Abstract
A rapid and simple method for the determination of polyhexamethylene biguanide (polyhexanide, PHMB) in liquid and gel-like pharmaceutical formulations by means of high performance liquid chromatography coupled to diode-array detection (HPLC–DAD) was developed. Best separation was achieved using a cyanopropyl bonded phase (Agilent Zorbax Eclipse XDB-CN column 4.6 mm×75 mm with particle size of 3.5 μm) as well as gradient elution consisting of acetonitrile/deionized water at a flow rate of 1.0 mL/min. The optimized and applied chromatographic conditions permitted separation of polyhexanide from interacting matrix with subsequent detection at a wavelength of 235 nm with good sensitivity. The method validation was carried out with regard to the guidelines for analytical procedures demanded by the International Conference on Harmonisation (ICH). Mean recoveries of 102% and 101% for gel-like as well as liquid preparations were obtained. Suitable repeatability as well as intermediate precision could be achieved with limits of detection ≤0.004 mg/mL for both formulations, equivalent to ≤0.004% PHMB concerning sample preparation. Determination of PHMB was accomplished without tedious sample preparation. Interacting matrix could be eliminated by the chromatographic procedure with excellent performance of system suitability. All analytical requirements were fulfilled permitting a reliable and precise determination of PHMB in pharmaceuticals. Furthermore, the developed method was applied to stability testing of pharmaceutical preparations containing PHMB.
Keywords: Polyhexamethylene biguanide, Polyhexanide, Pharmaceuticals, High performance liquid chromatography
1. Introduction
Polyhexamethylene biguanide hydrochloride (Polyhexanide, PHMB) is a chemical biocide. Therefore, it is used as an active ingredient in a lot of products such as wet wipes, wound irrigation solutions, sterile dressings as well as disinfectants. Due to its excellent properties the usage increased for application to personal care products and pharmaceuticals in wound management, for instance treatment of chronic wounds and burns [1]. This widely used biocide has been reviewed by US Environmental Protection Agency (EPA) and noted, with the exception of occupational users, as having very low aggregate risk of adverse health effects to the public or environment [2].
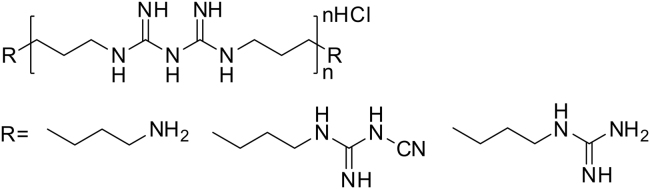
PHMB binds to the negatively charged phosphate head groups of phospholipids at bacteria cell wall, causing increased rigidity, sinking nonpolar segments into hydrophobic domains, disrupting the membrane with subsequent cytoplasmic shedding culminating in cell death [3]. The antibacterial activity of PHMB depends on the molecular structure. Minimum requirements are met by more than 2 biguanide moieties and 5–7 methylene groups as a spacer. Therefore, PHMB represents an oligomeric substance with number-average degree of polymerization of 2–5 [3]. The chemical structure of PHMB is shown in Fig. 1. It is a cationic biocide marketed worldwide, because of its excellent antimicrobial activity, chemical stability, low toxicity and reasonable cost [4]. PHMB is highly soluble in water (20%, w/v) and aliphatic alcohols but poorly soluble in nonpolar liquids. The biguanide moieties are strong bases and monoprotonated at a pH value of 7 (pKa1=2–3; pKa2=10.5–11.5) resulting in a polycation with a positive charge at each biguanide moiety [3].
Fig. 1.
Chemical structure of PHMB and possible terminal groups.
