Abstract
Inosiplex is a compound formulation composed of inosine and p-acetaminobenzoic acid (PABA) salt of N,N-dimethylamino-2-propanol (DIP). This study was to investigate the clinical plasma pharmacokinetic properties of DIP and PABA after single and multiple oral doses of inosiplex tablets in healthy Chinese volunteers. The established LC/MS/MS method for plasma DIP determination had a linear range of 0.02–10 µg/mL, and the HPLC method for plasma PABA determination had a linear range of 0.05–40 µg/mL. Linear pharmacokinetic characteristics were found with single oral doses of 0.5, 1.0 and 2.0 g. No obvious accumulation effects were observed for DIP and PABA.
Keywords: Inosiplex; N,N-dimethylamino-2-propanol; p-Acetaminobenzoic acid; LC/MS/MS; HPLC; Pharmacokinetics
1. Introduction
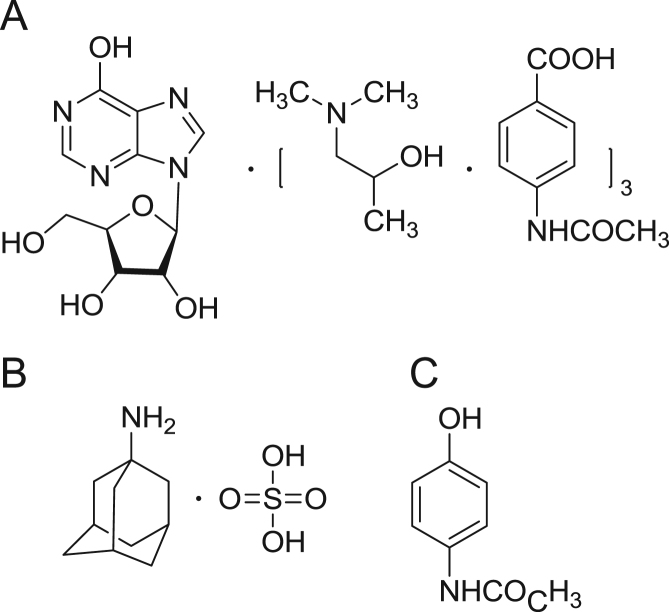
The antiviral agent inosiplex is composed of inosine, p-acetaminobenzoic acid (PABA), and N,N-dimethylamino-2-propanol (DIP) in molar ratio of 1:3:3 (Fig. 1). Although little is known about the precise mechanism of the action of inosiplex, numerous studies have shown its ability to potentiate certain aspects of the cellular immune response [1] both in vitro and in vivo. Inosiplex was found to have a broad spectrum of antiviral activity, including alleviating symptoms of subacute sclerosing panencephalitis, symptomatic subclinical human papillomavirus infection, and cervical condylomata acuminata because of genital human papillomavirus [2], [3], [4]. Inosiplex could also delay the progression of HIV infection to overt AIDS [5]. In addition, inosiplex has been employed as an immunoregulating agent for the treatment of immunopathological disorders, such as rheumatoid arthritis and alopecia areata [6], [7]. However, limited data was published up to now on the bioassay and pharmacokinetics of inosiplex [8]. And providing an integrated measure of inosiplex pharmacokinetics is of great importance in rational clinical therapeutic application of inosiplex. Therefore, endeavor has been done in this work to carry out the plasma pharmacokinetic studies of inosiplex tablets in Chinese volunteers.
Fig. 1.
Chemical structures of (A) inosiplex, (B) amantadine (internal standard for DIP), and (C) paracetamol (internal standard for PABA).
To meet the demands for pharmacokinetic studies, a selective, sensitive and robust analytical method is highly desirable for each active component. It was reported that the concentration of inosine did not increase obviously with dose enhancement and was maintained in the range of 100–1000 ng/mL in healthy volunteers [9], [10]. Although it has been reported [8], [11], [12] that PABA is extensively metabolized to p-aminohippuric acid and p-acetaminohippuric acid, for they are not pharmacologically as active as PABA and readily eliminated into the urine, the study here did not emphasize the determination of inosine, but focused on the analysis of DIP and the unconjugated PABA in the biological samples. In this paper, an LC/MS/MS determination method [13], [14] for DIP and an HPLC method for PABA have been set up, respectively, and the established methods were applied to plasma pharmacokinetic studies of DIP and PABA in 30 healthy Chinese volunteers after oral administration of inosiplex tablets. Meanwhile, the food and gender effects on the pharmacokinetic properties have been investigated as well. Therefore, this research is of a great importance on rational and safe clinical therapeutic application of inosiplex tablets.
