Abstract
Herbal medicines are highly complex and have unknown mechanisms in diseases treatment. Saraca asoca (Roxb.), De. Wild has been recommended to treat gynecological disorders and used in several commercial polyherbal formulations. In present study, efforts have been made to explore antimicrobial activity and its co-relation with the distributions of catechins in the organs of S. asoca using targeted MS/MS. Eight extracts (cold and hot water) from four different organs of S. asoca and two drugs were prepared and antimicrobial activity was assessed by microbroth dilution assay. Quantitative and qualitative analysis of catechins in crude extracts was done by using targeted and auto-MS/MS and correlated with antimicrobial activity. (+)-Catechin and (+)-epicatechin and their biosynthesis related compound were found to be up-regulated in regenerated bark and leaves extracts. (−)-Epigallocatechin was found to be significantly higher in bark water extract as compared to others but showed low antimicrobial activity. Result showed down-regulation of (−)-epigallocatechin and up-regulation of (+)-catechin and (+)-epicatechin in the regenerated bark and leaves of S. asoca. It might be the contributing factor in the antimicrobial activity of regenerated bark and leaves of the plant. The concentration of (+)-epicatechin in processed drugs (Ashokarishta) from Baidyanath was found to be seven times higher than that of Dabur Pvt. Ltd., but no antimicrobial activity was observed, indicating the variations among the plant based drugs. This will be helpful in rational use of S. asoca parts. Furthermore, the analytical method developed is sensitive, repeatable and reliable; therefore, it is suitable for quality control of herbal drugs.
Keywords: Mass spectroscopy, Phytochemistry, Quality control, Flavonoids, Ayurveda, Antimicrobial
1. Introduction
Saraca asoca (Roxb.), De. Wild (Ashoka) belongs to family Caesalpiniaceae. The earliest chronicled mention of this tree is in the ayurvedic treatise and later in Charka Samhita (100 A.D.), in which Ashoka has been recommended in formulations for the management of gynecological disorders as anodynes. Ashokarista is a very famous formulation from the bark of this plant which is available commercially from various reputed companies and used to treat menstrual disorders. Bark of S. asoca is reported to have stimulating effect on the endometrium and ovarian tissue and is useful in menorrhagia during uterine fibroids [1]. Antimicrobial [2], anti-estrogenic and anti-cancer [3] activities are reported for the plant. A number of compounds, including (+)-catechin (CA), (+)-epicatechin (EC) and (−)-epigallocatechin (EGC) [3], [4], [5], have been reported from the plant. These compounds are widely distributed in plant derived foods and herbal medicines. (+)-Catechins are well-known flavonoids known for antioxidant activity and also used for the symptomatic treatment of several gastrointestinal, respiratory and vascular diseases.
Most of the herbal medicine preparations are prepared by the decoction process. Therefore, a number of polar compounds present in the drugs make quality control of herbal drugs more difficult. Due to this reason, even after existence and continued use over centuries, traditional medicines have not been officially recognized in most countries. The quality data on the safety and efficacy of traditional medicine are far from sufficient to meet the criteria needed to support its world-wide use. This is due to lack of adequate or accepted research methodology to evaluate traditional medicine and use of adulterants/substituent.
Most recommended techniques for quality control of herbal drugs are chemical fingerprints obtained by chromatographic and electrophoretic techniques, since they might represent appropriately the “chemical integrities” [6]. However, the combination of chromatographic fingerprints, chemometric evaluation and quantization of selected metabolites (markers) of herbal medicines might be a powerful tool for quality control of herbal products [7]. Qualitative liquid chromatography mass spectroscopy analysis with principal component analysis and multivariate statistical analysis predicts the variations among the raw drug material and final products [8], [9]. In addition, quantitative analysis of biomarkers will ensure the presence of most of the metabolites related to their pathways. The liquid chromatography–mass spectrometer fingerprints and quantized metabolites of clinically proven efficient drugs may be the best option for the standardization of herbal drugs.
