Abstract
Antioxidant supplements from plants are vital to count the oxidative damage in cells. We assessed the antioxidants and antibacterial activity of green hull of Juglans regia in this study. According to our results the maximum antibacterial activity was observed in ethanolic extract when compared to other extract. So, the ethanolic extract was studied for antioxidant activity which exhibited high antiradical activity against DPPH, hydroxyl, and nitric oxide radicals. In conclusion, green hull of J. regia showed strong reducing power activity and total antioxidant capacity. The results justify the therapeutic application of plant in the indigenous system of medicine.
Keywords: Juglans regia, Ethanolic extract, Antioxidants, DPPH, Antibacterial activity
1. Introduction
Herbal medicines (phytomedicine), refer to using a plant's seeds, berries, roots, leaves, bark or flowers with minimal or no industrial processing that have been used for medicinal purposes. It is becoming more main stream as improvements in analysis and quality control in clinical research to show the value of herbal medicine in the treatment and prevention of diseases. According to the World Health Organization, about 80% of world's population rely on HMs for some aspect of their primary healthcare, and the worldwide annual market for HM products approaches US$ 60 billion [1], [2].
Free radical mediated oxidation of cellular components is a well established mechanism of cellular injury in many of the diseases like hypertension, diabetes mellitus, cardiovascular diseases, atherosclerosis, myocardial damage and cardiac arrhythmics. Antioxidant is a substance that can inhibit reactions of free radicals such as reactive oxygen species [3].
Juglans regia is an evergreen tree found in the sub Himalayan tract mostly in the Kashmir region. It is a good source of essential fatty acids, lipids and proteins. The purpose of the study is to evaluate the free radical scavenging activity and the inhibitory effect of green hull of J. regia extract on bacteria.
2. Experimental design
2.1. Collection of plant material
The green hull of J. regia was collected during the month of September from Kashmir, India. Fresh green hull of J. regia was washed under running tap water, air dried and powdered in electric blender.
2.2. Sample extraction
25 g of powdered sample was mixed with 100 mL of various solvents (petroleum ether, chloroform, ethyl acetate, ethanol and distilled water). The extracts of J. regia were prepared by using soxhlet and orbitory shaker apparatus for 72 h then the extracts were collected, evaporated to dryness by using a rotary vacuum evaporator at 40–50 °C and stored in a vial at 0–4 °C for further studies.
After extraction process, the phytochemical screening was done by using standard procedure. In that, most of the phytochemicals like flavonoid, phenol, terpenoids, alkaloids were present in ethyl acetate, ethanol and water extracts. So further studies were carried out by using these three (ethyl acetate, ethanol and water) extracts.
2.3. Antibacterial activity of J. regia
2.3.1. Disc preparation
The 6 mm (diameter) discs were prepared from Whatmann no. 1 filter paper and which were sterilized by autoclave at 12 °C. After the sterilization the moisture discs were dried on hot air oven at 50 °C. The various extracts (ethyl acetate, ethanol and water) were dissolved in DMSO to get a concentration of 5 and 10 mg/mL and then the discs were flooded with 20 μL of sample solution in each concentration from all extracts. The plain discs are used as a control and Ampicillin disc was used as a standard.
2.3.2. Collection of organism
The bacterial cultures like Staphylococcus aureus, Klebsiella aerogenosa, Bacillus subtilis and Escherichia coli were collected from Department of Microbiology, Karpagam University, Coimbatore.
2.3.3. Antibacterial activity of green hull of J. regia
The antibacterial activity studies were carried out by the disc diffusion technique [4]. The sterile nutrient agar plates and potato dextrose agar plates were prepared. The bacterial test organisms like S. aureus, K. aerogenosa, B. subtilis and E. coli were spread over the nutrient agar plates by using separate sterile cotton buds. After the microbial lawn preparation three different extracts of plant disc were placed on the organism inoculated plates with equal distance and control discs were also prepared. All bacterial plates were incubated at 27 °C for 24 h. The diameter of the inhibition zone was measured in centimeters. For each test, three replicates were performed.
2.4. Determination of in vitro antioxidant activity
1 mg of ethanolic extract of J. regia was dissolved in 1.0 mL of distilled water and used for the in vitro antioxidant activity.
2.4.1. Reducing power activity
The various concentrations (0.1–0.5 mL) of prepared extract were mixed with 2.5 mL of 1% potassium ferricyanide and 2.5 mL of 0.2 M sodium phosphate buffer. The mixture was incubated at 50 °C for 20 min. After incubation, the reaction was terminated by the addition of 2.5 mL of 10% (w/v) trichloroacetic acid which was centrifuged at 3000 rpm for 10 min. After centrifugation 5.0 mL of the supernatant was taken and mixed with 5.0 mL of deionized water and 1.0 mL of 0.1% ferric chloride. The absorbance was measured at 700 nm against blanks that contained distilled water and phosphate buffer. The control contained all the reagents except the sample [5]. Increased absorbance indicates increased reducing power of the sample.
