Abstract
A selective, sensitive and high-throughput liquid chromatography–tandem mass spectrometry (LC–ESI-MS/MS) method has been developed and validated for the quantitation of Guanfacine in rat plasma. Sample clean-up involved liquid–liquid extraction (LLE) and 100 μL of rat plasma was used. YMC BASIC column (50 mm×2.0 mm, 3.5 µm) was used. Mobile phase used was 10 mM ammonium formate (pH 4.0):acetonitrile (70:30, v/v) at a flow rate of 0.3 mL/min. The parent→product ion transitions for the drug (m/z 246.0→159.0) and IS (m/z 252.0→161.1) were monitored on a triple quadrupole mass spectrometer, operating in the multiple reaction monitoring (MRM) and positive ion mode. The method was validated over the concentration range of 50.00–10,000.00 pg/mL for Guanfacine. The method was successfully applied into a pharmacokinetic study in rat plasma.
Keywords: Guanfacine, LC–ESI-MS/MS, Rat plasma, Liquid–liquid extraction, Pharmacokinetic study
1. Introduction
Guanfacine is a centrally acting antihypertensive with alpha2-adrenoceptor agonist properties in a tablet form for oral administration. The chemical name of Guanfacine hydrochloride is N-Amidino-2-(2,6-dichlorophenyl) acetamide monohydrochloride (Fig. 1) and its molecular weight is 282.55 [1]. Guanfacine principal mechanism of action appears to be stimulation of central alpha-adrenergic receptors. By stimulating these receptors, Guanfacine reduces sympathetic nerve impulses from the vasomotor center to the heart and blood vessels. This results in a decrease in peripheral vascular resistance and a reduction in heart rate. Relative to an intravenous dose of 3 mg, the absolute oral bioavailability of Guanfacine is about 80%. Peak plasma concentrations occur from 1 to 4 h with an average of 2.6 h after single oral dose or at steady state [2]. The area under the concentration–time curve (AUC) increases linearly with the dose. In individuals with normal renal function, the average elimination half-life is approximately 17 h (range 10–30 h). Younger patients tend to have shorter elimination half-lives (13–14 h) while older patients tend to have half-lives at the upper end of the range [3]. Steady state blood levels were attained within 4 days in most subjects. The drug is approximately 70% bound to plasma proteins, independent of drug concentration. The whole body volume of distribution is high (a mean of 6.3 L/kg), which suggests a high distribution of drug to the tissues. Patients on dialysis also can be given usual doses of Guanfacine as the drug is poorly dialyzed [4], [5].
Fig. 1.
Chemical structures of Guanfacine and Guanfacine 15N313C1.
Nowadays, it is important to develop a most sensitive method by using advanced instrument LC–MS/MS for clinical pharmacokinetics application and bioanalysis [6], [7], [8]. Literature survey reveals that there are few methods reported for quantitation, identification of Guanfacine in biological matrices [9], [10], [11], [12], [13], [14], [15], [16], pharmaceutical formulations [17]. These methods are developed with different analytical instruments like HPLC–MS [12], GC–MS [14], electron-capture gas liquid chromatography [15], [16], spectrophotometry [17], and HPLC [18]. Among all the reported methods for quantification of Guanfacine in rats and human plasma, dried blood spots methods are helpful for clinical pharmacokinetics.
The reported methods [12], [13], [14], [15], [16], [17], [18] do not show high sensitive and rugged. It is required to develop and validate the most economical, simple, rugged and reproducible bioanalytical method for the quantification of Guanfacine in biological matrices for its clinical pharmacokinetics.
The objective of the present study is to develop a simple, sensitive, selective, rapid, rugged and reproducible method by using LC–ESI-MS/MS method for the quantitation of Guanfacine in rat plasma.
2. Experimental
2.1. Chemicals and materials
Reference standards of Guanfacine HCl (99.7%) and Guanfacine 15N3 13C1 HCl (99.89%, IS) were procured from United States of Pharmacopia (USP). HPLC grade methanol and acetonitrile were obtained from Mallinckrodt Baker (S.A.de C.V. Mexico). Reagent grade ammonium formate, sodium carbonate and formic acid were obtained from Merck Specialties Pvt. Ltd., (Mumbai, India). HPLC grade methyl tertiary butyl ether was obtained from RCI Labscan (Mumbai, India). Water used in the entire analysis was prepared from Milli-Q water purification system procured from Millipore (Bangalore, India). Rats and blank rat plasma were procured from Bioneeds (Bangalore, India).