2. Materials and methods
2.1. Chemicals and reagents
Inosiplex tablets (500 mg/tablet; Batch no. 100202S), inosine, DIP, and PABA reference standards were supplied by Shenzhen Salubris Pharmaceuticals Co., Ltd., China. Amantadine (from the Jiangsu Chia-tai Tianqing Pharmaceutical Co., Ltd., China, Batch no. 20040905) and paracetamol (from the National Institute for the Control of Pharmaceutical and Biological Products, China, Batch no. 10018-200408) were used as the internal reference standards (IS) for the determination of plasma DIP and PABA, respectively. Methanol and acetonitrile were chromatographic pure grades and purchased from Merck, Darmstadt, Germany. Other chemicals were all of analytical grades. Water was double-distilled and the mobile phase was filtered through 0.22 μm film before use.
2.2. Instrumentation
A Finnigan Surveyor system (Thermo Finnigan, San Jose, CA) containing a Surveyor LC pump, a Surveyor auto-sampler, and a TSQ Quantum Ultra AM triple-quadrupole tandem mass spectrometer with an ion max source was used for DIP determination. The LC/MS/MS system was operated with the Xcalibur 1.1 software. The HPLC analysis for PABA was carried out using an Agilent Liquid Chromatography System series 1100 (Agilent Technologies, USA), with a binary pump, an auto-sampler, a solvent degasser and a column oven.
2.3. Chromatographic and MS/MS conditions
HPLC separation for DIP was performed on a Phenomenex Luna 5u CN analytical column (250 mm×4.6 mm, 5 μm) at 30 °C with a mixture of acetonitrile–0.5% formic acid and 0.5% ammonium acetate solution (75:25, v/v) as a mobile phase which was delivered at 1 mL/min. Thirty percent of the eluent was split into the inlet of the MS spectrometer using an electrospray ionization (ESI) source.
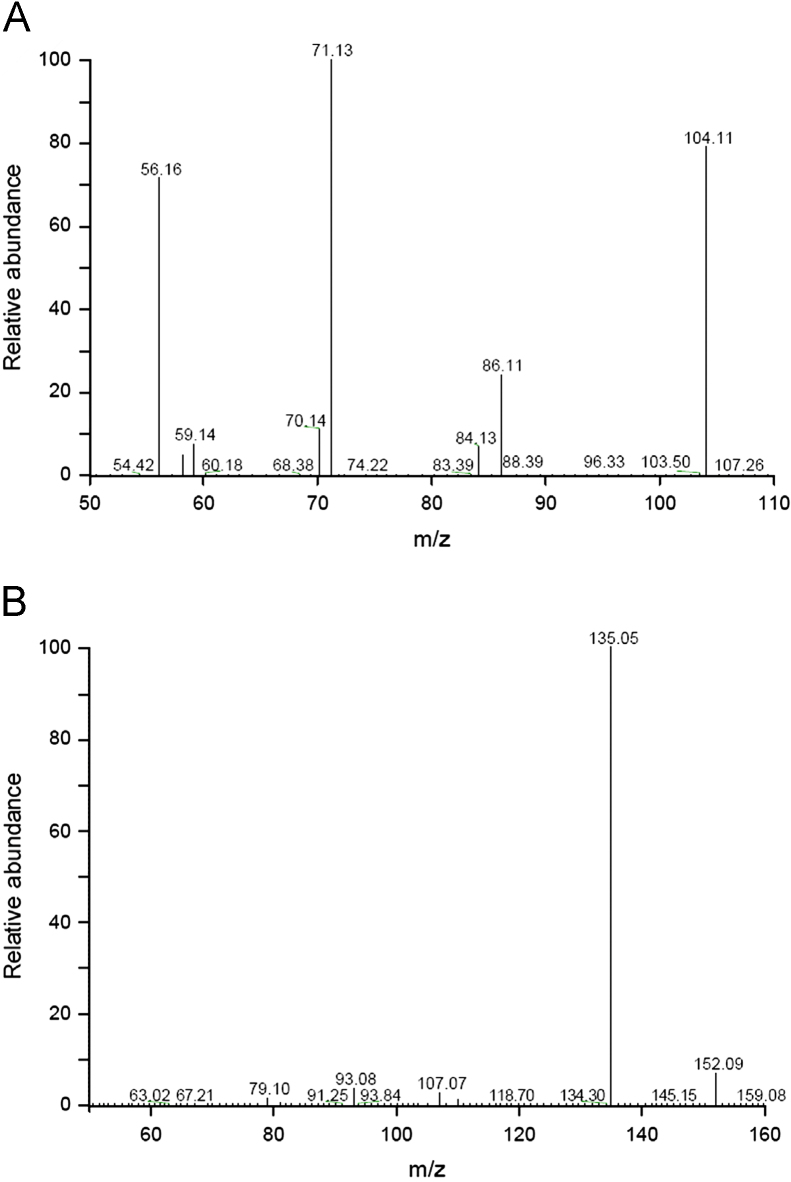
The TSQ Quantum parameters were optimized and set as following: positive electrospray ionization with spray voltage of 5000 V, capillary temperature of 350 °C, nitrogen sheath gas pressure of 240 kPa, auxiliary gas pressure of 35 kPa, argon collision gas pressure of 0.2 Pa and collision energy of 25 eV. Quantifications were performed by using selected reaction monitoring (SRM) with ion transitions of m/z 104.1→71.1 for DIP, and m/z 152.1→135.1 for amantadine. Fig. 2 shows the full scan positive ESI product ion mass spectra and the daughter ions selected for MRM were [M+H-H2O-CH3·]+· for DIP and [M+H-NH3]+ for amantadine.