2.4.2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical is scavenged by antioxidants through the donation of a proton forming the reduced DPPH. Various concentrations of sample (0.1–0.5 mL) were mixed with 1.0 mL of methanolic solution containing DPPH radicals, mixed well and left to stand for 30 min. Then the absorbance was measured at 517 nm. BHT was used as control [6]. The percentage of DPPH decolorization of the sample was calculated according to the equation
2.4.3. Ferric reducing antioxidant power (FRAP) assay
The stock solution of 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, 20 mM FeCl3, 6H2O and 0.3 M acetate buffer (pH 3.6) were prepared. The FRAP reagent contained 2.5 mL TPTZ solution, 2.5 mL ferric chloride solution and 25 mL acetate buffer. It were freshly prepared and warmed to 37 °C. 900 μL FRAP reagent was mixed with 90 μL water and 30 μL test sample/methanol/distilled water/standard antioxidant solution. The reaction mixture was then incubated at 37 °C for 30 min and the absorbance was recorded at 595 nm. An intense blue color complex was formed when ferric tripyridyl triazine (Fe3+–TPTZ) complex was reduced to ferrous (Fe2+) form. The absorption at 540 nm was recorded [7].
2.4.4. Hydroxyl radical scavenging assay
The varying concentrations of the extract were taken and mixed with 1 mL FeSO4 (1.5 mM), 0.7 mL hydrogen peroxide (6 mM) and 0.3 mL sodium salicylate (20 mM) then the samples were incubated for 1 h at 37 °C. After incubation, the absorbance of the hydroxylated salicylate complex was measured at 562 nm [8]. The percentage scavenging effect was calculated as
where A0 is the absorbance of the control (without extract), A1 is the absorbance in the presence of extract, and A2 is the absorbance without sodium salicylate.
2.4.5. Nitric oxide scavenging activity
Various concentrations of the extract were mixed with 2.5 mL of sodium nitroprusside and made upto 3.0 mL with PBS. Then the mixture was incubated for 15 min at 25 °C. After incubation, 0.5 mL of the reaction mixture was removed and 0.5 mL of the Griess reagent was added. Then the absorbance was measured at 546 nm [9]. The percentage inhibition was calculated by comparing the results of the test with those of controls not treated with the extract, as per the following formula:
3. Results and discussion
3.1. Evaluation of antibacterial activity
Free radicals are implicated for many diseases including diabetes mellitus, arthritis, cancer, ageing, etc. In the treatment of these diseases, antioxidant therapy has gained a great importance due to the protective effect in human body from free radicals and retards the progress of many chronic diseases as well as lipid oxidative rancidity in foods [10]. The present study was carried out to find the antibacterial and in vitro antioxidant activity of green hull extract of J. regia. So the Juglone (5-hydroxy-1,4-napthalenedione) occurs naturally in the leaves, green hull and bark of plants in the Juglandaceae family. Juglone has been shown to be active against tumors as well as many types of microorganisms. The crude extracts of the hull of J. regia with ethanol, water and ethyl acetate were screened for the presence of antibacterial activity using the strains such as E. coli, B. subtilis, K. aerogenosa and S. aureus. Ampicillin was used as a standard. The results are presented in Table 1.
Table 1.
Antibacterial activity of different extracts of green hull of Juglans regia on selected strains.
| S. no. | Name of the organism | Zone of inhibition (diameter in cm) |
||||||
|---|---|---|---|---|---|---|---|---|
| Control ampicillin | Ethanol extract |
Ethyl acetate extract |
Water extract |
|||||
| 5 mg/mL | 10 mg/mL | 5 mg/mL | 10 mg/mL | 5 mg/mL | 10 mg/mL | |||
| 1 | Escherichia coli | 2.2 | 1.62 | 3.45 | 1.55 | 3.06 | 1.67 | 3.35 |
| 2 | Bacillus subtilis | 2.7 | 1.60 | 3.15 | 1.62 | 3.20 | 1.39 | 2.85 |
| 3 | Klebsiella aerogenosa | 3.0 | 1.68 | 3.30 | 1.65 | 3.24 | 1.63 | 3.20 |
| 4 | Staphylococcus aureus | 2.5 | 1.64 | 3.28 | 1.62 | 3.23 | 1.52 | 2.96 |
The antibacterial activity of J. regia hull extracts was determined by the disc diffusion method (zone of inhibition), and it exhibits good antibacterial activity against all the bacterial species (E. coli, B. subtilis, K. aerogenosa and S. aureus) which we have tested in this study. Result confirmed that J. regia green hull extract may be beneficial in treating acne especially when they are known to have anti-inflammatory activities [11].