2.2. Instrumentation
The 1200 Series HPLC system (Agilent Technologies, Waldbronn, Germany) connected to the API 4200 triple quadrupole mass spectrometer (ABI-SCIEX, Toronto, Canada) with a turbo electrospray interface in a positive-ion mode was used for detection. Data processing was performed on Analyst 1.5.1 software package (SCIEX).
2.3. Standard stock, calibration standards and quality control sample p,reparation
The standard stock solutions of 100.00 µg/mL of Guanfacine and Guanfacine 15N3 13C1 were prepared by dissolving requisite amount in methanol. Calibration standards and quality control (QC) samples were prepared with blank plasma from standard stock solution of Guanfacine. Calibration standards were made at concentrations of 50.00, 100.00, 500.00, 1000.00, 2000.00, 4000.00, 6000.00, 8000.00 and 10,000.00 pg/mL while QC samples were prepared at four levels, via., 7000.00 pg/mL (HQC, high quality control), 5000.00 pg/mL (MQC, middle quality control), 150.00 pg/mL (LQC, low quality control) and 50.00 pg/mL (LLOQC, lower limit of quality control) for Guanfacine. From internal standard stock solution 50.00 ng/mL of IS dilution was prepared with 30% MeOH in 0.1% formic acid and stored at 2–8 °C in the refrigerator. Calibration standards and QC samples were stored at −30 °C until use.
2.4. Chromatographic condition
Chromatographic separation was carried out on a reversed-phase YMCBASIC column (50 mm×2.0 mm, 3.5 µm) using the mixture of 10 mM ammonium formate buffer (pH 4) and acetonitrile (70:30, v/v) as the mobile phase at a flow rate of 0.3 mL/min at 50 °C. Retention time of Guanfacine and Guanfacine 15N3,13C1 was found to be approximately 1.1±0.2 min for both the drug and IS with a total runtime of 3.0 min.
2.5. Mass spectrometric conditions
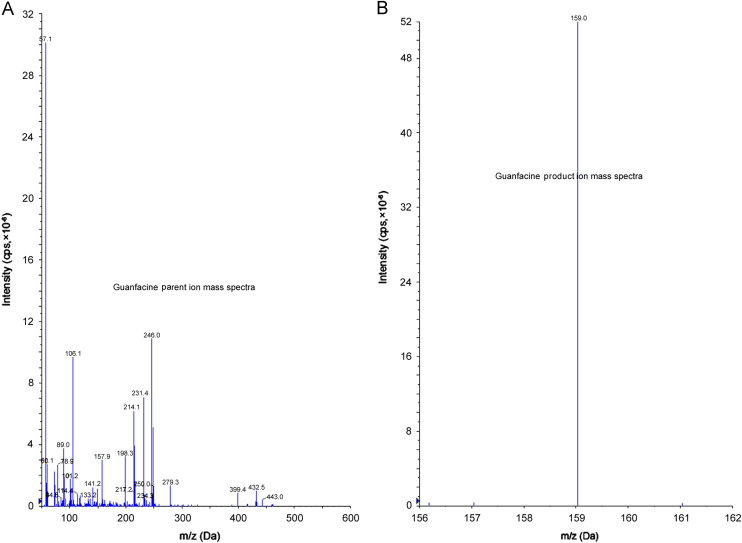
Ionization and detection of the analyte and IS were carried out on a triple quadrupole mass spectrometer, AB-SCIEX, (Toronto, Canada) equipped with electrospray ionization and operated in positive ion mode. Quantitation was performed using multiple reaction monitoring (MRM) mode to monitor parent→product ion (m/z) transition for Guanfacine at m/z 246.0→159.0 (Fig. 2A and B).
Fig. 2.
Parent ion mass spectra (A) and product ion mass spectra (B) of Guanfacine.
For internal standard, the MH+ (m/z 252.0) was monitored as the precursor ion (Fig. 3A) and a fragment at m/z 161.1 was monitored as the product ion (Fig. 3B). Mass parameters were optimized as source temperature 500 °C, nebulizer gas 30 (nitrogen) psi, heater gas 1 40 (nitrogen) psi, curtain gas 25 (nitrogen) psi, CAD gas 3 (nitrogen) psi, ion spray (IS) voltage 5500 V, source flow rate 0.3 mL/min without split, entrance potential 10 V, declustering potential 40 V for both the analyte and IS, collision energy 16 V for both the analyte and IS, collision cell exit potential 12 V and the dwell time was set at 200 ms for the analyte and 10 V for IS.