Fig. 2.
Product ions spectra of (A) DIP and (B) amantadine.
Plasma PABA was separated on an Agilent HC-C18 analytical column (150 mm×4.6 mm, 5 μm) with the mobile phase of methanol–0.2% ammonium acetate and 0.2% acetic acid solution (15:85, v/v). The UV detection wavelength was chosen at 266 nm.
2.4. Preparation of stock and working standard solutions
Primary stock solutions of DIP and PABA were prepared by dissolving appropriate amounts of the chemical reference substances in methanol, which were found to be stable for 30 days stored at 4 °C. Appropriate dilutions were made in methanol to produce a series of working standard solutions in the range of 0.2–100 µg/mL and 0.5–400 µg/mL for DIP and PABA, respectively. The amantadine and paracetamol solutions were prepared in the same way with the concentrations of 4 and 50 µg/mL. All the working standard solutions were freshly prepared, and stored at 4 °C.
2.5. Sample preparation
A protein precipitation method was employed for the DIP plasma sample pretreatment. Aliquot of 0.4 mL plasma sample was spiked with 40 μL of the IS solution (amantadine in methanol, 4.0 µg/mL) and then protein precipitation was induced by the addition of 0.8 mL acetonitrile and vortex mixing for 1 min. After centrifugation at 15,000g for 10 min, 10 µL supernatant was injected into the LC/MS/MS system for the quantification of DIP.
Liquid–liquid extraction was used for plasma sample work-up for the determination of PABA. Aliquot of 0.5 mL plasma sample was spiked with 50 μL of the IS solution (paracetamol in methanol, 50 µg/mL) and then mixed with 0.10 mL of hydrochloric acid (2.0 M) for acidification. The mixture was then extracted with 5.0 mL ethyl acetate by vortex-mixing for 2 min. After centrifugation at 1000g force for 10 min, the organic layer was transferred to a new tube and evaporated to dryness under a stream of nitrogen. The residual was reconstituted in 0.15 mL of the mobile phase and centrifuged at 15,000g for 10 min and 20 μL of the supernatant obtained was injected into the HPLC system for the analysis of PABA.
2.6. Assay validation
The LC/MS/MS and HPLC methods were validated according to the Food and Drug Administration guidelines for bioanalytical method validation [15]. Selectivity was performed using six different sources of blank plasma. They were extracted and analyzed, and the responses at the retention time of the analytes and IS were assessed. Calibration curves were created by plotting the peak area ratios of the corresponding analyte versus the concentrations. To assess linearity, the coefficient of correlation (r2) should be more than 0.99 and deviations should be within ±15% from nominal concentrations except for the LOQ level, at which a deviation of ±20% is permitted. Accuracy and precision were determined by assaying five replicates of quality control samples at the low, middle and high levels. Accuracy and precision were calculated in terms of relative error (RE, %) and relative standard deviation (RSD, %), respectively. The LOQ is defined as the lowest concentration. The recovery was determined at three quality control levels by comparing the peak areas obtained from plasma samples to those from spike-after samples. To evaluate the matrix effect of DIP, chromatographic peak areas of DIP from the spike-after protein precipitated samples were compared to the neat standards at the quality control concentrations. The variability in matrix factors should be less than 15%. Long-term storage stability, short-term temperature stability, auto-sampler rack stability, post-preparative stability, freeze-thaw stability and stock solution stability of DIP and PABA plasma samples were also investigated.
2.7. Pharmacokinetic study design
Thirty healthy Chinese volunteers, ranging in age from 30 to 40 years (34±3.3 years), in weight from 50 to 65 kg (60.5±4.1 kg), and in height from 150 to 175 cm (164.9±6.5 cm) were recruited. All subjects gave their written consent for their participation in the study after having been informed all aspects of the study, especially the potential risks. The study protocols were approved by the relevant Ethical Review Committee in Xijing hospital, Xi'an, China, in accordance with the principles of the Declaration of Helsinki and the recommendations of the State Food and Drug Administration of China.
The study was carried out with an open-label, randomized, three periods design. Subjects were divided into three groups with male and female equally assigned in each group (Groups I, II, and III). During the first period, Groups I–III were given a single oral dose of 0.5, 1.0 and 2.0 g inosiplex, with 250 mL of water after an over-night 12 h fast. The food effect study was conducted with Group II after a 7-day wash-out period and a single dose of 1.0 g inosiplex tablets was administered immediately following an FDA defined high fat breakfast consumed within 30 min. In the third period, multiple oral doses (1.0 g inosiplex t.i.d.) were administered to Group II for 7 consecutive days except for the 7th day with only a morning dose after a 7-day wash-out period. Venous blood samples each of 4 mL were collected in heparinized tubes at 0, 5, 10, 30, 45, 60, 90, 120, 180, 240, 360, 480, 720, and 1440 min, after dosing and on the 7th day for multiple doses. Venous blood samples were also collected prior to the morning dose (0 h) on days 4, 5, and 6 to affirm the steady states of plasma concentrations of DIP and PABA. All plasma samples were separated immediately by centrifugation at 1000g force for 10 min at 4 °C and stored at −80 °C until analysis.