With regard to its application in surgical dressing the need of pharmaceutical preparations containing PHMB on the basis of liquid and gel-like dosage forms increased clearly over the last years in our hospital. Therefore, products from our own manufacture were established for application to our hospital's wards requiring examinations in quality control to ensure the pharmaceutical drug safety. Hence, beside physico-chemical specifications, quantification of the PHMB content is essential. For those liquid and gel-like preparations of PHMB a sensitive and specific method of determination should be used. Several methods for analyzing PHMB in chemical disinfectants, eye drops or further personal care products are described in literature [5], [6], [7], [8], [9], [10], [11], [12]. Chromatographic applications like liquid chromatography or capillary electrophoresis as well as titrimetric methods were used. Most applications exhibited high limits of detection (LOD) insufficient for the referred applications. In addition, PHMB only shows applicable UV absorption at 235 nm attributed to the π–π⁎ transition of 3CåN3 in the biguanide group [4], [13] that was used in determination of PHMB in disinfectants by high performance liquid chromatography coupled to diode-array detection (HPLC–DAD) but a high LOD was also observed. Nevertheless, all described methods in literature report on the determination of PHMB in non-pharmaceutical formulations (medical devices as well as personal care products). However, there are no reports of HPLC methods in literature that were developed and validated for the analysis of pharmaceutical formulations as well as stability testing. Moreover, as reported by Al-Rimawi et al. [14], methods of Pharmacopeia for raw material analysis sometimes provide procedures which either do not include pharmaceutical formulations or cannot be applied to them due to missing selectivity concerning degradation products and interfering excipients present in the pharmaceutical dosage forms. In terms of polyhexanide, only one monograph (namely polihexanide solution 20% used as a raw material for drug manufacturing as well as extemporaneous products) is listed using a gravimetric analysis which is unsuitable for the specific analysis of polyhexanide in pharmaceutical formulations.
Thus, the aim of this study was to develop and implement an analytical procedure for PHMB in liquid and gel-like dosage forms with the required sensitivity and specificity for quality control in drug manufacturing. Typical concentrations of PHMB in those formulations are 0.04% (w/w). Liquid chromatography coupled to diode-array detection should be used as a commonly applied system. The guidelines of the International Conference on Harmonisation (ICH) were adopted for method validation [15]. In order to implement an efficient, economic and simple analytical procedure both sample preparation and analysis should be accomplished in one step separating matrix compounds and quantifying the analyte.
This paper presents a rapid, simple and stability-indicating method based on HPLC–DAD that was developed for the separation and quantification of PHMB in liquid and gel-like pharmaceutical dosage forms without tedious sample preparation for the first time.
2. Experimental
2.1. Chemicals and reagents
HPLC-grade water (deionized water) was obtained by purifying demineralized water in a Milli-Q system (Millipore, Bedford, MA, USA). Acetonitrile (gradient grade) was purchased from Sigma Aldrich (Weinheim, Germany). PHMB reference substance (200 mg/mL; certified regarding the requirements of DAC (German Pharmaceutical Codex) for impurities [16]) was bought from B. Braun Melsungen AG (Melsungen, Germany). The stock standard solution of PHMB (1 mg/mL) and further dilutions were prepared with HPLC-grade water.
The quantitative composition of used products manufactured in the hospital's preparation department was as follows.
PHMB wound irrigation solution (liquid matrix) contains 0.04 g PHMB, 0.86 g sodium chloride, 0.033 g calcium chloride, 0.03 g potassium chloride as well as purified water added to 100 g.
PHMB wound gel (gel-like matrix) contains 0.04 g PHMB, 6 g hydroxyethyl cellulose 400 as well as water for injection added to 100 g.
After drug filling both preparations are sterilized subsequently (autoclaving at 2 bar and 121 °C for 30 min).
2.2. Instrumentation
The LC system consisted of an Agilent 1100 HPLC system equipped with degasser and autosampler, coupled to a diode-array detector set at 235 nm (bandwidth 5 nm; reference 360 nm, bandwidth 50 nm) (Agilent Technologies, Waldbronn, Germany). The injection volume was set to 100 μL and the separation was carried out on an Agilent Zorbax Eclipse XDB-CN (4.6 mm×75 mm) with particle size of 3.5 μm and guard column (4.6 mm×12.5 mm). The mobile phase was composed of deionized water (solvent A) and acetonitrile (solvent B). The column temperature was kept at 20 °C and controlled by an oven. A constant flow rate of 1.0 mL/min was applied throughout the analysis.
