Version Changes
Revised. Amendments from Version 1
In this new version, I have tried to address all the comments and suggestions raised by both of my referees. I have therefore changed the manuscript’s contents, structure and figures accordingly. Therefore, the new version has now three main figures whereas the previous version had only one figure. I have additionally made some changes to the R code and updated the online git project. To address R1’s key comments, earlier in the manuscript, I have acknowledged that this work has been motivated by a previous similar publication. I have made the distinction between ANNs and DNNs clearer throughout the manuscript. All minor points by R1 has been also addressed. To address R2’s key concerns: I have added proper referencing to the R code to prevent from the error. Both ‘naïve bayes classifier’ and ‘logistic regression’ models have been re-added to the manuscript and to the figures. The issue of counting hits for DNN has been resolved and manuscript has been updated accordingly. Figures have been re-structured and now I have three figures instead of only one. The PR has been illustrated in one separate figure. I have also tried to address all the possible minor points. I have added a full detailed response to each of the referees in online ‘Respond or Comment’ section of the manuscript.
Abstract
In the era of explosion in biological data, machine learning techniques are becoming more popular in life sciences, including biology and medicine. This research note examines the rise and fall of the most commonly used machine learning techniques in life sciences over the past three decades.
Keywords: machine learning, linear regression, support vector machine, random forest, deep neural network, principal component, t-SNE, hierarchical clustering
Introduction
Over the past three decades, biological data have grown dramatically in both size and complexity. The major contributors to the growth in size of computation biology data include, but not are not limited to, the ability of biologists to sequence complex genomes such as the human genome (1990–2003) ( Lander et al., 2001), the advent of new high throughput sequencing techniques (around 2008) ( Marx, 2013), and most recently the very rapid advancements in single cell technologies, introduced in 2009 ( Wang & Navin, 2015).
The complexity of biological data has been growing even faster, and doesn’t seem to be linearly dependent on the size of data. Examples of complexity in the field of computational genomics include multiple diverse sources of technical noise, low signal to noise ratio, low numbers of biological replicates in comparative approaches, rare and usually hardly detectable mutations in non-coding regions and rare and barely identifiable cell types in complex heterogeneous systems such as the immune system and/or the brain.
At the intersection of mathematics, statistics and computer science is machine learning (ML), the de facto tool box in data science for deciphering the relationship between the input and output as well as detecting significant patterns within large, complex data sets. These quantitative approaches have been shown to be effective and are becoming increasingly popular in addressing challenges such as those outlined above. Highlights of their successful applications in functional genomics include, but are not limited to, learning and characterizing chromatin states by employing unsupervised approaches such as chromHMM ( Ernst & Kellis, 2012), predicting sequence specificities of DNA- and RNA-binding proteins using convolutional neural networks such as DeepBind ( Alipanahi et al., 2015), and employing a combination of supervised and unsupervised approach to determine the genetic and epigenetic contributors of antibody repertoire diversity ( Bolland et al., 2016). Nowadays it is almost impossible to publish a study on single cell assays without using dimensionality reduction methods such as Principal Component Analysis or t-SNE.
One indirect measure of the success of these techniques in extracting scientific insights from biological data is to measure the popularity and usage of machine learning algorithms in life sciences research over time ( Jensen & Bateman, 2011). Motivated by Jensen et al., I therefore set out to update machine learning usage in life sciences. For this I quantified what fraction of published papers in the PubMed database mention a particular technique and how these number of citations are changed each year (see methods).
Methods
For this analysis, I used the R RISmed package ( Kovalchik, 2015) to parse the publication data from NCBI. I examined publications in PubMed from 1990 to 2017 using a metric that measures the proportion of publications per year that mention the technique in the full text (Hits Per Year per Million articles published, or HPYM). The Popularity Rate (PR) of a technique was then defined as the difference between HPYMs in any two consecutive years. A positive PR shows an increase in popularity, whereas a negative PR reflects a decrease in popularity. I limited this note to 12 models listed in Table 1 which have been the most common or which showed a sharp change in popularity rate at a particular time. However, the R code is available with which any particular model during a specific period of time can be easily measured.
Table 1. Common Machine Learning Techniques in Life Sciences.
This table shows 12 machine learning techniques whose popularity in life sciences have been investigated in this study. Technical note: Supervised means that the model requires training data to learn its parameters. A supervised model is used to predict the future instances. An unsupervised model doesn’t require any training data and is used to detect patterns within a dataset. Dimensionality reduction models are used to project high-dimensional datasets into lower dimension space where new variables are more interpretable.
