Abstract
A rapid, novel spectrofluorimetric method to determine epristeride (EP) in biological fluids and a pharmaceutical formulation was developed, based on the fact that fluorescence intensity of l-tryptophan could be quenched by EP in the medium of pH=9.0. The various factors influencing fluorescence quenching were discussed. The quenching mechanism was investigated with the quenching type, synchronous fluorescence spectra and quantum efficiency. Under the optimized conditions, fluorescence quenching value (ΔF=Fl-tryptophan−FEP–l-tryptophan) showed a good linear relationship with the EP concentration ranging from 0.4 to 12.0 μg/mL. The linearity, recovery and limit of detection demonstrated that the proposed method was suitable for EP determination in biological fluids and EP tablets. The method was successfully applied to the analysis of EP in real samples and the obtained results were in good agreement with the results of the official method.
Keywords: Epristeride, l-tryptophan, Fluorescence quenching method
1. Introduction
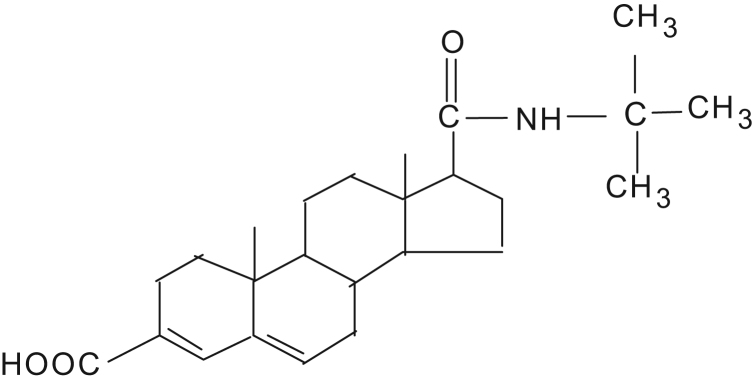
Benign prostatic hyperplasia is a disease of older men, and over 50% of men above 50 years have been found at autopsy, to have histological evidence of prostatic enlargement. Epristeride [17 β-(N-tert.-butyl carboxamido)-androst-3,5-diene-3- carboxylic acid] (EP), a 5α-reductase inhibitor (Fig. 1), through inhibiting the activity of 5α-reductase in the human body, can restrain testosterone from being reduced to dihydrotestosterone, so as to lower the concentration of dihydrotesterone and thus treat benign prostate hyperplasia [1]. Epristeride is the second inhibitor of 5α-reductase following finasteride for the treatment of benign prostate hyperplasia. Finasteride is the competitive inhibitor of 5α-reductase while epristeride is a non-competitive one and has a more rapid clinical onset than finasteride [2]. Therefore, epristeride has a broader prospect of application, and an analytical procedure is needed for quality assurance on pharmaceutical preparations.
Fig. 1.
The structure of epristeride.
Literature survey reveals several methods for the determination of EP in serum or EP tablets with high-performance liquid chromatography [3], [4]. However, analytical techniques based on HPLC involve a complex procedure and costly solvents and produce a lot of waste liquid. Compared with chromatography, spectrofluorimetry attracts more and more interest with its high sensitivity and simplicity, and thus has been used extensively in the analysis of drugs and food [5], [6], [7]. In our previous publication, the interaction of EP and bovine serum albumin with fluorescence quenching method and determined EP in EP tablet and human serum were developed, but the limit of detection was relatively high (0.28 μg/mL) [8].
Tryptophan consists of d-tryptophan and l-tryptophan. d-tryptophan is hardly utilized by organisms [9]. l-tryptophan is one of the necessary amino acids for life activities of creatures and is widely applied in medicine, food and feedstuff. Investigating the interaction of drugs and l-tryptophan is significant for knowing the transport and distribution of drugs in body in order to clarify the action mechanism and pharmaceutical dynamics of drugs. For example, the interaction of l-tryptophan and p-hydroxyphenylpyruvic acid or penicillin G with fluorescence quenching method was studied [10], [11]. In our previous publication, the interaction of tropisetron hydrochloride and l-tryptophan was considered [12].