For liquid and gel-like samples two different chromatographic conditions were chosen.
The following LC gradient was used for the separation of liquid preparations: isocratic from 0 to 1 min (95% A/5% B); from 1 to 7 min a linear increase of B to 50% that was held for further 3 min. Initial conditions were re-established immediately and the column equilibrated for 5 min.
For gel-like preparations the separation was obtained with isocratic elution for 1 min (95% A/5% B) followed by subsequent linear increase of B to 40% within 9 min. Initial conditions were re-established immediately with an equilibration time of 5 min.
2.3. Sample preparation
2.5 g of each matrix (gel-like or liquid formulation) was weighted into a 25 mL volumetric flask. For evaluating the recovery blank matrices were used and required volumes of standard solution were added at this point to obtain spiked samples at levels of 60%, 100% as well as 140% of the active ingredient. The volumetric flask was filled up with deionized water and homogenized by vigorous shaking. 1.5 mL of the sample solution was filled into a screw-capped vial and analyzed by high performance liquid chromatography with diode-array detection. Five calibration standards with concentrations of 0.02–0.06 mg/mL were prepared similarly in deionized water. Blank solutions of each matrix were analyzed as well.
2.4. Validation study
The method was validated considering the guidelines of ICH by determining specificity, linearity and range, precision (repeatability and intermediate precision), accuracy and robustness [15]. Evaluation of limits of detection (LOD) and quantitation (LOQ) is not part of ICH for methods determining the content or potency but these were ascertained within the validation study.
Furthermore, the stability of standard and sample solutions was investigated as a function of storage time over the period of validation as proposed by ICH.
The developed method should fulfill the following requirements as specification of performance.
Specificity: the UV-spectra of PHMB in samples may differ up to 5% from library UV-spectra.
Accuracy: the results should be within the range of 95–105%, tested with 3 concentration levels covering the specified range.
Precision: repeatability and intermediate precision are to result in less than ±5% in relation to the content of PHMB (0.04±0.002 mg/mL).
Linearity and range: the linear relationship should be achieved across the range of 60–140% in relation to the content of PHMB with a correlation coefficient R2≥0.995.
Moreover, system suitability tests according to the Reviewer Guidance of the Center for Drug Evaluation and Research (CDER) [17] were carried out to establish a reliable chromatographic system. These tests were determined by injecting sample solutions of each matrix (consisting of 0.04 mg/mL PHMB) six times. From this data set system suitability specifications such as repeatability of retention time and analyte peak area, theoretical plates as well as asymmetry (tailing factor; Tf) were calculated.
2.5. Stability assessment
For the evaluation of stability several forced degradation procedures were carried out with separately prepared PHMB solutions 0.04%. Degradation was initiated by dissolving 100 mg of PHMB solution (20%) in 50 mL of the respective buffer solution or 3% hydrogen peroxide solution as outlined below. The solutions were equilibrated to 50 °C and samples of the reaction solutions (1 mL) were analyzed daily in order to determine degree of degradation. All studies were obtained using the developed and validated chromatographic method.
Hydrolysis study was performed with pH 3 (acetate buffer solution), 7 and 10 (phosphate buffer solution) at 50 °C within 7 days. Oxidative degradation was initiated by 3% hydrogen peroxide solution for 7 days at 50 °C. Furthermore, influence of heat was investigated due to the sterilization process during the manufacturing of PHMB wound irrigation solution and PHMB wound gel at 121 °C (2 bar) for 30 min. Susceptibility to light was not performed due to storage with light protection (cardboard box) and known photostability [16], [18]. Moreover, PHMB shows no absorbance above 260 nm [4]. For testing the long-term stability 3 batches were analyzed at intervals of 3 months over a period of more than 12 months. These batches were stored at 25±2 °C; 60%±5% relative humidity in their marketing packs. Physical properties like appearance, clarity as well as color of solutions were monitored as further test attributes.