2.8. Pharmacokinetic analysis
The plasma pharmacokinetic parameters including Cmax, Tmax, t1/2 and AUC were estimated by standard non-compartmental methods using a DAS2.0 software for both DIP and PABA. An analysis of variance (ANOVA) was performed for Cmax and AUC0−τ after natural logarithmic transformation using a general linear model procedure for randomized crossover design to evaluate the effects of periods, food, and subjects at the significance level (α) of 0.05. The Wilcoxon signed rank test was used for the non-parametric analysis to determine differences in Tmax. Effects were considered statistically significant if the probability obtaining the calculated F test was ≤0.05.
3. Results and discussion
3.1. Analysis of DIP
Method development for the analysis of DIP in plasma began with the optimization of chromatographic conditions including column type and mobile phase composition. The feasibility of various mixtures of solvents, such as acetonitrile and methanol, using different buffers, such as ammonium acetate, ammonium formate and formic acid with various pH ranging from 3.0 to 7.0, along with altered flow-rates was tested for optimum chromatographic retention of the analytes and IS. Finally, a Phenomenex Luna CN (250 mm×4.6 mm, 5 μm) column with a mobile phase consisting of 75 volumes of acetonitrile and 25 volumes of 0.5% formic acid and 0.5% ammonium acetate solution was selected for the separation since good chromatographic resolution and symmetrical peak shapes of the analytes were obtained. They were also compatible with the determination of the analytes by positive electrospray ionization MS detection.
Mass spectrometric conditions were optimized so as to achieve maximum and stable responses of the parent and the major product ions of the analytes. The predominately protonated molecule ions were obtained by the positive ESI scan at m/z 104.1 and 152.1 for DIP and amantadine, respectively. They were apt to form major product ions at m/z 71.1 and 135.1 with the optimum collision energies of 25 and 22 eV, respectively.
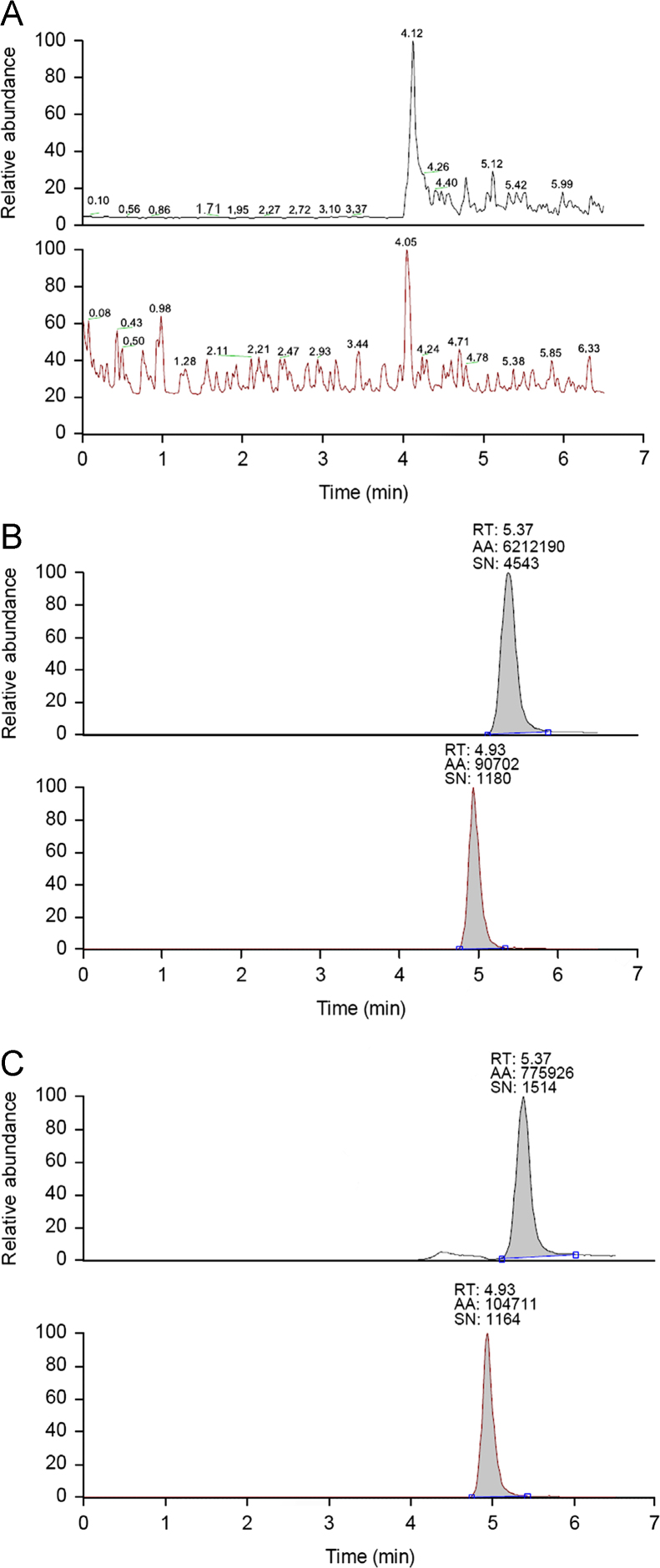
Fig. 3 shows the typical chromatograms of drug-free plasma, drug-free plasma spiked with DIP and the IS, and plasma sample from a volunteer after oral administration. No interference from endogenous substances with analytes or the IS was detected. The calibration curves of DIP in human plasma were linear in the concentration range of 0.02–10 μg/mL with correlation coefficient values >0.995. The LOQ for plasma DIP was 0.02 μg/mL. Table 1 summarizes the intra- and inter-batch precision and accuracy for DIP. It can be seen that the RSD values were less than 8.1% and the RE values were all within ±6.2%. Mean extraction recoveries were 89.0±3.6%, 96.0±3.7% and 