Fig. 3.
Parent ion mass spectra (A) and product ion mass spectra (B) of Guanfacine 15N313C1.
2.6. Sample preparation
Prior to analysis, all frozen subject samples, calibration standards and QC samples were thawed and allowed to equilibrate at room temperature. To an aliquot of 100 μL of spiked plasma sample, 50 μL internal standard was added and vortexed briefly. Further, 200 μL of 1 M sodium carbonate solution was added and vortexed briefly. To these samples, 3 mL of extraction solvent (methyl tertiary butyl ether) was added, capped and the samples were vortexed for 15 min. Centrifugation of the samples was done at 4000g, for 5 min at 20 °C. Supernatant from each sample was transferred into respective tube and evaporated to dryness under nitrogen at 40±2 °C. The dried samples were reconstituted with 200 μL of acetonitrile:10 mM ammonium formate (20:80, v/v). All the tubes containing samples were vortexed briefly and transferred into autosampler vials for injection into the chromatographic system.
2.7. Method validation
The method validation was performed as per the USFDA guidelines [19]. The method was validated for system suitability, carryover, linearity, precision and accuracy, selectivity, sensitivity, matrix effect, recovery, stability, ruggedness and dilution integrity.
2.7.1. System suitability and autosampler carryover
System suitability experiment was performed by injecting three consecutive injections using aqueous solution of Guanfacine and internal standard at the start of each batch during the method validation. The carryover effect of the autosampler was evaluated by injecting a sequence of injections solutions of aqueous standard, mobile phase, standard blank and extracted standard equivalent to the highest standard in the calibration range. As per the acceptance criteria, the response in the blank should not be greater than 20% of LOQ response.
2.7.2. Linearity and LLOQ
The linearity of the method was determined by analysis of five linear curves containing 10 non-zero concentrations. The ratio of analyte to IS area versus analyte concentration was used for regression analysis. Each calibration curve was analyzed individually by using least square weighted (1/x2) linear regression. The lowest standard on the calibration curve was accepted as the limit of quantitation (LOQ), if the analyte response was at least five times more than that of drug-free (blank) extracted plasma. The deviation of standards other than LLOQ from the nominal concentration should not be more than ±15.0%. For LLOQ it should not be more than ±20.0%.
2.7.3. Precision and accuracy
For determining the intra-day accuracy and precision, replicate analysis of plasma samples of Guanfacine was performed on the same day. The run consisted of a calibration curve, and six replicates of LLOQC, LQC, MQC and HQC samples. The inter-day accuracy and precision were assessed by analysis of five precision and accuracy batches on four consecutive validation days. The precision of the method was determined by calculating the percent coefficient of variation (% CV) for each level. The deviation at each concentration level from the nominal concentration was expected to be within ±15.0% except LLOQ, for which it should be within ±20.0%.
2.7.4. Selectivity
The selectivity of the method towards endogenous plasma matrix components was assessed in 10 lots (five K2 EDTA plasma lots, two hemolyzed lots, two lipimic lots and one heparinised lot) of blank rat plasma. This was done to estimate the extent to which endogenous plasma components contribute towards interference at the retention time of analytes and IS. The cross talk of MRM for analytes and IS was checked using the highest standard on calibration curve and working solution of IS.
2.7.5. Matrix effect
Ion suppression/enhancement effects on the MRM LC–MS/MS sensitivity were evaluated by the post-column analyte infusion experiment. A standard solution containing Guanfacine (at MQC level) and IS was infused post-column into the mobile phase at 10 μL/min employing the in-built infusion pump. Aliquots of 10 μL of extracted control plasma were then injected into the column by the autosampler and MRM LC–MS/MS chromatogram was acquired for the analytes and IS. Any dip in the baseline upon injection of double blank plasma (without IS) would indicate ion suppression, while a peak at the retention time of analyte and IS indicates ion enhancement.
2.7.6. Recovery
The relative recovery (RE) and process efficiency were assessed; all three parameters were evaluated at HQC, MQC and LQC levels in six replicates. Matrix effect was assessed at LQC and HQC levels in six replicates.
RE was calculated by comparing the mean area response of extracted samples (spiked before extraction) to that of un-extracted samples (spiked after extraction) at each QC level. The recovery of IS was similarly estimated.