The present study reports high performance liquid chromatography–quadruple time of flight mass spectrometry (HPLC–QTOFMS) method for the qualitative and quantitative analysis of CA, EC and EGC in various parts of plants and commercial formulations prepared from the bark extracts. The quantitative analysis of CA, EC and EGC in the extracts was carried out using external standard calibration which may further be useful for quality control as well authentication of plant and plant based drug samples.
2. Materials and methods
2.1. Plant material used
Various plant parts (bark, regenerated bark, leaves and flowers) of S. asoca were collected in February 2012 from the garden of National Research Institute of Basic Ayurvedic Sciences, CCRAS (Department of AYUSH), Nehru Garden, Kothrud, Pune. The collected plant materials were identified and voucher specimens (No. 207) were kept at the medicinal plant museum of the Institute. The Ashokarista formulations of Baidyanath Pvt. Ltd. (Batch No. 110085, mfg April 2011) and Dabur Pvt. Ltd. (Batch No. BD1049, mfg Sept 2010) were purchased from authorized medical stores.
2.2. Chemicals used
Standard compounds lidocaine, d-camphor, 5,7-isoflavone, (+)-catechin, (+)-epicatechin, and (−)-epigallocatechin were purchased from Sigma-Aldrich. Acetonitrile, formic acid and water of liquid chromatography–mass spectrometer grade were purchased from Sigma-Aldrich.
2.3. Method of extraction
The plant materials (20 g each) were washed and crushed. The crushed material was mixed with equal quantity of deionized water (Direct-Q, Millipore) and incubated with continuous shaking overnight at two different temperatures i.e. 25 °C and 70 °C (which were named water extract and hot water extract respectively) and this step was repeated three times to ensure complete recovery. The samples were named as bark water extract (BWE), bark hot water extract (BHWE), regenerated bark water extract (RBWE), regenerated bark hot water extract (RBHWE), leaves water extract (LWE), leaves hot water extract (LHWE), flower water extract (FEW) and flower hot water extract (FHWE). Samples were centrifuged at 10,000g for 10 min and filtered through 0.22 μm filters (Hi-media). The extracts were lyophilized using a lyophilizer (Freezone 4.5 Labconco, CA, USA) and stored at −80 °C till further use. The plant extracts were reconstituted in LC/MS grade water (5 mg/mL) for further analytical study. Ashokarista formulations of Baidyanath (BA) and Dabur (DA) were purchased from local market and per milliliter residual activity for both the formulations was measured. The Ashokarista formulations were centrifuged at 10,000g for 20 min at 4 °C to get rid of the residues and filtered through 0.22 μm membrane. The filtrates of S. asoca and commercial drugs were used for metabolomic studies.
2.4. Microorganisms used
Clinical isolates of the microorganisms were used along with the standard strains. Quality control strains of Aspergillus fumigatus (ITCC 4517) and Aspergillus flavus (ITCC 5192) were obtained from Indian Type Culture Collection, IARI, Delhi. Standard strains of Escherichia coli (MTCCB 82), Pseudomonas aeruginosa (MTCCB 741), Staphylococcus aureus (MTCCB 737) and Klebsiella pneumoniae (99/209) were included in each test as recommended by the National Committee for Clinical Laboratories Standards (NCCLS), purchased from Institute of Microbial Technology, Chandigarh, India.
2.5. Antibacterial screening
Antibacterial activities of the extracts were determined by the microbroth dilution assay [2], [10]. The plant extracts were dissolved in deionized water at a concentration of 2500 μg/mL. Proper controls were kept for each experiment. The bacterial strains used as inocula were grown at 37 °C to get OD 0.6 at 600 nm and used in susceptibility testing. The lowest concentration, which inhibited any visual growth, was considered to be minimum inhibitory concentration (MIC).