In this paper, the interaction of EP and l-tryptophan was studied with spectrofluorimetry. It was found that the native fluorescence intensity of l-tryptophan was distinctly quenched by EP. Under experimental conditions, the fluorescence quenching degree of l-tryptophan (ΔF) had a linear relationship with the concentration of EP. Hence, a new spectrofluorimetry was developed to determine EP with a relatively low detection limit. The proposed method was successfully applied to determine EP in real samples and the obtained results were in good agreement with that of the official method (titration method) [13]. The mechanism of action was also formulated.
2. Experimental
2.1. Apparatus and chemicals
A Hitachi F-4500 spectrofluorimeter (Japan) was used for all the fluorescence measurement, with excitation slits at 2.5 nm and emission slits at 5.0 nm, λex=281 nm and 1-cm quartz cell. The pH measurements were carried out with a pHS-25B pH-meter (Shanghai, China). All absorption spectral recordings and absorbance measurements were performed on a UV 2501 spectrophotometer (Shimadzu, Japan).
Epristeride standard and epristeride tablets (Batch number: 20120101, Labeled value: 5 mg/tablet) were kindly provided by Jiangsu Lianhuan pharmaceutical Co., Ltd., Jiangsu, China. l-tryptophan and other materials were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Double distilled water was used throughout.
An epristeride standard solution of 1.00 mg/mL was prepared by dissolving 0.0500 g of epristeride in 50 mL of anhydrous ethanol and kept in a cool and a dark place.
A standard l-tryptophan solution of 1.00 mg/mL was prepared by dissolving 0.0500 g of l-tryptophan in 50 mL of 0.1 M NaOH aqueous solution and kept in a cool and a dark place.
The buffer solution of ammonia–ammonium chloride (0.1 M, pH 9.0) was employed to control the pH of the sample solutions.
2.2. Sample preparation
2.2.1. Epristeride tablets treatment
Ten tablets of epristeride were weighed and crushed, and then sample powder of about five tablets was accurately weighed and placed in a 50 mL of beaker and dissolved with anhydrous ethanol. Insoluble excipient was removed by filtration through a 0.45 μm membrane filter. The filtered solution was diluted to 50 mL with anhydrous ethanol.
2.2.2. Urine and serum treatment [5]
First, the urine and the serum samples of healthy volunteers were collected from local hospitals and stored in polyethylene bottles after the dilution of 10- and 100-fold with double distilled water, respectively. Then urine or serum was spiked with convenient amounts of EP stock. The final EP concentration was 1.00 mg/mL.
2.3. Fluorescence measurements
In a 25 mL volumetric flask, 0.25 mL 1.00 mg/mL of l-tryptophan solution, 3.0 mL of NH3–NH4Cl buffer solution (pH=9.0) and adequate EP standard or sample solution were added; the solution was diluted to the mark with distilled water. Then l-tryptophan fluorescence spectra were recorded in the range of 250–550 nm upon excitation at 281 nm.
2.4. Determination of relative fluorescence quantum yield
Fluorescence quantum yield of l-tryptophan with or without EP was measured using 1.0×10−6 g/mL quinine sulfate as a reference material.
Under the same apparatus conditions, according to the equation ϕ2=(ϕ1A1F2)/(F1A2) [14], the quantum yield of the analyte was calculated. In brief, ϕ1 and ϕ2 correspond to the standard and unknown fluorescence quantum yield, and F1 and F2 are the integral areas of two calibration fluorescence emission curves; A1 and A2 are the absorbance (λabsorbance=λexcitation) of the standard and unknown, and ϕ1=0.55 (25 °C) is known.
3. Results and discussion
3.1. Quenching spectra of l-tryptophan with EP
The fluorescence spectra of l-tryptophan were recorded before and after incubation with a series concentration of EP in pH=9.0 at λex=281 nm (Fig. 2). It can be seen from Fig. 2 that (1) the fluorescence intensity of l-tryptophan decreased gradually with the increase of EP concentration without changing the emission maximum and shape of the peaks (curve 1–curve 9); (2) the fluorescence intensity of l-tryptophan was almost completely quenched when the concentration of EP was up to 200.0 μg/mL (Fig. 2 curve 9), and there was no fluorescence emission for EP at the range measured. These results indicated that there was an interaction between EP and l-tryptophan.
Fig. 2.
Fluorescence quenching spectra of l-tryptophan in the presence of EP. cl-tryptophan: 10.0 μg/mL; 1–9: cEP is 0.0, 4.0, 8.0, 12.0, 16.0, 40.0, 48.0, 80.0, 200 μg/mL.