The in vitro inhibitory effect of J. regia leaf extracts on the main developer of acne lesions, P. acnes and other organisms that are isolated from acne lesion has shown its antimicrobial activity [12]. The presence of antifungal and antimicrobial substances in higher plants is well inspiration for novel drug compounds as plants derived medicines which have made significant contribution toward human health. Based on antibacterial result, the ethanolic extract possessed very good activity when compared with ethyl acetate and water extracts. Hence the further studies were carried out by using ethanolic extracts of J. regia.
3.2. In vitro antioxidant activity of ethanolic extract of J. regia
Antioxidants play an important role in inhibiting and scavenging radicals, thus providing protection to humans against infections and degenerative diseases. The presence of antioxidants leads to the disappearance of these radical chromogens, the most widely used ones are hydroxyl radicals, DPPH, FRAP assay etc. [13]. Antioxidant potential of various extracts is determined by using spectrophtometric methods. Results of DPPH and nitric oxide assay confirm that the extract obtained from J. regia green hull possess significant antioxidant property.
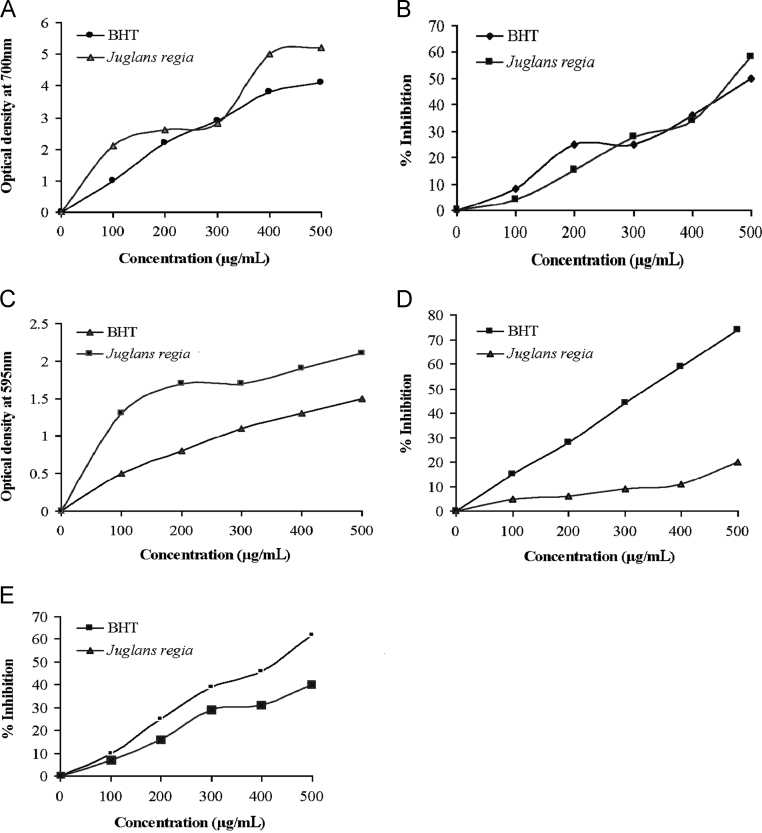
The reducing power capacity of the plant was evaluated in the ethanolic extract of green hull of J. regia and the results are represented in Fig. 1A. At a concentration of 500 μg/mL, the absorbance of the ethanolic extract of J. regia was found to be 5.2. The reduction power activity of ethanolic extract of J. regia expressed the absorbance of 700 nm at a concentration of 400 μg/mL which is almost near the standard BHT. This indicates that the extract seems to have antioxidant capacity due to the presence of polyphenols, which may act on a similar fraction reduction by donating the electrons [14]. The higher values of reducing power indicate that some components are electron donors which react with the free radicals.
Fig. 1.
In vitro antioxidant activity of ethanolic extract of Juglans regia.
Finding of antioxidant activity is necessary to establish the free radical scavenging activity of extract of green hull of J. regia which was performed using DPPH and the percentages of inhibition at five different concentrations 100–500 μg/mL were found to be 5%, 19%, 32%, 43%, and 63% (Fig. 1B). DPPH radical scavenging ability is widely used as an index to evaluate the antioxidant potential of medicinal plants. In the present study, the potential of ethanolic extract of J. regia in DPPH radical scavenging activity is comparable with BHA and it shows high scavenging activity. The FRAP radical scavenging activity of J. regia was also determined and the values are graphically represented in Fig. 1C. The optical densities of the extract were found to be 1.3, 1.7, 1.7, 1.9 and 2.1 at a concentration 100–500 μg/mL.