2.6. Antifungal screening
All the extracts were dissolved in water to achieve a concentration of 2500 μg/mL. Aspergillus species cultures were grown on Sabouraud dextrose agar at 37 °C until sporulation occurs, typically for 5 days. The spores were harvested in sabouraud dextrose broth and the numbers of colony forming units (CFU) per milliliter were determined by plating serial dilutions on Sabouraud dextrose agar plates. For susceptibility tests, serial two-fold dilutions of extracts were made using Sabouraud dextrose in 100 μL volumes and were inoculated with 100 μL of the spore suspensions having 2×104–2×105 CFU/mL in Sabouraud dextrose broth. The cultures were incubated for 48 h at 37 °C [11]. MICs were determined at the lowest concentration that inhibited visible fungal growth.
2.7. Auto-MS/MS
MS/MS experiments were performed on an Agilent 1290 Infinity Series HPLC interfaced to an Agilent 6538 Accurate-Mass QTOFMS. A volume of 20 μL of each sample was injected into ZORBAX 300SB reversed phase column (C-18, 4.5 mm×250 mm) of 5 μm particle size. The column temperature was maintained at 40 °C. Mobile phase comprised solvent A (water containing 0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid) and used the following iFn gradient mode {time/concentration (min/%)} for solvent B: 8/5%; 15/10%; 22/45%; 30/65%; 35/90%; and 40/5%, with flow rate of 0.4 mL/min. The mass spectrometer was operated in positive ion polarity mode with following parameters: gas temperature 350 °C, nebulizer 50 psi, gas flow 8 L/min, capillary voltage 3500 V, nozzle 500 V and fragmentor voltage 175 V. The QTOF was operated in the extended dynamic range (1700m/z, 2 GHz). To assure the mass accuracy of recorded data, continuous internal calibration was performed using standards of signals and lidocaine (234.3 m/z); 5,7-isoflavone (284.3m/z) was infused with samples as external standards.
2.8. Auto-MS/MS data analysis
Initial processing of HPLC–QTOFMS data e.g., baseline correction, noise reduction, and background contaminants removal, were performed by MassHunter Workstation Software (Version 3.1 Qualitative Analysis, Agilent Technologies). The molecular features of spectra were extracted using auto-MS/MS extraction tool of MassHunter Software. The molecular formulas were generated and searched in a specific library generated in house at the Institute.
2.9. Targeted QTOF MS/MS
Quantization was done by the standard addition method by spiking control plant samples with standard solutions of CA, EC and EGC (ranging from 0.08 to 10 μg/mL). Crude extracts of various plant parts as well as standard metabolites were introduced into the HPLC–QTOFMS operated in the positive mode. Parameters for targeted MS/MS were the same as in auto-MS/MS. However, collision energies for MS/MS spectra for CA, EC and EGC were adjusted to 25, 35 and 26 V, respectively.
2.10. Targeted MS/MS data analysis
All the three standards and metabolites of extracts were quantified by targeted MS/MS analysis. Agilent Mass Hunter Qualitative Analysis Software (Version B 2.0.0.2) was used to process the data. A window of 100 μg/mL was set for fragment identification. Standards and their corresponding metabolites in the extracts were quantified using the peak size of the fragment of the extracted ion chromatogram function. Metabolites were quantified by the formation of metabolite specific fragments.
3. Results and discussion
3.1. HPLC–QTOFMS
A solution of 5.0 mg/mL of all the samples from S. asoca was used for targeted and non-targeted analyses. As the water extracts contained polar compounds, the reversed phase chromatographic system was employed for rapid separation of a wide range of compounds. All the metabolites ionizable and detectable with the ESI (+)-MS were eluted in 35 min on a C-18 column of 5 μm particle size. The elution was continued for additional 10 min to ensure the complete removal of sample from the column. Retention time variability across the samples was found to be 3 s or a relative standard deviation (RSD) of less than 6%. The QTOFMS was calibrated before analysis and the mass accuracy achieved was below 2.0 μg/mL calculated by using reference ions.
A visual examination shows the differences between the total ions chromatograms of samples employed in the study. Along with several unique peaks across the samples, a prominent peak at 39.9 min in the chromatograms was observed only in regenerated bark samples (data not shown) which can be further exploited as a marker peak of regenerated bark during the UPLC analysis of samples.