3.2. Effect of l-tryptophan concentration on fluorescence quenching
The effect of l-tryptophan on fluorescence quenching value (ΔF=Fl-tryptophan−FEP–l-tryptophan) was investigated. Fig. 3 shows the effect of concentration of EP on ΔF in different concentrations of l-tryptophan (curves 1–6). It can be seen that ΔF was enhanced with increasing l-tryptophan concentration and reached the maximum value at 10.0 μg/mL of l-tryptophan and then remained constant (auxiliary Fig. 3).
Fig. 3.
Effect of concentration of EP on fluorescence quenching in different concentrations of l-tryptophan.
The linearity parameters and limit of detection (LOD) of the proposed method in different concentrations of l-tryptophan are listed in Table 1. It can be seen from Table 1 that (1) the LOD was decreased with increasing l-tryptophan concentration and was similar when the concentration of l-tryptophan was up to 8.0–12.0 μg/mL; (2) the linear range was narrower in 8.0–12.0 μg/mL of l-tryptophan than in 2.0–6.0 μg/mL of l-tryptophan. To decide on the concentration of l-tryptophan, the LOD and the linear range had to be taken into consideration. 10.0 μg/mL of l-tryptophan was adopted as an optimum concentration for this study.
Table 1.
Linearity parameters and limit of detection of the proposed method in different concentrations of l-tryptophan.
| Sample | l-tryptophan (μg/mL) | The linear range of EP (μg/mL) | Linear equation | R | LOD (μg/mL) [15] |
|---|---|---|---|---|---|
| Standard solution | 2.0 | 0.4–16.0 | ΔF=19.98cEP+30.1 | 0.9952 | 0.030 |
| 4.0 | 0.4–20.0 | ΔF=26.16cEP+31.8 | 0.9991 | 0.022 | |
| 6.0 | 0.4–20.0 | ΔF=38.05cEP+92.0 | 0.9927 | 0.016 | |
| 8.0 | 0.4–12.0 | ΔF=64.25cEP+94.7 | 0.9937 | 0.009 | |
| 10.0 | 0.4–12.0 | ΔF=76.5cEP+100.5 | 0.9979 | 0.008 | |
| 12.0 | 0.4–12.0 | ΔF=80.1cEP+107.7 | 0.9957 | 0.007 | |
| Serum | 10.0 | 0.4–12.0 | ΔF=77.15cEP+116.0 | 0.9935 | 0.025 |
| Urine | 0.4–12.0 | ΔF=77.82cEP+135.5 | 0.9942 | 0.020 |
3.3. Effect of pH on fluorescence quenching
The effect of pH on fluorescence quenching value ΔF=Fl-tryptophan−FEP–l-tryptophan is shown in Fig. 4. From Fig. 4, maximum ΔF can be obtained at pH=9.0. After that, ΔF decreased with a further increase of pH.
Fig. 4.
Effect of pH on fluorescence quenching (cl-tryptophan: 10.0 μg/mL, cEP: 8.0 μg/mL, curve 1: cl-tryptophan: 10.0 μg/mL).
The probable reason is that pH had an important effect on Fl-tryptophan from the results concluded in auxiliary Fig. 4. As shown in auxiliary Fig. 4, the Fl-tryptophan reached a maximum value at the pH of 9.0 and obviously reduced at higher or lower pH values. The above results indicated that pH exerts its effect on ΔF mainly through influencing the Fl-tryptophan.
The effect of buffer solution volume was also studied. Fig. 5 shows that ΔF changed very little with the buffer solution volume ranging from 1.0 to 7.0 mL. In this study, a 3.0 mL NH3–NH4Cl buffer solution (pH=9.0) was selected as suitable for the optimized method.
Fig. 5.
Effect of buffer solution volume on fluorescence quenching (cl-tryptophan: 10.0 μg/mL, cEP: 8.0 μg/mL).
3.4. Effect of reaction time on fluorescence quenching
At room temperature, the quenching reaction could reach equilibrium immediately after EP and l-tryptophan were mixed. The fluorescence intensity of the mixture varied very little in 2 h.