Generally the reducing properties are associated with presence of compounds which exert their action by breaking the free radical chain by donating an H-atom. The results obtained in the present study are shown in the Fig. 1C which indicates that the values obtained for the extract are close to the values observed in standard BHT. Rahimipanah et al. [15] have shown that the FRAP value of J. regia green hull extract was comparable to trolox at a concentration of 100 μg/mL. Hydroxyl radical is the major active oxygen centered radical which can cause oxidative damage to lipids, DNA and proteins [16]. The hydroxyl radical scavenging capacity of ethanolic extract of green hull J. regia was studied at different concentrations 100–500 μg/mL, which shows that the ethanolic extract has slighter hydroxyl radical scavenging activity when compared with BHT (Fig. 1D).
Nitric oxide is a diffusible free radical which is an important effecter molecule in diverse biological systems. It is generated by the endothelial cells and macrophages, which are mediators of various physiological processes [17]. The nitric oxide radical scavenging was studied by the spectrophotometric method in which the absorbance of chromophore formed during the diazotization of the nitrite with sulfanilamide and the subsequent coupling with naphthalene diamine dihydrochloride was measured. The results for nitric oxide radical scavenging activity of ethonolic extract are represented in Fig. 1E which confirms the presence of free radical scavenging activity of green hull J. regia. From the above results we concluded that the ethanolic extract of J. regia is a good source of antioxidant and antimicrobial activity. Hence it may be used as pharmacotherapeutic agent in future.
Acknowledgment
We are thankful to the Registrar, Vice-chancellor and Chancellor of Karpagam University for doing this research as part of our work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.WHO Issues Guidelines for Herbal Medicines, Bulletin of the World Health Organization, vol. 83, 2004, p. 238. [PMC free article] [PubMed]
- 2.Chen X.G., Wu H.T., Tan G.G. Liquid chromatography coupled with time-of-flight and ion trap mass spectrometry for qualitative analysis of herbal medicines. J. Pharm. Biomed. Anal. 2011;1(4):235–245. doi: 10.1016/j.jpha.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hans C.P., Chaudhary P.D., Bansal D.D. Magnesium deficiency increase oxidative stress in Juglans regia treated rats. Indian J. Exp. Biol. 2002;40:1275–1279. [PubMed] [Google Scholar]
- 4.Newall C.A., Anderson L.A., Phillipson J.D. The Pharmaceutical Press; London: 1996. Herbal Medicines. p. 25. [Google Scholar]
- 5.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- 6.Murthy C.K., Vanitha A., Swamy M.M. Antioxidant and antimicrobial activity of Cissus quadrangularis L. J. Med. Food. 2003;6:99–105. doi: 10.1089/109662003322233495. [DOI] [PubMed] [Google Scholar]
- 7.Benzie I.F.F., Strain J.J. Ferric reducing antioxidant power (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 8.Klein S.M., Cohen G., Cederbaum A.I. Production of formaldehyde during metabolism of dimethyl sulphoxide by hydroxyl radical generating system. Biochemistry. 1991;20:6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- 9.Green L.C., Wagner D.A., Glogowski J. Analysis of nitrate, nitrite and nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 10.Kale A.A., Torane R.C., Deshpande N.R. Antioxidant potential from stem bark of Juglans regia L. Ann. Biol. Res. 2011;2(1):176–180. [Google Scholar]
- 11.Qadan F., Thewaini A., Ali D.A. The antimicrobial activities of Psidium guajava and Juglans regia leaf extracts to acne developing organisms. Am. J. Chin. Med. 2005;33(2):197–204. doi: 10.1142/S0192415X05002783. [DOI] [PubMed] [Google Scholar]
- 12.Arima H., Danno G. Isolation of antimicrobial compounds from guava (Psidium guajava L.) Biosci. Biotechnol. Biochem. 2002;66:1727–1730. doi: 10.1271/bbb.66.1727. [DOI] [PubMed] [Google Scholar]
- 13.Ali S.S., Kasoju N., Luthra A. Indian medicinal herbs as sources of antioxidants. Food Res. Int. 2008;41:1–15. [Google Scholar]
- 14.Yang J.H., Huang L.C. Antioxidant properties of fermented soyabean broth. Food Chem. 2000;71:249–254. [Google Scholar]
- 15.Rahimipanah M., Hamedi M., Mirzapour M. Antioxidant activity and phenolic contents of Persian walnut (Juglans regia L.) green husk extract. Afr. J. Food Sci. Technol. 2010;1(4):105–111. [Google Scholar]
- 16.Jayaprakasha G.K., Selvi T., Sakaria K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003;36(2):117–122. [Google Scholar]
- 17.Hagerman A.E., Riedl K.M., Jones G.A. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]