3. Results and discussion
3.1. Optimization of the chromatographic conditions
Optimization of the method was started with selection of the analytical column. Due to the highly polar character of PHMB several analytical columns of different suppliers using nonpolar as well as more polar bonded phases such as cyanopropyl were examined. The use of reversed phases (C8 and C18, respectively) resulted in both poor separation of PHMB and matrix compounds and asymmetry of analyte's peak. Best performance could be achieved using cyano bonded phases such as Zorbax Eclipse XDB-CN. Excellent separation and peak symmetry were obtained. These findings were verified by analyses of degraded samples resulting in good resolution (Rs≥1.5).
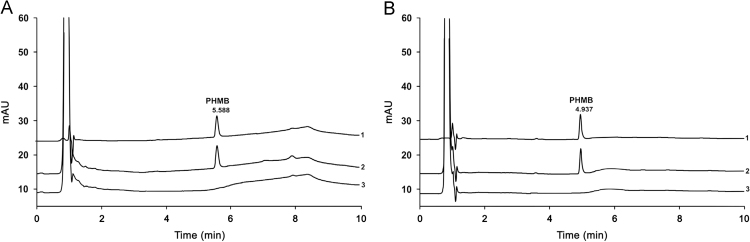
Furthermore, observed matrix interactions during chromatography could be minimized using the optimized chromatographic conditions. With regard to the different matrices two chromatographic methods had to be used in order to obtain efficient separation. Gelling agent from gel-like matrix interfered with PHMB during chromatography when the content of acetonitrile increased excessively. Therefore, using moderate elution conditions this influence could be prevented. Representative chromatograms of samples are shown in Fig. 2. The peak eluted with retention time at approximately 0.7 min was expected to be macrogol 4000, an excipient in polyhexanide concentrate which is used for manufacturing. This result agrees with occurrence in chromatograms of sample and blank but not in standard.
Fig. 2.
Chromatograms of polyhexanide wound gel (A, gel-like matrix) and polyhexanide wound irrigation solution (B, liquid matrix). 1=standard; 2=sample; 3=blank sample. The concentration amounts to 0.04 mg/mL PHMB for standard and sample.
The chromatographic conditions were evaluated and optimized regarding theoretical plates, asymmetry and repeatability of retention time as demanded by CDER [17]. The results of this system suitability evaluation (system suitability test; SST) are shown in Table 1.
Table 1.
Repeatability of retention time and area, calculated theoretical plates as well as asymmetry obtained from system suitability tests (SST) for the developed method as recommended by CDER [17].
| Matrix | Retention time (min) | Repeatability (retention time) (RSD %) | Repeatability (area) (RSD %) | Theoretical plates | Asymmetry (Tf) |
|---|---|---|---|---|---|
| Gel-like | 5.588 | 0.01 | 0.05 | 8229 | 1.3 |
| Liquid | 4.937 | 0.06 | 0.05 | 6963 | 1.6 |
| Criteria | – | <5 | <5 | >2000 | <2 |
The SST was carried out by injecting one sample per matrix (0.04 mg/mL) six times and repeated every week during the validation study.
3.2. Sample preparation
Due to chromatographic conditions interfering matrix could be separated. Thus, particular sample preparation was not required. To achieve suitable sample solutions gel-like formulations had to be diluted tenfold with deionized water. In order to obtain both comparable sample preparation procedure and use of single calibration liquid samples were prepared similarly. Typical chromatograms of liquid and gel-like dosage forms are shown in Fig. 2.