3.5. Effect of potential interferences
The potential interference of the proposed method was evaluated by analyzing the standard solution of EP in the presence of tablet excipients such as lactose, microcrystalline cellulose, sucrose, magnesium stearate, colloidal silicon dioxide, and other possible coexisting substances. It was found that the solubility of microcrystalline cellulose, croscarmellose sodium, magnesium stearate or colloidal silicon dioxide was very small under the examination condition and could be eliminated as sediment from solution.
The effects of other substances were discussed in the determination of 0.2 mg EP in a 25 mL volumetric flask. The level of tolerated concentrations of foreign substances was considered as the maximum concentration found to cause a change in signal, less than ±5%, compared with the signal without foreign substances. The results are shown in Table 2. The tolerance limits of most tested substances exceeded the quantity coexisting with the studied drug EP, suggesting that the method possessed a good selectivity.
Table 2.
Tolerance limits of interfering substances.
| Tested substances | Tested substances to analyte ratio (w/w) | Tested substances | Tested substances to analyte ratio (w/w) |
|---|---|---|---|
| Ethanol | Over 8000 | Mn2+ | 1 |
| Glucose | 2500 | Al3+ | 0.9 |
| Sucrose | 1500 | Pb2+ | 0.5 |
| Lactose | 1000 | Zn2+ | 0.5 |
| Na+ | 150 | Co2+ | 0.2 |
| Citric acid | 62.5 | Fe3+ | 0.1 |
| Mg2+ | 5 | Cu2+ | 0.1 |
| Ca2+ | 5 |
3.6. Analytical performance
The calibration graphs for determining EP were obtained under the experimental conditions described above by plotting l-tryptophan fluorescence intensity quenching values versus EP concentration in standard solution, human serum and human urine matrices. The analytical parameters obtained are given in Table 1.
For comparative studies, the results obtained by this method were compared with those by other methods for the determination of EP (Table 3). Venkata et al. [3] and Yan et al. [4] determined EP with HPLC, in which operation was complicated and consumed a lot of organic reagents and the recovery was not as satisfactory as expected in spite of lower LOD (0.0025 μg/mL). Compared with Ref. [8], which also used spectrofluorimetry to determine EP, the detection limit of this work was lower. The results indicate that this work has advantages such as good accuracy, high sensitivity, broad linear range as well as simple and rapid determination.
Table 3.
Comparison of methods for the determination of EP.
| Technique | Linearity range (µg/mL) | LOD (μg/mL) | Recovery (%) | Reference |
|---|---|---|---|---|
| HPLC+SPEa | 0.001–0.5 | 0.0025 | 90.2±2.96 | [3] |
| HPLC | 0.5–20 | – | 98.3 | [4] |
| Fluorescence | 0.0–40.0 | 0.28 | 95.0–102.5 | [8] |
| Fluorescence | 0.4–12.0 | 0.008 | 98.3–101.2 | This work |
SPE: solid phase exteaction.
3.7. Determination of EP in real sample
The method was applied for the determination of EP in commercial tablets. The data are shown in Table 4. The results obtained by the proposed method (5.02 mg/tablet) were in good agreement with those obtained by the official method—titration method (4.99 mg/tablet). The statistical t-test (P=0.95) was used to compare the results from both the methods, which showed that there was no significant difference between them.
Table 4.
The determination of EP in real sample and recovery.a
| Sample | EP added (μg/mL) | EP found (μg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| EP tablets | 4.00 | 4.05 | 101.2 | 0.38 |
| 8.00 | 7.86 | 98.2 | 0.43 | |
| 12.00 | 11.80 | 98.3 | 0.58 | |
| Serum | 0.00 | ND | – | – |
| 4.00 | 4.28 | 107.0 | 1.0 | |
| 8.00 | 8.40 | 105.1 | 1.8 | |
| 12.00 | 12.55 | 104.6 | 2.2 | |
| Urine | 0.00 | ND | – | – |
| 4.00 | 4.30 | 107.5 | 1.5 | |
| 8.00 | 8.28 | 103.5 | 1.8 | |
| 12.00 | 12.40 | 103.3 | 2.0 |
ND: Not detected.
Average value of three determinations.
The method was also applied for determining EP in spiked human urine and serum (Table 4). Accuracy was assessed with the recovery of the studied drug. Obtained mean values of the recoveries ranged from 98.2% to 101.2%, from 104.6% to 107.0%, and from 103.3% to 107.5% for EP tablets, human serum and human urine respectively; the RSD ranged from 0.38% to 0.58%, from 1.0% to 2.2%, and from 1.5% to 2.0%, respectively, indicating both good accuracy and precision.