The overall ‘process efficiency’ (% PE) was calculated as (ME×RE)/100. The assessment of relative matrix effect was based on the direct comparison of the MS/MS responses (peak areas) of the analytes spiked into extracts originating from different lots of plasma. The variability in these responses, expressed as % CV was considered as the measure of relative matrix effect.
Absolute matrix effect (ME) was assessed by comparing the mean area response of unextracted samples (spiked after extraction) with that of neat standard solutions.
2.7.7. Stability
Stability experiments were carried out to examine the analyte stability in stock solutions and in plasma samples under different conditions. Short term stability at room temperature and long-term stability of spiked solution stored at −30 °C were assessed by comparing the area response of stability sample of analyte and IS with that of sample prepared from fresh stock solutions. The solutions were considered stable if the deviation from nominal value was within ±10%.
Autosampler (wet extract) stability, bench top stability, freeze–thaw stability and long-term stability were performed at LQC and HQC levels using six replicates at each level. The samples were considered stable if the deviation from the mean calculated concentration of freshly thawed quality control samples was within ±15%.
2.7.8. Ruggedness
To authenticate the ruggedness of the proposed method, it was done on two precision and accuracy batches. The first batch was analyzed by two different analysts while the second batch was analyzed on a different column.
2.7.9. Dilution integrity
Dilution integrity experiment was conducted by diluting the stock solution prepared as spiked standard at concentration of 15,000.00 pg/mL for Guanfacine. The precision and accuracy for dilution integrity standards at 1/2 (7500.00 pg/mL) and 1/4th (3750.00 pg/mL) dilutions for Guanfacine were determined by analyzing the samples against calibration standards.
2.8. Study design
The validated method has been successfully applied to quantify Guanfacine concentrations in rat plasma. The study was conducted according to the current GCP guidelines [20], [21]. Before conducting the study it was approved by an authorized animal ethics committee. Six healthy rats (200–400 g) were used for conducting in-vivo studies. After an initial period of acclimatization for 1 week to laboratory conditions, the rats were randomly selected and administered the dose. The study was designed as a single dose, one-way cross over study with a washout period 14 days. The protocol followed in Experimental was in accordance to Animal Ethical Guidelines for investigations in laboratory animal and approved by the Animal Ethics Committee (No. BION/COP/GF-2012-6-5F), Bangalore. Animal had access to food 2 h after dose administration. There were a total of 13 blood collection time points including the predose sample (0, 0.5, 1.5, 3, 4, 8, 14, 20, 32, 44, 56, 68, 92 h). The blood samples were collected in separate vacutainers containing K2EDTA as an anticoagulant. The plasma from these samples was separated by centrifugation at 4000 rpm within the range of 2–8 °C. The plasma samples thus obtained were stored at −30 °C until analysis. After analysis the pharmacokinetic parameters were computed using WinNonlin® software version 5.2.
3. Results and discussion
3.1. Method development
During method development, different options were evaluated to optimize mass spectrometry detection parameters, chromatography and sample extraction.
3.1.1. Mass spectrometry detection parameters optimization
Electrospray ionization (ESI) provided a maximum response over atmospheric pressure chemical ionization (APCI) mode, and was chosen for this method. The instrument was optimized to obtain sensitivity and signal stability during infusion of the analyte in the continuous flow of mobile phase to electrospray ion source operated at both polarities at a flow rate of 20 μL/min. Guanfacine gave more responses in positive ion mode as compared to the negative ion mode. The collisionally activated dissociation (CAD) mass spectrum of Guanfacine shows formation of characteristic product ions at m/z 159.0. The major product ion at m/z 159.0 for Guanfacine could be from the protonated precursor molecule. The CAD mass spectrum of Guanfacine 15N3 13C1 shows formation of characteristic product ions at m/z 159.1, 161.1, 162.0 and 163.0. The major product ion at m/z 161.1 arose from the protonated precursor molecule. The predominant peaks in the primary ESI spectra of Guanfacine and Guanfacine 15N3 13C1 correspond to the [M+H]+ ions at m/z 246.0 and 252.0 respectively. (Figs. 2A and 3A). Product ions of Guanfacine and Guanfacine 15N3 13C1 were m/z of 159.0 and 161.1 respectively (Figs. 2B and 3B).