| Technique | Abbreviation | Category |
|---|---|---|
| Random Forest | RF | Supervised |
| Support Vector Machine | SVM | Supervised |
| Artificial Neural Network | ANN | Supervised |
| Deep Neural Network | DNN | Supervised &
Unsupervised |
| Principal Component Analysis | PCA | Dimensionality
Reduction |
| Linear Regression | LR | Supervised |
| Markov Model | MM | Unsupervised |
| Decision Tree | DT | Supervised |
| Hierarchical Clustering | HC | Unsupervised |
| t-Distributed Stochastic
Neighbour Embedding |
t-SNE | Dimensionality
Reduction |
| Logistic Regression Model | LogReg | Supervised |
| Naïve Bayes Classifier | NBC | Supervised |
Results
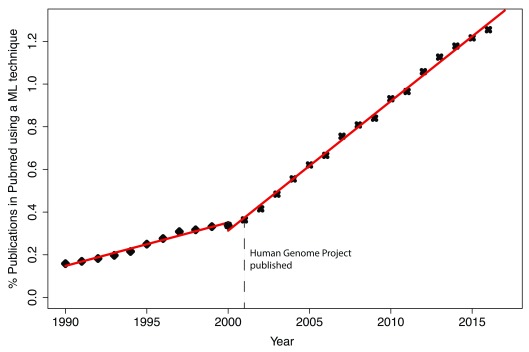
This analysis demonstrates that the overall popularity of machine learning methods in biomedical research has linearly increased since 1990 to 2017, but with two different slopes. From 1990 to 2000 the slope is 0.02, meaning that popularity increased only 2% per year. In 2001 (when sequencing big genomes became possible) the slope increased to 0.06, and since then it has remained constant. A maximum of 1.2% of all papers published in PubMed in any calendar year have mentioned one of the machine learning methods investigated in this study ( Figure 1). I was expecting to see a higher usage of ML in life sciences, but without a gold standard set to compare with, I would not be able to judge if this is too high or low or just about right.
Figure 1. Cumulative usage of all 12 machine-learning techniques used in this manuscript.
Two different linear regression models have been fitted to this data. The first one covers years from 1990 to 2000. The second one that shows a triple increase in its slope covers from 2001 till 2017. Y-axis shows the number of hits per 100 publications.
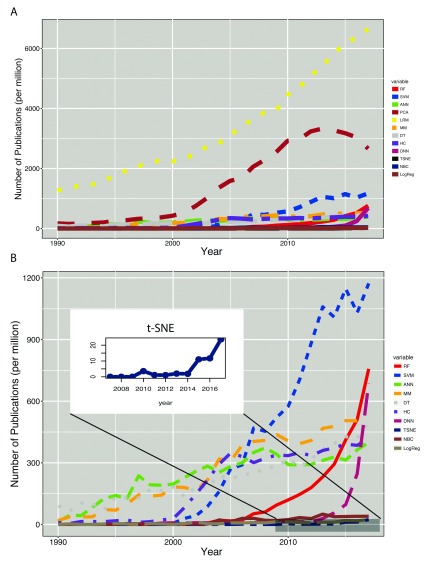
The Linear Regression (LR) models have been the most dominant machine learning techniques in the life sciences over the past three decades ( Figure 2A). It is interesting to see that LR models are still highly in used despite recent appearance of sophisticated ML techniques such as ensemble-based approaches and/or Support Vector Machines and even with very recent and state of the art deep learning techniques. Although, its popularity rate has been plateaued over the past few years ( Figure 3) meaning that its usage is increased linearly with a constant slope. With a constant increase of 300 HPYM, and considering its higher intercept at 1990, the linear regression models is predicted to be one of the most popular techniques over the next few years.
Figure 2.
A: Trends of individual machine-learning techniques defined as per million hits in y-axis. B: Similar to A but without the two very highly used techniques Linear Regression and Principal Components Analysis in order to enhance clarity in usage of other not-very-commonly used techniques that were overshadowed by LRs and PCAs.
Figure 3. An illustration of popularity rate of all 12 techniques used in this manuscript.
The PR has been defined as differences of HPYMs in each two-consecutive year for each model. This number have been further re-scaled to vary only between -1 and 1.
Perhaps a very surprising observation of this study is the rise and fall of Principle Component Analysis (PCA). PCA became very fashionable during 2000 to 2013. In fact, 3329 per million papers published in 2013 mentioned PCA which was the highest number of PCA usage. Since then it has been used less, although it still is the second most popular tool ( Figure 2A).
In early 2000s, unsupervised Hierarchical Clustering alongside newly introduced supervised techniques Support Vector Machines (SVMs) and Random Forests (RFs), showed a sharp rise in usage, which was mainly associated to microarray data analysis. Usage of hierarchical clustering plateaued shortly after its sharp popularity rise in 2000. SVMs kept their popularity longer, for almost a decade in fact, but subsequently dropped to an almost negligible popularity rate ( Figure 3). RFs on the other hand, showed less popularity at the beginning of their arrival, but later on (after 2013) they were ranked the second highest in popularity after Deep Neural Networks (DNN) ( Figure 2A, 2B and Figure 3).
During the period of 1990–2017, Artificial Neural Networks (ANNs) have demonstrated considerable fluctuations in popularity ( Figure 2B and Figure 3). ANNs in the early 1990’s after Linear Regression and PCA, were the most commonly used techniques until early 2000, when they lost their popularity to MMs, HCs and SVMs and even later to RFs. However, since 2013, a sub-family of ANN known as Deep Neural Networks (DNNs) made their way into the life sciences, and their usage since then has increased remarkably, so that DNNs currently have the highest popularity rate ( Figure 3).