3.8. Discussion of the mechanism of quenching effect
3.8.1. Quenching type
Generally, quenching types of fluorescence often include static and dynamic quenching. The quenching type is differentiated as follows:
-
1.
The fluorescence quenching spectra of substance
The fluorescence quenching spectra and quenching type could be analyzed by Stern–Volmer equation:Among them, F0 and F are the fluorescence intensities of l-tryptophan in the absence and presence of EP, respectively; Ksv is the Stern–Volmer quenching constant; CQ is the concentration of quencher [8].
The Stern–Volmer curve would be linear if the quenching type is single static or dynamic quenching and it is an upward curvature [12], [16] if the quenching type is combined quenching (both static and dynamic).
A linear Stern–Volmer plot, however, does not define the quenching type. One of the ways to distinguish dynamic from static quenching is to examine temperature's effect on quenching constant Ksv. The Ksv values will be enhanced with an increase in temperature for dynamic quenching, but the reverse effect would be observed for static quenching [12].
The Stern–Volmer quenching constant Ksv and relative coefficients at three temperatures are listed in Table 5. The linearity of F0/F versus cEP revealed that the quenching type was single, static or dynamic.
Table 5 shows that the values of Ksv were enhanced when increasing temperature, indicating that the possible fluorescence quenching mechanism of l-tryptophan by EP was a dynamic quenching procedure.
-
2.
The UV absorption spectra of fluorescence substance in the presence of quencher
For dynamic quenching, the absorption spectra of fluorescence substance are not changed [8], [17]. The UV absorption spectra of 4.0 μg/mL of l-tryptophan with or without EP were recorded under pH=9.0 (Fig. 6).
Table 5.
Stern–Volmer quenching constant Ksv and correlation coefficients (R).
| T (K) | Ksv×10−4 (L/mol) | R |
|---|---|---|
| 286.15 | 3.42±0.25 | 0.992 |
| 293.15 | 3.57±0.18 | 0.996 |
| 303.15 | 3.61±0.20 | 0.995 |
Fig. 6.
Effect of EP on UV absorption spectrum of l-tryptophan. cl-tryptophan: 4.0 μg/mL, cEP: 8.0 μg/mL.
The examination demonstrated that there was almost no difference between the absorption spectra of l-tryptophan and that of l-tryptophan–EP mixture. From this, it can be deduced that the fluorescence quenching type of l-tryptophan initiated by EP is dynamic quenching.
3.8.2. Synchronous fluorescence spectra
The synchronous fluorescence spectra give the information on the molecular microenvironment, particularly in the vicinity of the fluorophore functional groups [18]. Therefore, the synchronous fluorescence spectra of l-tryptophan were measured at Δλ=80 nm (Fig. 7). As shown in Fig. 7, the position of the maximum wavelength produced a red shift, which indicates that the microenvironment of l-tryptophan system was affected by EP.
Fig. 7.
Synchronous fluorescence spectra of l-tryptophan at Δλ=80 nm. 1–7: cl-tryptophan=4.0 μg/mL, cEP=2.0, 4.0, 8.0, 12.0, 16.0, 20.0 μg/mL.
3.8.3. Relative fluorescence quantum yield
The fluorescence quantum yield Φf of l-tryptophan with or without 8.0 μg/mL EP was determined, and the Φf was 0.49 and 0.34, respectively. The results showed that the quantum yield Φf was approximately 30% times lower in the presence of EP than that in the absence of EP. The Φf value is closely related to chemical structure and microenvironment of the system. The drop of Φf indicated that in the presence of EP, the microenvironment of the system was changed and the excited-state l-tryptophan molecules collided with EP, so the energy of excited-state l-tryptophan molecules was transferred to EP molecules and the quantum yield fell.