3.1.2. Chromatography optimization
Initially, a mobile phase consisting of ammonium formate and methanol in varying combinations was tried, but a low response was observed. The mobile phase containing ammonium acetate:acetonitrile (20:80, v/v) gave a better response, but poor peak shape was observed. A mobile phase with various strengths of ammonium formate in water at pH 4 in combination with methanol and acetonitrile with varying combinations was tried. Using a mobile phase containing ammonium formate (pH 4) in water in combination with acetonitrile (70:30, v/v), the best signal along with a marked improvement in the peak shape was observed for Guanfacine and Guanfacine 15N3 13C1.
Short length columns, such as Symmetry Shield RP18 (50 mm×2.1 mm, 3.5 μm), Inertsil ODS-2V (50 mm×4.6 mm, 5 μm), Hypurity C18 (50 mm×4.6 mm, 5 μm) and Hypurity Advance (50 mm×4.0 mm2, 5 μm) were tried during the method development. Symmetry Shield RP18 column gave a relatively good peak shape, but the response was low. Using Hypurity C18 column poor chromatography was observed. The best signal was obtained using the YMC BASIC (50 mm×2.0 mm, 3.5 µm) column. It gave satisfactory peak shapes for both Guanfacine and Guanfacine 15N3 13C1. Flow rate of the mobile phase was adjusted and optimized at 0.3 mL/min without splitter. Both the drug and IS were eluted at 1.1 min with the total run time of 3 min. For an LC–MS/MS analysis, utilization of stable isotope-labeled or suitable analog drugs as an internal standard proves helpful when a significant matrix effect is possible. In our case, Guanfacine 15N3 13C1 was found to be the best for the present purpose. The column oven temperature was kept at a constant temperature of about 50 °C. Injection volume of 20 µL sample was adjusted for better ionization and chromatography.
3.1.3. Extraction optimization
Prior to load the sample for LC injection, the co-extracted proteins should be removed from the prepared solution. For this purpose, initially we tested with different extraction procedures like protein precipitation (PP), liquid–liquid extraction (LLE), and solid phase extraction (SPE). We found ion suppression effect in PP method for drug and internal standard. Further, we tried with SPE and LLE. Among all we found that LLE is suitable for extraction of drug and IS. We tried with several organic solvents (ethyl acetate, chloroform, n-hexane, dichloromethane and methyl tertiary butyl ether) individually as well with combinations in LLE to extract analyte from the plasma sample. In our case methyl tertiary butyl ether:dichloromethane (80:20) combination served as a good extraction solvent. Auto sampler wash is optimized as 50% acetonitrile in 0.1% formic acid. High recovery and selectivity were observed in the LLE method. These optimized detection parameters, chromatographic conditions and extraction procedure resulted in reduced analysis time with accurate and precise detection of Guanfacine in rat plasma.
3.2. Method validation
The method was validated in terms of system suitability, carryover, linearity, selectivity, precision and accuracy, sensitivity, matrix effect, recovery, stability, ruggedness and dilution integrity [19].
3.2.1. System suitability and autosampler carryover
Throughout the method validation, the %CV of the system suitability was observed below 5.0% at the retention time of Guanfacine and the IS. Carryover evaluation was performed in each analytical run to ensure that it does not affect the accuracy and the precision of the proposed method. There was a negligible carryover (≤5% of the LLOQ response) observed during autosampler carryover experiment. No enhancement in the response was observed in double blank after subsequent injection of the highest calibration standard (aqueous and extracted) at the retention time of analyte and IS.
3.2.2. Linearity
The calibration curves were linear over the concentration range of 50.00–10,000.00 pg/mL with the correlation coefficient r≥0.9850 for Guanfacine Fig. 4 (Table 1).
Fig. 4.
LLOQ chromatogram of Guanfacine (A) and Guanfacine 15N313C1 (B) in rat plasma.
Table 1.
Calibration curve details.
| Concentration (pg/mL) | Mean±S.D. | %CV | Accuracy (%) |
|---|---|---|---|
| 50.00 | 50.54±0.44 | 0.88 | 101.08 |
| 100.00 | 98.10±1.90 | 1.94 | 98.10 |
| 500.00 | 484.20±6.38 | 1.32 | 96.84 |
| 1000.00 | 1024.00±15.17 | 1.48 | 102.40 |
| 2000.00 | 2022.00±8.37 | 0.41 | 101.10 |
| 4000.00 | 3998.00±20.49 | 0.51 | 99.95 |
| 6000.00 | 6032.00±42.07 | 0.70 | 100.53 |
| 8000.00 | 8036.00±57.71 | 0.72 | 100.45 |
| 10,000.00 | 9960.00±56.57 | 0.57 | 99.60 |
S.D. Standard deviation; CV=Coefficient of variation.