103.3±2.0% at the concentrations of 0.04, 0.4 and 4 μg/mL, respectively. Average matrix effect values obtained varied from 95.7% to 96.9%, which were found to be within the acceptable limit. The stock solution was stable for at least 30 days at 4 °C. The stability experiment confirmed that DIP plasma samples were stable for over 60 days when stored at −20 °C and through three freeze-thaw cycles. The worked out plasma samples were stable for at least 24 h at 4 °C.
Fig. 3.
Typical MRM chromatograms of DIP and IS (amantadine) in human plasma. (A) Blank plasma sample; (B) plasma sample spiked with 10.0 μg/mL DIP and 4.00 μg/mL amantadine (IS); and (C) plasma sample 4 h after an oral administration of 1.0 g inosiplex tablets to a volunteer (DIP 1.01 μg/mL and amantadine 4.00 μg/mL).
Table 1.
Precision and accuracy of the LC/MS/MS method to determine DIP in human plasma (n=3 days, five replicates per day).
| Spiked concentration (μg/mL) | Intra-batch |
Inter-batch |
||||
|---|---|---|---|---|---|---|
| Mean (μg/mL) | RSD(%) (n=5) | Relative error (%) | Mean (μg/mL) | RSD (%) (n=15) | Relative error (%) | |
| 0.0400 | 0.04247 | 5.3 | 6.2 | 0.04127 | 8.1 | 3.1 |
| 0.400 | 0.4043 | 1.9 | 1.1 | 0.4097 | 4.0 | 0.9 |
| 4.00 | 3.983 | 2.2 | 0.42 | 4.063 | 5.0 | 1.5 |
3.2. Analysis of PABA
The determination of PABA in plasma has been investigated before by our and other groups [16], [17], [18]. Fig. 4 shows the typical chromatograms of blank plasma, spiked plasma sample with PABA and the IS, and the plasma sample from a volunteer after oral administration. No significant interference from endogenous substances was observed at the retention time of PABA or IS. The calibration curves were linear over the concentration range of 0.05–40.0 μg/mL and the LOQ was 0.05 μg/mL. Table 2 summarizes the intra- and inter-batch precision and accuracy for PABA. It can be seen that the RSD values were less than 5.7% and the RE values were all within ±11.7%. Mean values of absolute recoveries for PABA at 0.10, 2.00 and 20.0 μg/mL were 101.8±3.3%, 104.3±5.0% and 111.0±3.0%, respectively. The stock solution was stable for at least 30 days at 4 °C. The stability experiment confirmed that PABA plasma samples were stable for over 60 days when stored at −20 °C and through three freeze-thaw cycles. The worked out plasma samples were stable for at least 24 h at 4 °C.
Fig. 4.
Typical chromatograms of PABA and IS (paracetamol) in human plasma. (A) Drug-free plasma sample; (B) plasma sample spiked with PABA (tR=7.7 min, C=1.00 μg/mL) and IS (paracetamol, tR=5.5 min, C=5.00 μg/mL); and (C) plasma obtained from a volunteer at 0.5 h after oral administration of 0.5 g inosiplex with PABA (tR=7.7 min, C=2.22 μg/mL) and IS (paracetamol, tR=5.5 min, C=5.00 μg/mL).
Table 2.