As filtration is often recommended in pharmaceutical analyses this approach was studied using common polyester membrane filters with 0.45 μm pore size (Macherey & Nagel, Düren, Germany). As a result, no adsorption of PHMB could be observed enabling filtration if necessary.
3.3. Method validation and performance
The validation of the proposed method was based on the ICH guideline for validation of analytical procedures [15]. Hence, specificity, linearity and range, LOD, LOQ, accuracy, precision, robustness as well as stability of standard and sample solutions were investigated in terms of parameters to be considered within the validation study. The results are shown in Table 2.
Table 2.
Accuracy and precision data, LOD and LOQ for polyhexamethylene biguanide as results of validation study considering the ICH guideline for validation of analytical procedures [15].
| Matrix | Concentration levela (mg/mL) | Recovery (Accuracy) (%) | Intermediate precision (%) | Repeatability (%) | LODa (mg/mL) | LOQa (mg/mL) |
|---|---|---|---|---|---|---|
| Gel-likeb | 0.02 | 102.7 | 6.3 | 5.0 | 0.004 | 0.013 |
| 0.04 | 102.2 | 5.2 | 2.9 | |||
| 0.06 | 101.2 | 2.1 | 1.7 | |||
| Liquidc | 0.02 | 101.1 | 4.9 | 5.3 | 0.001 | 0.003 |
| 0.04 | 100.7 | 0.5 | 0.5 | |||
| 0.06 | 100.3 | 0.7 | 0.6 |
(mg/mL) correspond to (%) PHMB concerning sample preparation.
Number of samples per level n=12 (analyzed in triplicate).
Number of samples per level n=9 (analyzed in triplicate).
3.3.1. Specificity
Specificity was accomplished using UV-spectra obtained from diode-array detector by comparing the chromatograms of liquid and gel-like formulations obtained for spiked as well as blank samples. Peak purity was calculated by means of HPLC software (ChemStation; Agilent, Waldbronn) for these spectra and compared to spectra library. No interactions could be observed resulting in similarity factors >995 in either case. Therefore, we conclude that this method is selective and suitable for the identification and quantification of PHMB in the different tested pharmaceutical formulations.
3.3.2. Linearity, LOD and LOQ
The linearity was studied by analyzing standard solutions with each analysis in triplicate at five concentration levels in the range of 0.02–0.06 mg/mL (60–140% of PHMB equivalent to 0.02–0.06% PHMB considering sample preparation). The correlation coefficient (R2) was found to be 0.999 in general. The respective linear regression equations for liquid and gel-like matrices were found to be y=867.91x+0.37 and y=859.73x+0.29, respectively.
The LOD and LOQ for each matrix were determined from signal-to-noise ratios (S/N) of 3:1 and 10:1 by injecting calibration solutions. The LOQ was verified by analysis of spiked samples defined as a minimum precision <30% at this level. The confirmation of comparable LOD and LOQ in both calibration standard and sample underscores the excellent separation of matrix and permits the precise determination of PHMB over the linearity range. Nevertheless, in the case of gel-like matrix an increased LOD and LOQ was detected as a result of matrix influence increasing noise.
3.3.3. Accuracy and precision
Accuracy was established by recoveries at three concentration levels achieving excellent precisions and recoveries for both matrices. For calculation of recoveries as well as repeatability and intermediate precision three samples of each matrix per day as well as concentration level (repeated in triplicate) were analyzed and this procedure was carried out at further days (four and three times regarding gel-like and liquid preparations, respectively). In particular for gel-like samples, recoveries of more than 100% were observed. The influence of the matrix was ascertained as the probable cause of these results as such effects could not be detected in liquid preparations.
3.3.4. Robustness
Assessment of robustness was investigated by system suitability tests and varying the flow rate (±0.1–0.2 mL/min), column oven temperature (±1–2 °C) and proportion of acetonitrile in the mobile phase (±0.5–1%). Influence on robustness was only observed with variations in content of acetonitrile resulting in poor peak shape and insufficient resolution (due to decreasing theoretical plates) whereas other parameters did not change appreciably. Furthermore, the observed intermediate precision proved a robust procedure.