4. Conclusion
A new fluorimetric method was developed and validated for the determination of EP in human serum, urine and EP tablets. The obtained results of interference examination showed that the common substances from dosage forms and biological samples did not affect the determination of EP. The biological significance of this work is evident since l-tryptophan is one of the necessary amino acids and the interaction of EP and l-tryptophan has not been characterized so far. Hence, the report has a great significance in pharmacology and clinical medicine as well as methodology.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (20875082and 21155001) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Foundation of the Excellence Science and Technology Invention Team in Yangzhou University.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Audet P.R., Baine N.H., Benincosa L.J. Epristeride steroid 5α- reductase inhibitor treatment for benign prostatic hyperplasia. Drugs Future. 1994;19(7):646–650. [Google Scholar]
- 2.Lü J.L. The compliance of finasteride and epristeride in the treatment of benign prostatic hyperplasia. Chin. J. Med. Guide. 2010;12(10):1731–1732. [Google Scholar]
- 3.Venkata K.B., Cynthia M.S., Gerald R.R. Normal-phase high-performance liquid chromatographic determination of epristeride, a prostatic steroid 5α-reductase enzyme inhibitor, in human plasma. J. Chromatogr. A. 1993;631(1):251–254. doi: 10.1016/0021-9673(93)80529-h. [DOI] [PubMed] [Google Scholar]
- 4.Yan D.M., Tu L.L, Song Z.Y. HPLC determination of the content and dissolution of epristeride tablers, Chin. J. Pharm. Anal. 2007;8(27):1264–1266. [Google Scholar]
- 5.Manzoori J.L., Abolghasem J., Mohammad A. Spectrofluorimetric determination of buparvaquone in biological fluids, food samples and a pharmaceutical formulation by using terbium–deferasirox probe. Food Chem. 2011;126:1845–1849. doi: 10.1016/j.foodchem.2010.11.160. [DOI] [PubMed] [Google Scholar]
- 6.Wang F., Huang W. Determination of curcumin by its quenching effect on the fluorescence of Eu3+–tryptophan complex. J. Pharm. Biomed. Anal. 2007;43:393–398. doi: 10.1016/j.jpba.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Guo C.C., Chu Z.J. Luminescence enhancement effect for the determination of balofloxacin with balofloxacin–europium (III)–sodium dodecylbenzene sulfonate system. J. Lumin. 2009;129:90–94. [Google Scholar]
- 8.Gong A.Q., Zhu X.S., Hu Y.Y. A fluorescence spectroscopic study of the interaction between epristeride and bovin serum albumine and its analytical application. Talanta. 2007;73:668–673. doi: 10.1016/j.talanta.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y.H., Wang Y.H., Yu H.S. Advance of l-tryptophan application and production technology. J. Jilin Agric. Univ. 2008;30:586–589. [Google Scholar]
- 10.Wu F.Y., Fu M.G., Wei X.S. Fluorescence quenching method for the determination of P-hydroxyphenylpyruvic acid. Spectrosc. Spectral Anal. 2001;21:359–361. [PubMed] [Google Scholar]
- 11.Kumar R.S., Prabhune A.A., Pundle A.V. A tryptophan residue is identified in the substrate binding of penicillin G acylase from Kluyvera citrophila. Enzyme Microb. Technol. 2007;40(5):1389–1397. [Google Scholar]
- 12.Zhu X.S., Gong A.Q., Wang B.S. Study on the interaction of tropisetron hydrochloride and l-tryptophan by spectrofluorimetry and its analytical application. J. Lumin. 2008;128(11):1815–1818. [Google Scholar]
- 13.SFDA, Chinese Pharmaceutical Standard WS-605 (X-531)-99, 2000.
- 14.Chen G.Z., Huang X.Z., Xu J.G. 2nd ed. Science Publishing House; Beijing: 1990. Spectrofluorimetric Analytical Method; p. 16. [Google Scholar]
- 15.IUPAC Method, Accessed Pathway: 〈http://www.doc88.com/p-802573471184.html〉.
- 16.Kandagal P.B., Ashoka S., Seetharamappa J. Study of the interaction of an anticancer drug with human and bovine serum albumin: spectroscopic approach. J. Pharm. Biomed. Anal. 2006;41:393–399. doi: 10.1016/j.jpba.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Ye L., Hu L.F., Du X.X. Study on the interaction of minocycline with human serum albumin by fluorescence spectrometry. Herald Med. 2011;30(12):1651–1654. [Google Scholar]
- 18.Fan Y.C., Zhang S.L., Kong J.C. Study on the interaction between an ionic liquid and l-tryptophan by fluorescence spectroscopic technique. J. Microchem. 2011;99(2):439–442. [Google Scholar]