3.2. Auto-MS/MS
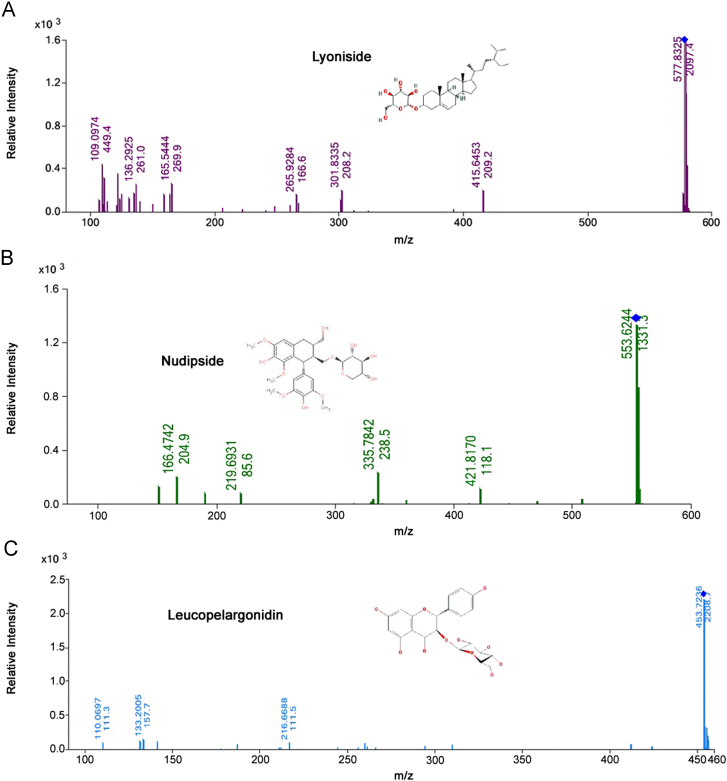
Auto-MS/MS data sets were acquired in positive mode using the parameters described in the materials and methods section (Section 2). Auto-MS/MS data were processed by using qualitative MassHunter (B.04.00 Version) and more than 4000 molecular features were observed in each sample. Molecular formulae of the compounds were generated using the formula generator in the same software. An in-house generated library of compounds specific for S. asoca was used to identify the metabolites present in the different extracts. In qualitative analysis of samples, lyoniside was found to be present in the leaves and flowers extracts (Fig. 1 A). Nudiposide was found to be present only in regenerated bark extracts (Fig. 1B). Leucopelargonidin-3-glucoside was found only in the water extract of leaf (Fig. 1C). In qualitative analysis of samples, lyoniside was found to be present in all the plant parts except in drug formulations used in the study. Nudiposide was found in DA but not in the BA formulation. Data showed inconsistency in the herbal drug preparation and indicate the requirement of standardization of drug preparation procedures and quality control parameters. Leucopelargonidin-3-glucoside was found only in the water extracts of leaf. Therefore, leucopelargonidin-3-glucoside can be used as specific marker for leaf samples and lyoniside for leaves and flowers.
Fig. 1.
MS/MS spectrums of (A) lyoniside; (B) nudiposide and (C) leucopelargonidin-3-glucoside.
3.3. Targeted MS/MS
To quantify (+)-catechin and (−)-epigallocatechin from different parts of S. asoca along with Ashokarista, targeted MS/MS was used successfully. Accurate MS/MS spectra of the compound provide a high level of confidence for the identification and quantification of raw and processed plant based drugs, which is rather difficult by other technologies.