3.3.5. Stability of standard and sample solutions
The stability of PHMB standard and sample solutions was investigated as short-term stability. For that purpose, calibration and sample solutions were stored at temperatures of 2–8 °C as well as ambient temperature (20 °C) for approximately 6 weeks (period of validation study). Results of 8 calibrations in repeated measurement during this period were achieved and calculated as overall slope with relative standard deviation. No significant degradation of PHMB (with relative standard deviation of overall slope <0.6%) was observed compared to results obtained for the corresponding daily prepared solutions. Similar results were observed for sample solutions.
This result emphasizes both the stability of PHMB under the selected storage conditions and the applicability of the method to routine analyses.
Summarized, the results for system suitability tests (shown in Table 1) as well as validation study (shown in Table 2) demonstrate a reliable, robust and precise procedure for the determination of PHMB using the proposed method fulfilling all performance specifications.
3.4. Application to real samples and stability assessment
The developed procedure was applied to the analysis of real samples from the manufacturing. All validation parameters could be confirmed and the method was proved to be suitable for routine analysis regarding both rapid and accurate results.
In addition, preparations stored under well-defined temperature (25±2 °C; 60%±5% relative humidity) within more than 12 months after manufacturing as long-term stability testing were analyzed as well. As a result, neither significant decrease of the active substance PHMB (<1%; 3 batches; specification for compliance: <2.5%) nor other variations could be observed. These data are consistent with literature and ascertain the excellent stability of PHMB in preparations [4], [16].
For pharmaceutical analysis stability indicating methods are important to investigate and consider the stability of the preparation in many cases, especially concerning specification of expiration date as well as storage conditions.
Therefore, the investigation of degradation products is very important. For instance, these could be generated by hydrolysis under tightened storage conditions.
For the evidence of a stability indicating performance of the used analytical procedure several stress tests were accomplished. These included susceptibility to pH value, temperature and oxidation in accordance with the intended storage conditions as well as manufacturing process.
With both acidic and alkaline conditions degradation of PHMB (5.2% and 9.4%) could be observed while temperature caused no degradation. Best stability was observed for pH values of 5–7. As a result of manufacturing development the pH range of both wound irrigation solution and wound gel was defined to be in a range of 5.5–6.5 as a specification parameter.
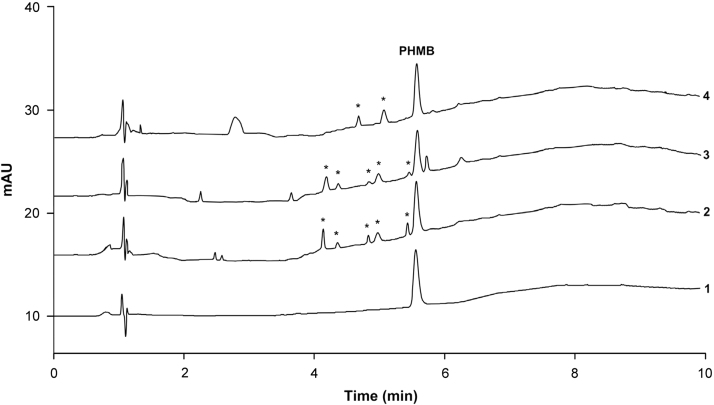
Oxidative conditions induced moderate degradation of PHMB (<5%). Typical chromatograms obtained following the assay of stressed samples are shown in Fig. 3.
Fig. 3.
Representative chromatograms of polyhexanide (0.04%) on thermal (1), acidic (2), alkaline (3) and oxidative (4) degradations. Peaks exhibiting spectra comparable with PHMB are marked by asterisks. Applied chromatographic conditions were the same as described for gel-like matrix. Using chromatographic conditions of liquid matrix no significant differences concerning resolution or peak shape were observed except for shortened retention times.
