[3.1.1]hept-2-ene (7.05%), p-menth-1-en-8-ol (6.68%), isocaryophillene (4.35%), cadina-1 (10),4-diene (3.89%), caryophyllene (3.05%) and (Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cyclound-ecatriene (2.73%).
3.3. Analysis of volatile components extracted with diethyl ether
Thirty-seven compounds were identified by GC/MS (Fig. 1(C) and Table 1) and the main volatile compounds were as follows: α-farnesene (15.80%), p-menth-1-en-8-ol (13.88%), benzyl acetone (13.50%), γ-muurolene (8.91%), cadinol (4.08%) and eucalyptol (2.95%).
3.4. Comparison of the three methods
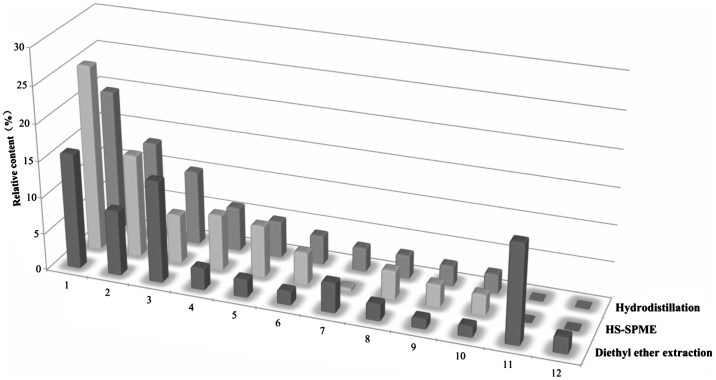
Twenty-two common volatile components extracted by hydrodistillation, HS-SPME and diethyl ether extraction were identical (Table 1). However, different extraction methods did reflect different selectivity (Fig. 2). Terpene was relatively concentrated by HS-SPME while enol was easily collected by hydrodistillation. Ketone and aldehyde, such as benzyl acetone and benzenepropanal, could be gathered easily by diethyl ether extraction. Most volatile components could be obtained from RAO by hydrodistillation with long time extraction. However, some heat-sensitive compounds, such as α-pinene and camphene, were easily lost during the heating procedure. They are heat-sensitive while their relative contents were higher by diethyl ether extraction. This revealed that diethyl ether extraction was beneficial to those volatile components of thermal instability. HS-SPME manifested better affinity to those principal components such as α-farnesene, γ-muurolene and eucalyptol, even if total amounts of volatile compounds were lower than those extracted by other methods.
Fig. 2.
Comparison of relative contents of some volatile components of Rhizoma Alpiniae Officinarum by hydrodistillation, HS-SPME and diethyl ether extraction. 1: α-farnesene; 2: γ-muurolene; 3: p-menth-1-en-8-ol; 4: eucalyptol; 5: 2,6-dimethyl-6-(4-methyl-3-pentenyl) bicyclo[3.1.1]hept-2-ene; 6: isocaryophillene; 7: cadinol; 8: cadina-1(10),4-diene; 9: caryophyllene; 10: (Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene; 11: benzyl acetone 12: benzenepropanal.
4. Conclusions
According to the study, a total of 40 (hydrodistillation), 32 (HS-SPME) and 37 (diethyl ether extraction) volatile components were extracted and identified successfully including α-farnesene, γ-muurolene, 2,6-dimethyl-6-(4-methyl-3-pentenyl)bicyclo [3.1.1] hept-2-ene, eucalyptol and cadina-1(10), 4-diene and so forth, respectively. In terms of analysis, HS-SPME has the advantages of faster, better affinity, small amount sample, and solvent-free, so it can be as first choice for extracting volatile components from medicinal plants.
Acknowledgments
This study was financially supported by the Industry-University-Research Cooperation Program from Science and Technology Department of Guangdong Province (No: 2010B090400533) and the International Scientific and Technological Cooperation Program of China (No: 2009DFA31230).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Deng Y.F., Feng L.N., Luo H. Study on HPLC fingerprint of Alpinia officninarum. J. Chin. Med. Mater. 2011;34(9):1351–1355. [PubMed] [Google Scholar]
- 2.Lee J., Kim K.A., Jeong S. Anti-inflammatory, anti-nociceptive, and anti-psychiatric effects by the rhizomes of Alpinia officinarum on complete Freund's adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2009;126(2):258–264. doi: 10.1016/j.jep.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Mayachiew P., Devahastin S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. Food Sci. Technol. 2008;41(7):1153–1159. [Google Scholar]
- 4.Sun Y., Matsubara H., Kitanaka S. Diarylheptanoids from the Rhizomes of Alpinia officinarum. Helv. Chim. Acta. 2008;91(1):118–123. doi: 10.1055/s-2008-1034345. [DOI] [PubMed] [Google Scholar]
- 5.Lu W., Jiang L.H. Chemical constituents and pharmacological activities of Alpinia officinarum Hance. Chin. Pharm. 2006;15(3):19–21. [Google Scholar]
- 6.Jia L.H., Liu Y., Li Y.Z. Rapid determination of volatile constituents in safflower from Xinjiang and Henan by ultrasonic-assisted solvent extraction and GC–MS. J. Pharm. Anal. 2011;1(3):213–218. doi: 10.1016/j.jpha.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawliszyn J. Chemical Industry Press; Beijing: 2009. Handbook of Solid Phase Microextraction. [Google Scholar]
- 8.Lord H., Pawliszyn J. Evolution of solid-phase microextraction technology. J. Chromatogr. A. 2000;885(1-2):153–193. doi: 10.1016/s0021-9673(00)00535-5. [DOI] [PubMed] [Google Scholar]
- 9.Setkova L., Risticevic S., Pawliszyn J. Rapid headspace solid-phase microextraction-gas chromatographic–time-of-flight mass spectrometric method for qualitative profiling of ice wine volatile fraction: II. Classification of Canadian and Czech ice wines using statistical evaluation of the data. J. Chromatogr. A. 2007;1147(2):224–240. doi: 10.1016/j.chroma.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 10.Huang B.K., Lei Y.L., Tang Y.H. Comparison of HS-SPME with hydrodistillation and SFE for the analysis of the volatile compounds of Zisu and Baisu, two varietal species of Perilla frutescens of Chinese origin. Food Chem. 2011;125(1):268–275. [Google Scholar]
- 11.National Commission of Chinese Pharmacopoeia. Chinese Pharmacopoeia, vol. 1, China Medical Science Press, Beijing, China, 2010, pp. Appendix 63.
- 12.Jirovetz L., Buchbauer G., Shafi M.P. Analysis of the essential oils of the leaves, stems, rhizomes and roots of the medicinal plant Alpinia galanga from southern India. Acta Pharm. 2003;53(2):73–81. [PubMed] [Google Scholar]
- 13.Mallavarapu G.R., Rao L., Ramesh S. Composition of the volatile oils of Alpinia galanga Rhizomes and leaves from India. J. Essent. Oil Res. 2002;14(6):397–399. [Google Scholar]
- 14.Rahman N., Kasimu R., Niyaz Z. Analysis of essential oil from Alpinia officinarum Hance by GC/MS. J. Xinjiang Med. Univ. 2008;31(4):441–442. [Google Scholar]
- 15.Risticevic S., Niri V., Vuckovic D. Recent developments in solid-phase microextraction. Anal. Bioanal. Chem. 2009;393(3):781–795. doi: 10.1007/s00216-008-2375-3. [DOI] [PubMed] [Google Scholar]