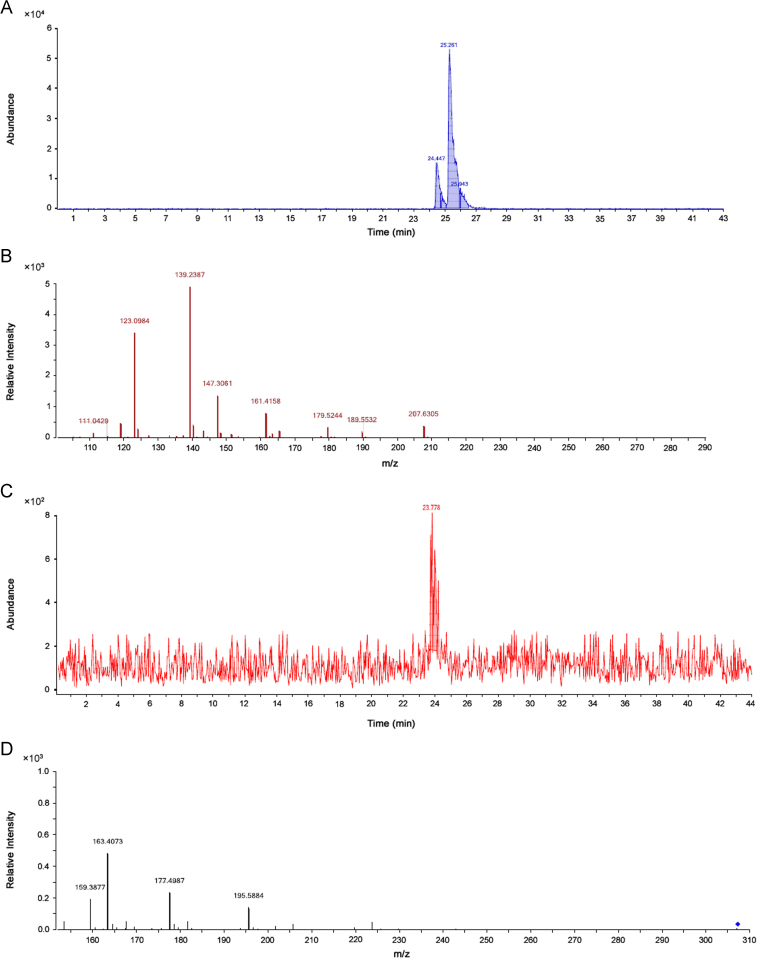
The gradient of water containing 0.1% formic acid and acetonitrile 0.1% formic acid method produced well-shaped peaks for EC, CA and EGC at 24.447, 25.261 and 23.8 min, respectively (Fig. 2A, C). The molecular transitions and collision energy employed in targeted MS/MS for different standards are shown in Table 1. Fragmentor voltage 75 V was kept constant throughout the study. MS/MS peak intensities were found to be reproducible after normalization to the same molar quantity. MS/MS revealed that the signals responses for compounds were comparable, and therefore no correction factor was required. A linear regression line was calculated for the standards used for each metabolite to account for the dependence of ionization on the size (m/z) of the parental ion. (Table 1)
Fig. 2.
(A) ESI chromatogram showing separation of isomers 291.27 m/z, (+)-catechin and (+)-epicatechin. The earlier peak represents (+)-catechin while the later is for (+)-epicatechin. (B) The product ion spectrum for (+)-catechin and (+)-epicatechin. (C) An ESI chromatogram representing the unique peak of (−)-epigallocatechin (307.27 m/z). (D) A product ion spectrum for (−)-epigallocatechin.
Table 1.
Transitions, retention time, collision energy, calibration range, regression coefficients and limit of quantization for the catechins.
| Compound | Transition | Retention time (min) | Collision energy (V) | Test range (ng) | R2 | LOD (ng) |
|---|---|---|---|---|---|---|
| (+)-Epicatechin | 291.27→139.2374 | 25.261 | 25 | 0.78–10.0 | 0.9837 | 0.781 |
| (+)-Catechin | 291.27→139.2387 | 24.447 | 25 | 0.78–10.0 | 0.9407 | 1.5625 |
| (−)-Epigallocatechin | 307.27→163.4057 | 23.800 | 26 | 0.78–10.0 | 0.9874 | 0.781 |
LOD= limit of detection.