3.2.3. Precision and accuracy
The accuracy and precision (% CV) observed for the calibration standards ranged from 96.84% to 102.40% and 0.41% to 1.94% for Guanfacine respectively. The lowest concentration (LLOQ) in the standard curve for Guanfacine was measured at a signal-to-noise ratio (S/N) of ≥20. The intra- and inter-batch precision and accuracy were established from validation runs performed at HQC, MQC, LQC and LLOQ QC levels. The intra- and inter-batch precision ranged from 0.93% to 3.24% and 1.50% to 3.38% respectively for Guanfacine. The accuracy values were within 97.89–102.76% and 97.98–101.39% for the analyte in intra- and inter-batches (Table 2).
Table 2.
Intra-batch and inter-batch precision and accuracy.
| Nominal added concentration (pg/mL) | Intra-batch(n=6) |
Inter-batch(n=36) |
||||
|---|---|---|---|---|---|---|
| Mean±S.D. | Precision (% CV) | Accuracy | (%) Mean±S.D. | Precision (% CV) | Accuracy (%) | |
| 50.0 | 50.75±1.06 | 2.08 | 101.50 | 50.67±1.71 | 3.38 | 101.35 |
| 150.0 | 146.83±4.75 | 3.24 | 97.89 | 146.97±3.65 | 2.49 | 97.98 |
| 5000.0 | 5021.67±46.65 | 0.93 | 100.43 | 4978.33±74.79 | 1.50 | 99.57 |
| 7000.0 | 7193.33±83.35 | 1.16 | 102.76 | 7097.33±126.43 | 1.78 | 101.39 |
S.D: Standard deviation, CV=coefficient of variation.
3.2.4. Selectivity
To establish the selectivity of the method for interference due to endogenous plasma components from haemolysed, lipidemic, heparinised and K2EDTA blank plasmas, the % change in the area ratio (analyte/IS) at LLOQ level was within 4–6%, while the precision (% CV) in their measurement varied from 2.0 to 4.5.
The extraction procedure together with mass detection gave a very good selectivity for the analysis of drug and IS in the blank plasma. No endogenous interferences were found at the retention times of analyte and IS.
3.2.5. Matrix effect
Matrix effect may be defined as a composite of some undesirable effects that originate from a biological matrix. These components may result in ion suppression/enhancement, decrease/increase in sensitivity of analytes over a period of time, increased baseline, imprecision of data, drift in retention time and distortion or tailing of a chromatographic output. Result of post-column infusion experiment indicates no ion suppression or enhancement at the retention time of analyte and IS as evident from the flat baseline. There is no ion suppression and enhancement observed at retention time of analyte and IS.
3.2.6. Recovery
The relative recovery and process efficiency for drug were observed as 79.96%. The recovery for IS in rat plasma was 87.50%.
3.2.7. Stability
Stock solution stability was performed to check stability of Guanfacine and Guanfacine 15N3 13C1 in stock solutions prepared in methanol and stored at 2–8 °C in a refrigerator. The freshly prepared stock solutions were compared with stock solutions prepared before 9 days. The % change for Guanfacine and Guanfacine 15N3 13C1 were less than 5% which indicated that stock solutions were stable at least for 9 days.
Bench top and autosampler stability for Guanfacine was investigated at LQC and HQC levels. The results revealed that Guanfacine was stable in plasma for at least 26 h at room temperature, and 46 h in an auto sampler. It was confirmed that repeated freezing and thawing (three cycles) of plasma samples spiked with Guanfacine at LQC and HQC levels did not affect their stability. The long-term stability results also indicated that Guanfacine was stable in a matrix up to 65 days at a storage temperature of −30 °C. The results obtained from all these stability studies are tabulated in Table 3.
Table 3.
Stability of Guanfacine in rat plasma samples.
| Stability | Spiked plasma concentration (pg/mL) | Concentration measured (pg/mL) (mean±S.D.; n=6) | CV (%) (n=6) |
|---|---|---|---|
| Bench-top stability (26 h) | 150.00 | 143.17±1.83 | 1.28 |
| 7000.00 | 6931.67±73.33 | 1.06 | |
| Autosampler stability (46 h) | 150.00 | 144.00±2.45 | 1.70 |
| 7000.00 | 7030.00±40.00 | 0.57 | |
| Long term stability (65 days) | 150.00 | 140.17±7.57 | 5.40 |
| 7000.00 | 7013.33±508.67 | 7.25 | |
| Freeze and thaw stability (Cycle 3, 48 h) | 150.00 | 148.67±2.16 | 1.45 |
| 7000.00 | 7038.33±68.53 | 0.97 | |
3.2.8. Ruggedness
The results of ruggedness study for Guanfacine were well within the acceptance limit of 15% in precision and 85.0–115.0% in mean accuracy. The precision and accuracy values for both experiments at LLOQ, LQC, MQC and HQC levels for Guanfacine ranged from 1.6% to 4.9% and 98.40% to 107.21% respectively.