The dimensionality reduction technique t-distributed Stochastic Neighbour Embedding (t-SNE) published in 2008, has become quickly tailored to all sorts of single cell techniques. It is therefore not surprising to see that t-SNE usage has also been very rapidly growing over the past few years ( Figure 2B).
Copyright: © 2018 Koohy H
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Discussion
I have illustrated the rise and fall of ML techniques in life sciences from 1990 to the present day. I chose this period because I believe this is the transition period for life scientists to join the big-data club. With the same R code used in this study to parse the publication data from NCBI, it would be possible to look at any period of time.
It was not very surprising to see LR models as the most commonly used model in the field, since:
a) LR models are one of the oldest ML methods that have been in use in almost any field,
b) Parameters in LR models can be learned by using a training data with just a few data samples.
c) A lot of other models can be placed under this umbrella, for instance by first applying a transformation function.
It was, however, surprising to see the sharp rise and fall of PCA. Perhaps a contributing factor to PCA being the most dominant dimensionality reduction method available in this period was its easy-to-use implementation in R. The question still remains as to why its popularity decreased from 2008 onwards. Perhaps the arrival of more versatile models such as RFs and SVMs which are very capable of handling high dimensionality and dealing with co-linearity in biological data eased the need to use PCA. Additionally, t-SNE as a tremendously growing dimensional reduction model in the field, is establishing itself as a strong competitor for the PCA.
ANNs have been fairly popular since the 1990s until around 2004. Around that time more readily useable and less complex techniques became available, such as SVMs, RFs and MMs. However, with the huge investments of giant information companies such as Google leading to very impressive applications of DNNs and other sub-families of ANNs, in various disciplines, DNNs, currently has the sharpest popularity rate ( Figure 3).
I appreciate that there are limitations to this study. For instance, for the majority of comparative analyses of gene expression, researchers use a differential expression software and/or package, but cite only the package name and not the underlying statistical or ML technique used in the package. These cases have not been covered in this study. However, this study can be considered as an approximation of the extent to which machine learning techniques are used in life sciences.
This note can be considered as an update of a similar study by Jensen et al. ( Jensen & Bateman, 2011), in which the authors investigated the rise and fall of a number supervised machine learning techniques in life sciences. Here, I have gone beyond the abstracts and searched the full text of each paper, for the usage of both supervised and unsupervised ML technique.
Data and software availability
Dataset 1: The text file contains the raw data underlying the results presented in this study, i.e. the number of publications in PubMed mentioning each machine learning technique from 1990–2017. These data is further normalized per million for downstream analysis. DOI, 10.5256/f1000research.13016.d184022 ( Koohy, 2017).
R code used to parse the publication data from NCBI is available at: https://github.com/hkoohy/Machine_Learning_in_Life_Sciences
Archived source code as at the time of publication: http://doi.org/10.5281/zenodo.1039642 ( hkoohy, 2017).
License: GNU GENERAL PUBLIC LICENSE
Acknowledgments
I am very grateful to David Sims, Edward Morrisey and Supat Thongjuea for critical reading of the manuscript and for their invaluable comments. I acknowledge both referees of this manuscript for their very fair and un-biased comments that have immensely improved the clarity and quality of this note.
Funding Statement
This work was supported by the Human Immunology Unit MRC Core grant (MC_UU_12010).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved]
References
- Alipanahi B, Delong A, Weirauch MT, et al. : Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning. Nat Biotechnol. 2015;33(8):831–838. 10.1038/nbt.3300 [DOI] [PubMed] [Google Scholar]
- Bolland DJ, Koohy H, Wood AL, et al. : Two mutually exclusive local chromatin states drive efficient V(D)J recombination. Cell Rep. 2016;15(11):2475–2487. 10.1016/j.celrep.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kellis M: ChromHMM: automating chromatin-state discovery and characterization. Nat Methods. 2012;9(3):215–216. 10.1038/nmeth.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- hkoohy: hkoohy/Machine_Learning_in_Life_Sciences: First release.(Version V1.0.0). Zenodo. 2017. Data Source [Google Scholar]
- Jensen LJ, Bateman A: The rise and fall of supervised machine learning techniques. Bioinformatics. 2011;27(24):3331–3332. 10.1093/bioinformatics/btr585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koohy H: Dataset 1 in: The rise and fall of machine learning methods in biomedical research. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchik S: RISmed: download content from NCBI databases. R package version. 2015. Reference Source [Google Scholar]
- Lander ES, Linton LM, Birren B, et al. : Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- Marx V: Biology: The big challenges of big data. Nature. 2013;498(7453):255–260. 10.1038/498255a [DOI] [PubMed] [Google Scholar]
- Wang Y, Navin NE: Advances and applications of single-cell sequencing technologies. Mol Cell. 2015;58(4):598–609. 10.1016/j.molcel.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]