The spectra generated for compounds in positive ion detection gave the protonated molecules and their transitions ions [M H+] (m/z) 291.27→139.237 for EC and CA (Fig. 2B), 307.27→163.405 for EGC (Fig. 2D). Identification of compounds was done on the basis of retention time and presence of peak in the trace compared with those of the standards. EC and CA isomers of 291.7 m/z were successfully separated from the mixture of three compounds (Fig. 2). Compounds used in the study were quantified (n=10) from 10 different samples using a standard calibration curve (from 0.08 to 10 ppm). Good correlation coefficients were obtained in the concentration range studied (Table 1). The results for reproducibility were relative standard deviations (RSD) of 1.5%, 1.5% and 2% for run-to-run precision, on CA, EC and EGC concentrations, respectively. The lowest detection limit based on a signal-to-noise ratio of 3:1 was calculated through the standard addition curves, giving a value of compounds studied in the samples.
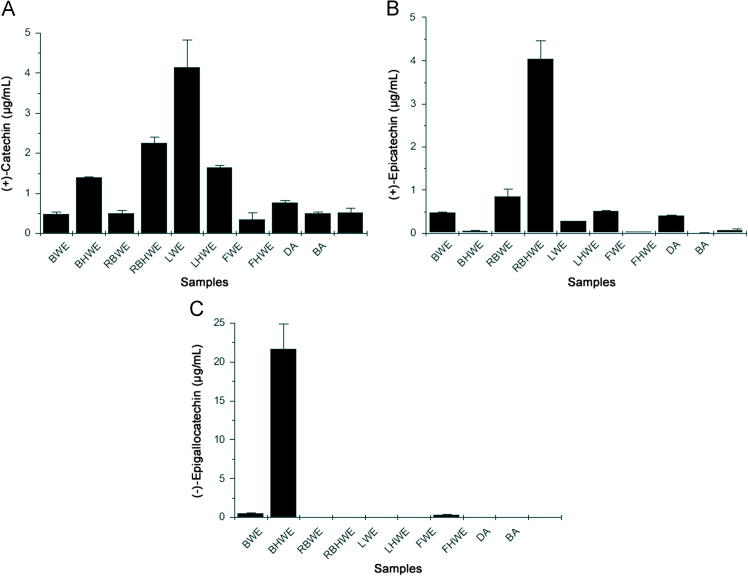
EC and CA were found to be universally present throughout the samples. Regenerated bark samples showed significantly higher amount of EC and CA (4.048 and 2.249 μg/mL) as compared to other samples. CA was found to be significantly elevated in BHWE, RBHWE, LWE and LHWE as compared to other parts of the plant (Fig. 3A). RBWE and RBHWE contain significantly high amount of EC (Fig. 3B). BHWE also contains significantly higher amounts of EGC (Fig. 3C). In the present study, leaf sample was found to have the highest amount of CA (4.14+0.698 μg/mL). The regenerated bark was found to contain 4.048+0.423 μg/mL EC which is almost two-fold higher than that CA. EGC was found to be present only in normal bark extracts. Ashokarista formulations used in the study did not show antimicrobial activity which may be due to lower amounts of catechins in drug samples. Besides this, Ashokarishta formulation from BA was found to have seven times higher EC than DA, showing the variations among the drugs (Fig. 3).
Fig. 3.
Quantitative analysis of (A) (+)-catechin, (B) (+)-epicatechin and (C) (−)-epigallocatchin in μg/mL.
Targeted MS/MS was found to be more accurate and sensitive to quantize catechins instead of other reported methods [14]. The method showed good results in terms of detection limits, repeatability and linearity (Table 1). As shown in Fig. 3, CA and EC were found to be elevated in RBHWE, LWE and LHWE which are crucial for the flavanoids and cutin biosynthesis. Correlating the above facts, RBHWE and LHWE were found to have better antimicrobial activity among the samples (Table 2).
Table 2.