3.2.9. Dilution integrity
The dilution integrity experiment was performed with an aim to validate the dilution test to be carried out on higher analyte concentration above the upper limit of quantification (ULOQ), which may be encountered during real subject sample analysis. The precision and accuracy values for 1/2th and 1/4th dilution ranged from 4.9% to 5.2% and 102.8% to 103.8% for Guanfacine.
3.3. Application of the method
The validated method has been successfully applied to quantify Guanfacine concentrations into a single dose (72 µg/200 g) in rats. Male Sprague-Dawley rats were obtained from Bioneeds, Bangalore. After i.v. administration of drug via left femoral vein 0.2 mL of blood samples for analytical determinations was collected via the right femoral vein at specific time intervals for 92 h. Plasma samples were stored at −30 °C until analysis. The study was carried out after the approval from an independent animal ethics committee. The pharmacokinetic parameters evaluated were maximum observed drug concentration during the study (Cmax), area under the plasma concentration–time curve measured 92 h, using the trapezoidal rule (AUC0–92), time to reach maximum drug concentration (Tmax), apparent first order terminal rate constant calculated from a semi-log plot of the plasma concentration versus time curve, using the method of least square regression (Kel) and terminal half-life as determined by quotient 0.693/Kel (T1/2) [21]. Both compartmental and a noncompartmental methods were used for analysis of kinetic parameters. Pharmacokinetic details are shown in Table 4. The mean concentration versus time profile of Guanfacine in rat plasma is shown in Fig. 5.
Table 4.
Mean pharmacokinetic parameters of Guanfacine in rat plasma after intravenous administration of 72 µg/200 g male rat.
| Pharmacokinetic parameter | Values |
|---|---|
| AUC0–t (pg h/mL) | 10,155±89 |
| Cmax (pg/mL) | 3275±52 |
| AUC0–∞ (pg h/mL) | 103,113±92 |
| Kel (h−1) | 0.04870 |
| Tmax (h) | 0.5 |
| t1/2(h) | 14.23 |
AUC0–∞: area under the curve extrapolated to infinity; AUC0–t: area under the curve up to the last sampling time; Cmax: the maximum plasma concentration; Tmax: the time to reach peak concentration; Kel: the apparent elimination rate constant.
Fig. 5.
Mean plasma concentrations versus time graph of Guanfacine after intravenous administration of 72 µg/200 g in male rat.
4. Conclusion
The proposed method exhibited excellent performance in terms of sensitivity, selectivity, ruggedness and efficiency (3.0 min/sample) due to cleaner extracts, with simplicity of sample preparation. This method was successfully applied in pharmacokinetics of rat plasma.