In either case peak purity was controlled by HPLC software during chromatography and resulted in similarity factors >990 demonstrating suitable resolution of the chromatographic system regarding occurring degradation products. Calculated resolutions of PHMB and closest eluting peaks were ≥1.5.
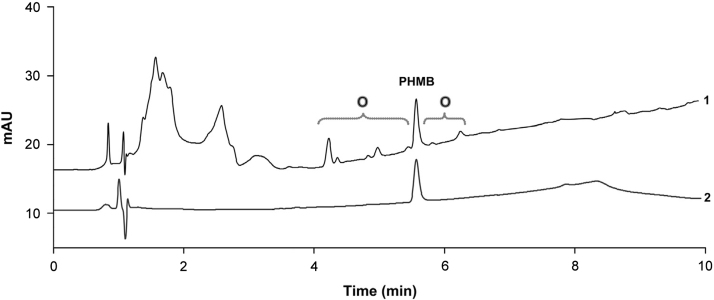
In addition, by the usage of reference substances obtained from several suppliers remarkable results were observed during validation study that related to the kind of PHMB chemical synthesis. PHMB can be synthesized by different routes resulting in various degree of polymerization depending on the manufacturer [3], [4]. The reference substance used for the method validation produced a single peak during chromatography while another one was separated into a multitude of peaks as shown in Fig. 4. This result seems to be a further clear proof for the suitability of the chromatographic method for stability testing assays enabling measurement of degradation products of PHMB. The UV-spectra of observed peaks were comparable to the reference spectra of PHMB demonstrating similar moieties. Similar results could be observed for degradation by acidic and alkaline conditions. This implicates hydrolysis of the biguanide moiety resulting in PHMB shortened for some monomer units. Under oxidative conditions two peaks eluting close to PHMB (4.7 min and 5.1 min) also showed similar reference spectra with the exception of the large peak eluting at 2.7 min. Peaks exhibiting spectra comparable with PHMB are marked by asterisks in Fig. 3.
Fig. 4.
Chromatograms of standard solutions at high (1) and low (2) content of oligomeric composition. Possible oligomers of PHMB were termed as “O”. These unknown compounds were characterized by UV-spectra, which corresponded to PHMB with high correlation indicating similar moieties. Chromatogram (1) was enlarged threefold. The concentration of PHMB represented 0.04 mg/mL in each solution. The UV-spectra of peaks in the area from 0 to 3.5 min were unspecific and not characterized closer.
For this reason, a stability indicating power of the used analytical procedure can be assumed.
Summarized, PHMB could be considered to be very stable in terms of thermal stress. Under strong acidic as well as alkaline conditions moderate degradation was observed whereas best stability was achieved at pH 6. PHMB was also proved to be fairly resistant to oxidative stress conditions.
4. Conclusions
Separation of hydrophilic and ionic compounds like PHMB can be achieved by usage of polar and special prepared cyano bonded phases such as Zorbax Eclipse XDB-CN. Due to the selection as well as optimization of chromatographic conditions both rapid and simple sample preparations as well as excellent system suitability were obtained. Moreover, the interfering matrix of samples could be separated from PHMB permitting a reliable and precise determination. As a result, an accurate, precise and robust analytical procedure was developed and validated considering the requirements of ICH. The proposed method permits the determination of PHMB in liquid and gel-like preparations with excellent results. In addition, the performance of the analytical method has been verified for application to routine analysis in quality control and assessment of stability.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Kramer A., Roth B., Müller G. Influence of the antiseptic agents polyhexanide and octenidine on FL cells and on healing of experimental superficial aseptic wounds in piglets. Skin Pharmacol. Physiol. 2004;17:141–146. doi: 10.1159/000077241. [DOI] [PubMed] [Google Scholar]
- 2.United States Environmental Protection Agency, Washington, D.C. 〈http://www.epa.gov/oppsrrd1/REDs/phmb_red.pdf〉, 2005 (accessed 14.12.12).