MIC (mg/mL) of plant extracts against the microorganisms by microbroth dilution broth assay.
| Extract | PA | KP | SA | EC | AF | AFL |
|---|---|---|---|---|---|---|
| BWE | 6.250 | 6.250 | 12.500 | 12.500 | – | – |
| HBWE | 1.065 | 1.065 | 3.125 | 1.065 | 6.250 | – |
| RBWE | 3.125 | 3.125 | 1.065 | 1.065 | 6.250 | – |
| RBHWE | 0.266 | 0.532 | 0.532 | 0.532 | 6.250 | – |
| LWE | 1.065 | 1.065 | 1.065 | 1.065 | 6.250 | – |
| LHWE | 0.5325 | 0.5325 | 0.5325 | 0.532 | 1.065 | 6.250 |
| FEW | 3.125 | 6.250 | 6.250 | 6.250 | 6.250 | – |
| FHWE | 1.065 | 1.065 | 1.065 | 1.065 | 3.125 | 12.50 |
| DA | – | – | – | – | – | – |
| BA | – | – | – | – | – | – |
PA=P. aeruginosa, KP= K. pneumoniae, SA= S. aureus, EC=E. coli, AF=A. fumigatus, and AFL= A. flavus.
3.4. Antimicrobial activity
Various parts of S. asoca were evaluated for their antimicrobial potential against six microorganisms using microbroth dilution assay (Table 2). LHWE and RBHWE were found to have better activity among the samples and MIC was recorded to be 0.266–0.5325 mg/mL against the bacterial species. Other samples showed variable activity in the range of 0.5325–6.25 mg/mL. MICs against the fungal species were found to be in the range of 12.5–1.065 mg/mL. LWE water extract exhibited better antifungal activity, where MIC was observed to be 1.065 mg/mL.
Catechins are essential components in foods and herbal medicines and well reported in S. asoca. Along with several other biological activities S. asoca is also reported for antimicrobial activity [2]. Puhl and Treutter [12] reported the anti-infective activity of catechins. However, contradictory reports have been published by Bais et al. [13]. In the present study, RBHWE got better activity among the samples may be because exposure of internal tissue of the plant to external environment increased the synthesis of molecules to combat infections.
Flavanoids and cutin protect the plant from various infections and might be elevated in response to infections and to avoid moisture loss [15]. The EGC was absent in the regenerated bark samples indicating the importance of CA and EC over it, in prevention of infections. However, EGC was present in significantly higher amounts in the bark hot water extract having less antimicrobial activity. It shows that down-regulation of EGC and up-regulation of CA and EC biosynthesis during the regeneration process of bark may be important in the healing process. The accumulation of catechin derived procyanidins is one fundamental factor inhibiting the growth of the pathogenic fungus Botrytis cinerea in immature strawberry fruits [12]. However, Bais et al. [13] contradict the reports. But in the present study, it is clear that CA and EC levels increase in the regenerated bark and leaves which shows importance of these metabolites in the prevention of infection.
4. Conclusion
The study has correlated antimicrobial activity with catechins present in S. asoca samples. For catechins quantization a convenient, sensitive, high-throughput, and reliable HPLC–QTOFMS method was developed which can also be used to identify morphologically same parts of S. asoca. A careful examination of data, in particular of LWE, shows that some other antimicrobial entities along with catechins are also present in the extracts which need to be explored.
Quality control of herbal drugs which is an important challenge in present scenario can be addressed with reliable and sensitive quantization of important biological active metabolites in the sample(s). Present study also demonstrates that quantization of catechins can be used as a powerful tool to profile and differentiate phytochemical compositions of S. asoca samples and herbal drugs. The identified specific marker compounds from qualitative analysis can be further utilized for authentication of plant parts. The method can also be used to separate and quantify CA and EC isomers along with EGC using a simple solvent scheme. Overall, this work can be utilized for the evaluation of quality of medicinal herbs having significance in the pharmacological and clinical investigation.