Acknowledgments
The authors are grateful to the Indian Institute of Chemical Technology, Hyderabad for Literature survey and Acron Accunova, Manipal, India for their Lab facility of this research work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Carchman S.H., Crowe J.T., Jr., Wright G.J. The bioavailability and pharmacokinetics of guanfacine after oral and intravenous administration to healthy volunteers. J. Clin. Pharmacol. 1987;27(10):762–767. doi: 10.1002/j.1552-4604.1987.tb02993.x. [DOI] [PubMed] [Google Scholar]
- 2.Kirch W., Kohler H., Axthelm T. Pharmacokinetics of guanfacine in patients undergoing haemodialysis. Eur J. Drug Metab. Pharmacokinet. 1982;7(4):277–280. doi: 10.1007/BF03189630. [DOI] [PubMed] [Google Scholar]
- 3.Kiechel J.R. Pharmacokinetics and metabolism of guanfacine in man: a review. Br. J. Clin. Pharmacol. 1980;10(1):25 S–32 S. doi: 10.1111/j.1365-2125.1980.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biederman J., Melmed R.D., Patel A, McBurnett K., Konow J., Lyne A., Scherer N. SPD503 Study Group, a randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121(1):73–84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 5.Swearingen D., Pennick M., Shojaei A., Lyne A., Fiske. K. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin. Ther. 2007;29(4):617–625. doi: 10.1016/j.clinthera.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Ponnuru Venkata Suresh, Challa B.R., Nadendla R. Quantification of desloratadine in human plasma by LC–ESI-MS/MS and application to a pharmacokinetic study. J. Pharm. Anal. 2012;2(3):180–187. doi: 10.1016/j.jpha.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponnuru V.S., Challa B.R., Nadendla R. Quantification of sibutramine and its two metabolites in human plasma samples by LC–ESI-MS/MS and its application to bioequivalence Study. J. Pharm. Anal. 2012;2(4):249–257. doi: 10.1016/j.jpha.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konda R.K., Chandu B.R., Challa B.R. Bio-analytical method development and validation of Rasagiline by high performance liquid chromatography tandem mass spectrometry: application to Pharmacokinetic study. J. Pharm. Anal. 2012;2(5):342–349. doi: 10.1016/j.jpha.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Henion J., Abbott R., Wang P. Dried blood spots as a sampling technique for the quantitative determination of guanfacine in clinical studies. Bioanalysis. 2011;3(22):2501–2514. doi: 10.4155/bio.11.262. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Henion J., Abbott R., Wang P. Semi-automated direct elution of dried blood spots for the quantitative determination of guanfacine in human blood. Bioanalysis. 2012;4(12):1445–1456. doi: 10.4155/bio.12.111. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Henion J., Abbott R., Wang P. The use of a membrane filtration device to form dried plasma spots for the quantitative determination of guanfacine in whole blood. Rapid Commun. Mass Spectrom. 2012;26(10):1208–1212. doi: 10.1002/rcm.6212. [DOI] [PubMed] [Google Scholar]
- 12.Wolf C.E., Kester-Florin S.J., Poklis A. A HPLC–MS method to detect and quantify guanfacine in urine. Clin. Chem. Lab. Med. 2011;50(3):535–537. doi: 10.1515/CCLM.2011.803. [DOI] [PubMed] [Google Scholar]
- 13.Uhlen S., Lindblom J., Tiger G. Quantification of alpha2A and alpha2C adrenoceptors in the rat striatum and in different regions of the spinal cord. Acta Physiol. Scand. 1997;160(4):407–412. doi: 10.1046/j.1365-201X.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- 14.Haglock C., Wolf C., Poklis A. A novel method for the determination of guanfacine in urine by gas chromatography–mass spectrometry. J. Anal. Toxicol. 2008;32(8):544–546. doi: 10.1093/jat/32.8.544. [DOI] [PubMed] [Google Scholar]
- 15.Guerret M., Julien-Larose C., Kiechel J.R. Determination of 3-hydroxy-guanfacine in biological fluids by electron-capture gas–liquid chromatography. J. Chromatogr. 1982;10(233):181–192. doi: 10.1016/s0378-4347(00)81745-4. [DOI] [PubMed] [Google Scholar]
- 16.Guerret M., Lavene D., Longchampt J. Determination of guanfacine in biological fluids by electron-capture GLC. J. Pharm. Sci. 1979;68(2):219–221. doi: 10.1002/jps.2600680225. [DOI] [PubMed] [Google Scholar]
- 17.Wahbi A.A, Bedair M.M., Galal S.M. Spectrophotometric analysis of some guanidino drugs by acid-dye and charge-transfer complexation methods. J. Pharm. Biomed. Anal. 1993;11(8):639–645. doi: 10.1016/0731-7085(93)80169-2. [DOI] [PubMed] [Google Scholar]
- 18.Ahirraoa K. Vinod, Sangalea R. Dnyaneshwar, Sonekara S. Vinayak, Thorata V. Vinod, Maratheb P. Rajendra, Nawaleb B. Rajesh, Pawara P. Rajendra. Stability-indicating RP-HPLC method for determination of Guanfacine hydrochloride in bulk drugs and in pharmaceutical dosage form. Int. J. Ind. Chem. 2011;2(2):69–77. [Google Scholar]
- 19.Guidance for Industry: Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), May 2001.
- 20.Guidance for Industry Food—Effect Bioavailability and Fed Bio Equivalence Studies, U.S Department of Health and Human services, Food and Drug Administration Centre for Drug Evaluation and research (CDER), December 2002.
- 21.Guidance for industry Bio availability and Fed Bio equivalence Studies for Orally Administered Drug Products-General Considerations, U.S .Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and research (CDER), March 2003.