Precision and accuracy of the HPLC-UV method to determine PABA in human plasma (n=3 days, five replicates per day).
| Spiked concentration (μg/mL) | Intra-batch |
Inter-batch |
||||
|---|---|---|---|---|---|---|
| Mean (μg/mL) | RSD (%) (n=5) | Relative error (%) | Mean (μg/mL) | RSD (%) (n=15) | Relative error (%) | |
| 0.100 | 0.1117 | 3.0 | 11.7 | 0.1107 | 5.7 | 10.2 |
| 2.00 | 2.028 | 1.3 | 1.4 | 2.016 | 2.0 | 0.4 |
| 20.0 | 18.74 | 1.4 | 6.3 | 18.77 | 1.2 | −6.4 |
3.3. Pharmacokinetic application
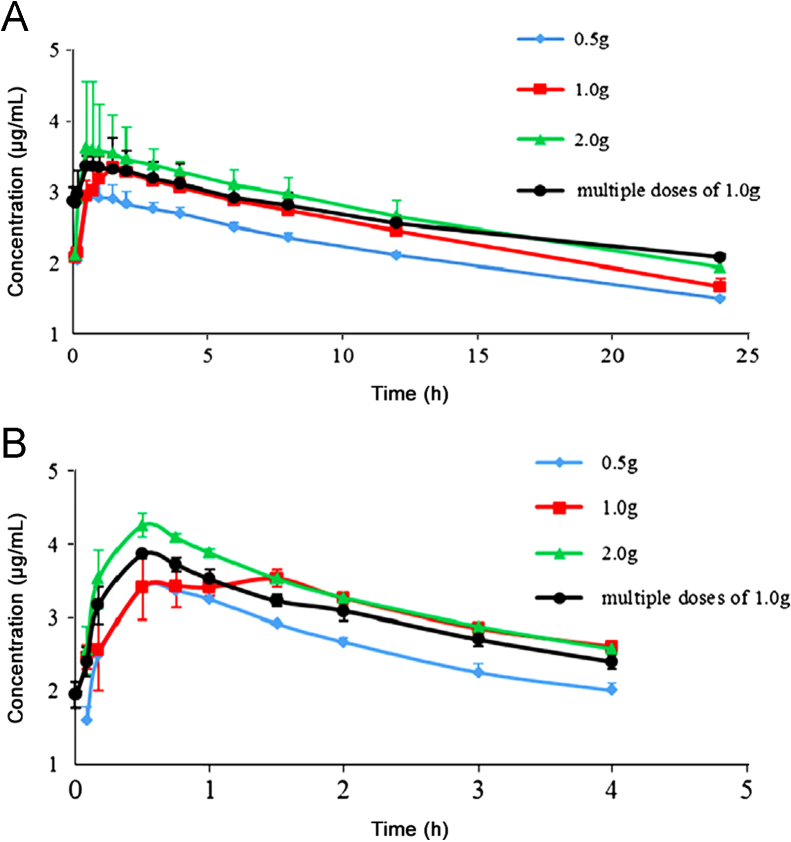
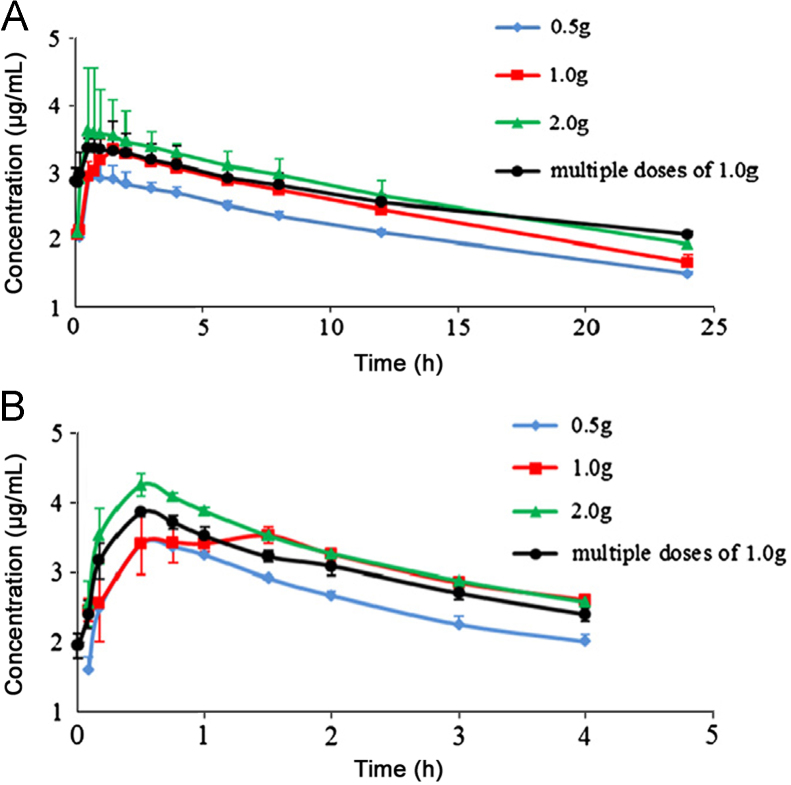
All 30 subjects completed the pharmacokinetic study. The mean plasma concentration–time profiles of DIP and PABA after a single oral dose of 0.5, 1.0 and 2.0 g inosiplex tablets, and at steady-state after multiple oral doses of 1.0 g inosiplex tablets are shown in Fig. 5. The pharmacokinetics parameters are shown in Table 3. Linear pharmacokinetics was found for both DIP and PABA based on these results. The plasma concentrations of DIP and PABA reached steady-state at days 4–7 with the average minimum concentration of DIP as 0.77±0.18 μg/mL and PABA as 0.09±0.02 μg/mL before the morning dose. The food effect study demonstrated that the food intake had no remarkable effects on the absorption of DIP, but led to significant decrease in the bioavailability of PABA with the reduction of the AUC of about 13%. This might be attributed to the reason that the food intake changes the pH values in gastric fluid, affects gastric emptying, and subsequently inhibits the absorption of the acidic compound, PABA. No significant difference was found between the pharmacokinetic parameters after single oral dose of 1.0 g inosiplex tablets and those at the steady-state. Therefore, there was no obvious accumulation when inosiplex tablets were orally given t.i.d., repeatedly. The results of ANOVA for assessment of subjects indicated that no significant differences in Cmax, AUC0−τ, and AUC0−∞ were found between the male and female groups. The obvious tmax differences between 1 g group and 0.5 g, 2 g groups may be due to the fact that the subjects used for the 1 g single and steady-state studies were different from the subjects that had taken part in the 0.5 g and 2.0 g single dose studies.