- 3.Kaehn K. Polihexanide—a new option for wound treatment. Skin Pharmacol. Physiol. 2010;23(Supplement 1):7–16. doi: 10.1159/000318237. [DOI] [PubMed] [Google Scholar]
- 4.De Paula G.F., Netto G.I., Mattoso L.H.C. Physical and chemical characterization of poly(hexamethylene biguanide) hydrochloride. Polymers. 2011;3:928–941. [Google Scholar]
- 5.Arora A., Ali A., Zzaman M.T. Simple and sensitive method for determination of polyhexanide in multipurpose solution. Electron. J. Biomed. 2009;2:30–37. [Google Scholar]
- 6.Lucas A.D., Gordon E.A., Stratmeyer M.E. Analysis of polyhexamethylene biguanide in multipurpose contact lens solutions. Talanta. 2009;89:1016–1019. doi: 10.1016/j.talanta.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Masadome T., Miyanishi T., Watanabe K. Determination of polyhexamethylene biguanide hydrochloride using photometric colloidal titration with crystal violet as a color indicator. Anal. Sci. 2011;27:817–821. doi: 10.2116/analsci.27.817. [DOI] [PubMed] [Google Scholar]
- 8.Lucas A.D. Environmental fate of polyhexamethylene biguanide. Bull. Environ. Contam. Toxicol. 2012;88:322–325. doi: 10.1007/s00128-011-0436-3. [DOI] [PubMed] [Google Scholar]
- 9.Rowhani T., Lagalante A.F. A colorimetric assay for the determination of polyhexamethylene biguanide in pool and spa water using nickel–nioxime. Talanta. 2007;71:964–970. doi: 10.1016/j.talanta.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Y. Che, X. Ding, S. Zhao, et al., Rapid determination of polyhexamethylenebiguanide in compound chemical disinfectants by reversed-phase high performance liquid chromatography. 〈http://www.chemistrymag.org/cji/2005/078055ne.htm〉, 2012 (accessed 14.12.12).
- 11.Abad-Villar E.M., Etter S.F., Thiel M.A. Determination of chlorhexidine digluconate and polyhexamethylene biguanide in eye drops by capillary electrophoresis with contactless conductivity detection. Anal. Chim. Acta. 2006;561:133–137. [Google Scholar]
- 12.Hattori T., Nakata Y., Kato R. Determination of biguanide groups in polyhexamethylene biguanide hydrochloride by titrimetric methods. Anal. Sci. 2003;19:1525–1528. doi: 10.2116/analsci.19.1525. [DOI] [PubMed] [Google Scholar]
- 13.Nandi S.D. Spectrophotometric (UV) investigation on biguanide and substituted biguanides. Tetrahedron. 1972;28:845–853. [Google Scholar]
- 14.Al-Rimawi F., Zareerb W., Rabieb S. Development and validation of a reversed-phase HPLC method for analysis of tetrahydrozoline hydrochloride in eye drop formulations. J. Pharm. Anal. 2012;2:67–70. doi: 10.1016/j.jpha.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ICH Guideline Validation of Analytical Procedures; Text and Methodology, Q2 (R1), International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonized Tripartite Guideline, 2005.
- 16.ABDA - Bundesvereinigung Deutscher Apothekerverbände, Deutscher Arzneimittel-Codex® (DAC)/Neues Rezeptur-Formularium® (NRF), Govi-Verlag, Eschborn, 1983.
- 17.Center for Drug Evaluation Research (CDER), Reviewer Guidance: Validation of Chromatographic Methods, FDA, USA, 1994.
- 18.Roth B., Holtz D., Mayer D. Recommendations for the use of polyhexanide-containing products for the treatment of wounds. Praxis. 2011;100:531–537. doi: 10.1024/1661-8157/a000517. [DOI] [PubMed] [Google Scholar]