Acknowledgments
This study was supported by Central Council for Research in Ayurvedic Sciences (CCRAS), Department of AYUSH, Government of India.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Aggarwal B.B., Prasad S., Reuter S. Identification of novel anti-inflammatory agents from ayurvedic medicine for prevention of chronic diseases: reverse pharmacology and bedside to bench approach, Curr. Drug Targets. 2011;12:1595–1653. doi: 10.2174/138945011798109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabur R., Gupta A., Mandal T.K. Antimicrobial activity of some Indian medicinal plants. Afr. J. Trad. Compl. Alter. Med. 2007;4:313–318. doi: 10.4314/ajtcam.v4i3.31225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradhan P., Joseph L., Gupta V. Saraca asoca (Ashoka): a review. J. Chem. Pharm. Res. 2009;1:62–71. [Google Scholar]
- 4.Jayita S., Taniya M., Kamala G. Phytoconstituents and HPTLC analysis in Saraca asoca (Roxb.)Wilde. Int. J. Pharm. Pharma. Sci. 2012;4:96–99. [Google Scholar]
- 5.Gahlaut A., Taneja P., Shirolkar A. Principal component and partial least square discriminant based analysis of methanol extracts of bark and re-generated bark of Saraca asoca. Int. J. Pharm. Pharma. Sci. 2012;4:331–335. [Google Scholar]
- 6.Gahlaut A., Gothwal A., Dabur R. TLC based analysis of allelopathic effects on tinosporoside contents in Tinospora cordifolia. J. Chem. Pharm. Res. 2012;4:3082–3088. [Google Scholar]
- 7.Zeng Z., Chau F.T., Chan H.Y. Recent advances in the compound-oriented and pattern-oriented approaches to the quality control of herbal medicines. Chin. Med. 2008 doi: 10.1186/1749-8546-3-9. 3:9, 10.1186/1749-8546-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Hou X., Zhang J. Applications of HPLC/MS in the analysis of traditional Chinese medicines. J. Pharm. Anal. 2011;1:81–91. doi: 10.1016/S2095-1779(11)70015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X.F., Wu H.T., Tan G.G. Liquid chromatography coupled with time-of-flight and ion trap mass spectrometry for qualitative analysis of herbal medicines. J. Pharm. Anal. 2011;1:235–245. doi: 10.1016/j.jpha.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tasleem A., Mandal T.K., Kumar N. In vitro and in vivo antimicrobial activities of seeds of Caesalpinia bonduc (Lin.) Roxb. J. Ethnopharma. 2009;123:177–180. doi: 10.1016/j.jep.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Dabur R., Sharma G.L. In vitro antifungal activity of 2-(3,4-dimethyl-2,5-dihydro-1H-pyrrol-2-yl)-1-methylethyl pentanoate, a dihydropyrrole derivative. J. Med. Microbiol. 2005;54:549–552. doi: 10.1099/jmm.0.45968-0. [DOI] [PubMed] [Google Scholar]
- 12.Puhl I., Treutter D. Ontogenetic variation of catechin biosynthesis as basis for infection and quiescence of Botrytis cinerea in developing strawberry fruits. J. Plant Dis. Prot. 2008;115:247–251. [Google Scholar]
- 13.Bais H.P., Venkatachalam L., Biedrzycki M.A. Stimulation or inhibition conflicting evidence for (±)-catechin's role as a chemical facilitator and disease protecting agent. Plant Signal. Behav. 2010;5:239–246. doi: 10.4161/psb.5.3.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhanga J., Yanga J., Duana J. Quantitative and qualitative analysis of flavonoids in leaves of Adinandra nitida by high performance liquid chromatography with UV and electrospray ionization tandem mass spectrometry detection. Anal. Chim. Acta. 2005;532:97–104. [Google Scholar]
- 15.Irit M., Faoro F. Chemical diversity and defence metabolism: how plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009;10:3371–3399. doi: 10.3390/ijms10083371. [DOI] [PMC free article] [PubMed] [Google Scholar]