Fig. 5.
The mean plasma concentration–time profiles of (A) DIP and (B) PABA in healthy volunteers (n=10) after a single oral administration of 0.5, 1.0, and 2.0 g and multiple oral doses of 1.0 g inosiplex tablets.
Table 3.
The main pharmacokinetic parameters for DIP and PABA after single oral doses of 0.5, 1.0, and 2.0 g and multiple doses of 1.0 g (t.i.d) inosiplex tablets to healthy volunteers (mean±SD, n=10).
| Compound | Dose (g) | Pharmacokinetic parameters |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax (mg/L) | Tmax (h) | AUC0–24 (mg h/L) | AUC0−∞ (mg h/L) | AUCss (mg h/L) | CL/F (L/h) | Vz/F (L) | t1/2z (h) | ||
| DIP | 0.5 | 1.04±0.18 | 0.98±0.78 | 5.45±0.92 | 5.76±0.82 | – | 24.52±3.24 | 160.3±27.85 | 4.569±0.743 |
| 1.0 | 2.24±0.18 | 1.55±0.16 | 12.93±2.11 | 13.28±2.31 | – | 21.57±4.27 | 135.27±25.61 | 4.417±0.812 | |
| 2.0 | 4.48±0.79 | 0.63±0.21 | 23.22±3.58 | 23.72±3.77 | – | 23.92±3.58 | 147.98±18.61 | 4.325±0.449 | |
| Steady state | 2.49±0.11 | 1.00±0.46 | 16.06±2.80 | 17.01±3.33 | 10.92±1.49 | 16.93±3.52 | 137.43±32.02 | 5.745±1.244 | |
| PABA | 0.5 | 2.63±0.22 | 0.58±0.12 | 3.09±0.21 | 3.17±0.23 | – | 76.30±5.31 | 77.46±8.85 | 0.707±0.097 |
| 1.0 | 5.18±1.80 | 1.03±0.51 | 7.69±1.13 | 7.83±1.16 | – | 62.82±9.30 | 85.36±10.20 | 0.952±0.126 | |
| 2.0 | 19.91±4.68 | 0.53±0.08 | 17.49±2.76 | 17.60±2.77 | – | 56.08±9.33 | 79.33±21.14 | 0.982±0.222 | |
| Steady state | 7.37±0.94 | 1.00±0.46 | 7.90±1.16 | 8.03±1.14 | 7.90±1.16 | 61.08±8.33 | 92.40±21.05 | 1.043±0.171 | |
The methods were also utilized for the urinary excretion studies of DIP and PABA. The DIP accumulate urinary excretion rates (0–24 h) for 0.5, 1.0 and 2.0 g dose groups were 32.4±21.4%, 38.4±10.7%, and 29.1±15.9%, respectively, while for PABA were 12.4±7.4%, 29.9±14.3%, and 15.8±11.2%, respectively.
4. Conclusion
The LC/MS/MS and HPLC-UV methods have been set up for the pharmacokinetic study of inosiplex tablets in healthy Chinese volunteers. The established LC/MS/MS and HPLC-UV methods were sensitive and selective for plasma DIP and PABA determination, respectively, and suitable for the pharmacokinetic study of inosiplex tablets. The pharmacokinetic characteristics found provide a useful reference for the rational and safe clinical therapeutic application of inosiplex tablets from both the dosage regimens and the therapeutic drug monitoring point of views.
Acknowledgments
This work was financially supported by the Fundamental Research Funds for the Central Universities (Grant no. JKP2011008), the Qing Lan Project, and the Program for New Century Excellent Talents in University (Grant no. NCET-10-0816).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Ya Ding, Email: ayanju@163.com.
Tai-Jun Hang, Email: hangtj@cpu.edu.cn.
References
- 1.Morin A., Ballet J.J. A recent overview on in vitro and in vivo immunological activities of methisoprinol. Allergol. Immunopath. 1982;10:109–114. [PubMed] [Google Scholar]
- 2.Gascon G.G. Randomized treatment study of inosiplex versus combined inosiplex and intraventricular interferon-alpha in subacute sclerosing panencephalitis (SSPE): international multicenter study. J. Child Neurol. 2003;18(12):819–827. doi: 10.1177/088307380301801201. [DOI] [PubMed] [Google Scholar]
- 3.Tay S.K. Efficacy of inosine pranobex oral therapy in subclinical human papillomavirus infection of the vulva: a randomized double-blinded placebo controlled study. Int. J. STD AIDS. 1996;7:276–280. doi: 10.1258/0956462961917960. [DOI] [PubMed] [Google Scholar]
- 4.Georgala S., Katoulis A., Befon A. Oral inosiplex in the treatment of cervical condylomata acuminata: a randomized placebo-controlled trial. BJOG. 2006;113:1088–1091. doi: 10.1111/j.1471-0528.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 5.Simone C.De, Famularo G., Tzantzoglou S. Inosine pranobex in the treatment of HIV infection: a review. Int. J. Immunopharmacol. 1991;13(Suppl. 1):S19–S27. doi: 10.1016/0192-0561(91)90120-v. [DOI] [PubMed] [Google Scholar]
- 6.Brzeski M., Madhok R., Hunter J.A. Randomised, double blind, placebo controlled trial of inosine pranobex in rheumatoid arthritis. Ann. Rheum. Dis. 1990;49:293–295. doi: 10.1136/ard.49.5.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgala S., Katoulis A.C., Befon A. Inosiplex for treatment of alopecia areata: a randomized placebo-controlled study. Acta Derm. Venereol. 2006;86:422–424. doi: 10.2340/00015555-0138. [DOI] [PubMed] [Google Scholar]
- 8.Streeter D.G., Pfadenhauer E.H. Inosiplex: metabolism and excretion of the dimethylaminoisopropanol and p-acetamidobenzoic acid components in rhesus monkeys. Drug Metab. Dispos. 1984;12:199–203. [PubMed] [Google Scholar]
- 9.Farthing D., Sica D., Gehr T. An HPLC method for determination of inosine and hypoxanthine in human plasma from healthy volunteers and patients presenting with potential acute cardiac ischemia. J. Chromatogr. B. 2007;854:158–164. doi: 10.1016/j.jchromb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Song J.F., Yu P.F. A novel electrochemical sensing system for inosine and its application for inosine determination in pharmaceuticals and human serum. Electrochem. Commun. 2006;8:1521–1526. [Google Scholar]
- 11.Chan K., Miners J.O., Birkett D.J. Direct and simultaneous high-performance liquid chromatographic assay for the determination of p-aminobenzoic acid and its conjugates in human urine. J. Chromatogr. 1998;426:103–109. doi: 10.1016/s0378-4347(00)81931-3. [DOI] [PubMed] [Google Scholar]
- 12.Wang L.H., Huang W.S., Tai H.M. Simultaneous determination of p-aminobenzoic acid and its metabolites in the urine of volunteers, treated with p-aminobenzoic acid sunscreen formulation. J. Pharm. Biomed. Anal. 2007;12:1430–1436. doi: 10.1016/j.jpba.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Li X.J., Sun Y.T., Yin L. Quantitation of bivalirudin, a novel anticoagulant peptide, in human plasma by LC–MS/MS: method development, validation and application to pharmacokinetics. J. Pharm. Anal. 2013;3(1):1–8. doi: 10.1016/j.jpha.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polagani S.R., Pilli N.R., Gajula R. Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC–MS/MS and its application to a human pharmacokinetic study. J. Pharm. Anal. 2013;3(1):9–19. doi: 10.1016/j.jpha.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah V.P., Midha K.K., Findlay J.W. Bioanalytical method validation—a revisit with a decade of progress. Pharm. Res. 2000;17:1551–1557. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 16.Mirzaei M., Khayat M., Saeidi A. Determination of para-aminobenzoic acid (PABA) in B-complex tablets using the multivariate curve resolution- alternating least squares (MCR–ALS) method. Sci. Iran. C. 2012;19:561–564. [Google Scholar]
- 17.O'kruk R.J., Adams M.A., Philp R.B. Rapid and sensitive determination of acetylsalicylic acid and its metabolites using reversed-phase high-performance liquid chromatography. J. Chromatogr. B. 1984;310:343–352. doi: 10.1016/0378-4347(84)80099-7. [DOI] [PubMed] [Google Scholar]
- 18.Que X.T., Ding Y., Hang T.J. Pharmacokinetics of inosiplex tablets in healthy Chinese volunteers. Chin. J. New. Drugs Clin. Rem. 2010;10:738–742. [Google Scholar]