Abstract
A simple, sensitive and specific liquid chromatography–tandem mass spectrometry (LC–MS/MS) method was developed for the quantification of milnacipran (MC) in rat plasma by using the liquid–liquid extraction method. Milnacipran-d10 (MCD10) was used as an internal standard (IS). Chromatographic separation was achieved on Zorbax SB-CN (4.6 mm×75 mm, 3.5 µm) column with an isocratic mobile phase composed of 10 mM ammonium acetate (pH 4.0) and methanol in the ratio of 25:75(v/v), at a flow-rate of 0.7 mL/min. MC and MCD10 were detected with proton adducts at m/z 247.2→230.3 and m/z 257.2→240.4 in multiple reaction monitoring (MRM) positive mode respectively. The method was validated over a linear concentration range of 1.00–400.00 ng/mL with a correlation coefficient (r2)≥0.9850. This method demonstrated intra- and inter-day precision within 5.40–10.85% and 4.40–8.29% and accuracy within 97.00–104.20% and 101.64–106.23%. MC was found to be stable throughout three freeze–thaw cycles, bench top and postoperative stability studies. This method was successfully applied to a pharmacokinetic study of rats through i.v. administration.
Keywords: Milnacipran, Pharmacokinetics, Rat plasma, LC–MS/MS
1. Introduction
Milnacipran (1-phenyl-1-diethylamino-carbonyl-2-amino-methylcyclopropane hydrochloride) is a new antidepressant which is characterized by serotonin and noradrenalin reuptake inhibitor (SNRI) and by virtual absence of postsynaptic effects. Chemically, it is (1R, 2S)-rel-2-(aminomethyl)-N, N-diethyl-1-phenylcyclopropane carboxamide hydrochloride. It has an empirical formula of C15H22N2O·HCl and a molecular weight of 282.8 g/mol (Fig. 1). The indications for these medicines include treatment of depression, Generalised Anxiety Disorder (GAD), Social Anxiety Disorder (SAD), panic disorder and diabetic peripheral neuropathy (duloxetine only). The exact mechanism of the central pain inhibitory action of MC and its ability to improve the symptoms of fibromyalgia in humans are unknown. Preclinical studies have shown that MC is a potent inhibitor of neuronal norepinephrine and serotonin reuptake; MC inhibits norepinephrine uptake with approximately 3-fold higher potency in vitro than serotonin without directly affecting the uptake of dopamine or other neurotransmitters. MC is well absorbed after oral dosing and has a bioavailability of 85%. Meals do not have an influence on the rapidity and extent of absorption. Peak plasma concentrations are reached 2 h after oral dosing. The Cmax of 50 mg oral dose is about 126.18±27.90 ng/mL. The elimination half-life of 8 h is not increased by liver impairment and old age, but by significant renal disease. MC is conjugated to the inactive glucuronide and excreted into the urine as unchanged drug and conjugate. Only traces of active metabolites are found. Enzymes of the CYP class do not play a role in the metabolism of MC so that the risk of interactions with drugs metabolized by CYP enzymes is minimal [1], [2], [3], [4].
Fig. 1.
Chemical structures of milnacipran and milnacipran-d10.
Literature survey reveals that, there are few methods reported for quantification of MC in pharmaceutical [5], [6], [7], [8] and biological fluids [9], [10], [11]. These are quantified by several techniques including capillary electrophoresis [5], micellar electro kinetic capillary [6], liquid chromatography (LC) [7], [8], [9], [10] and LC–MS/MS [11]. However, LC–MS/MS has played an important role for the quantitative estimation of drugs in various biological matrices, including plasma, serum, urine, and ocular fluids, due to its high sensitivity, selectivity and reproducibility. Most of the published methods in the literature are liquid–liquid [9], [10] and solid-phase extraction (SPE) [11] of MC in human plasma. Among all Shinozuka et al. [11] developed the most sensitive method in human plasma by using LC–MS/MS. However, it is required to develop the simplest, sensitive method with proper internal standard usage[12], [13], [14].
The aim of the proposed method is to develop the simplest, sensitive, high recovery and selective extraction method (liquid–liquid extraction). As per standard regulatory guidelines, it could be successfully employed in the analysis of rat plasma samples following i.v. administration in healthy rats.
2. Materials and methods
2.1. Chemicals and reagents
MC was obtained from MSN Pharmachem Pvt. Ltd. (MR0021011) (99.80% purity), Hyderabad, India and MCD10 was obtained from Clearsynth (AC0202158) (100.00% purity). Ammonium acetate and acetic acid were purchased from Merck, Mumbai, India. Methanol and acetonitrile were purchased from J.T BAKER, USA. Rats and rat plasma were obtained from Bioneeds, Bangalore.
2.2. LC–MS/MS instrument and conditions
The 1200 Series HPLC system (Agilent Technologies, Germany) was used. Mass spectrometric detection was performed on an API 4200 triple quadrupole instrument (ABI-SCIEX, Toronto, Canada) using MRM. Data processing was performed on Analyst 1.5.1 software package (SCIEX). Detection was performed by Turbo ionspray (API) positive mode with Unit Resolution. For MC, the MH+ (m/z 247.2) was monitored as the precursor ion and a fragment at m/z 230.3 was chosen as the product ion. For internal standard, the MH+ (m/z 257.2) was monitored as the precursor ion and a fragment at m/z 240.4 was monitored as the product ion. Mass parameters were optimized as source temperature 550 °C, heater gas 40 (nitrogen) psi, nebulizer gas 50 (nitrogen) psi, curtain gas 30 (nitrogen) psi, CAD gas 2 (nitrogen) psi, ion spray (IS) voltage 5500 V, source flow rate 600 µL/min without split, entrance potential 10 V, declustering potential 45 V for analyte and 45 V for IS, collision energy 20 V for analyte and 20 V for IS, collision cell exit potential 10 V for both analyte and IS.
2.3. Chromatographic conditions
ZORBAX SB-CN column (4.6 mm×75 mm, 3.5 µm) was selected as the analytical column. The mobile phase composition was 10 mM ammonium acetate (pH 4.0) and methanol in the ratio of 25:75 (v/v). The flow rate of the mobile phase was set at 0.7 mL/min. The column temperature was set at 40 °C. MCD10 was found to be an appropriate internal standard in terms of chromatography and extractability. The retention time of MC and MCD10 was found to be 1.7±0.2 min approximately at overall 3 min run time.
2.4. Preparation of standards and quality control (QC) samples
Standard stock solutions of MC (100.00 µg/mL), and the IS (100.00 µg/mL) master stock solutions were prepared in methanol. The IS spiking solutions (400.00 ng/mL) were prepared in 50% methanol from IS master stock. Master stock solutions and IS spiking solutions were stored in refrigerator at 2–8 °C until analysis. Master stock solutions were added to drug-free rat plasma to obtain MC concentration levels of 1.00, 2.00, 5.00, 20.00, 40.00, 80.00, 160.00, 240.00, 320.00 and 400.00 ng/mL for analytical standards and 1.00, 3.00, 200.00 and 300.00 ng/mL for quality control standards. These standards were stored in the freezer at −30 °C until analysis. The aqueous standards were prepared in reconstitution solution [75% methanol in 10 mM ammonium acetate (pH 4.0)] for validation exercises and stored in the fridge at 2–8 °C until analysis.
2.5. Sample preparation
The liquid–liquid extraction method was used to isolate MC, and its respective IS from rat plasma. For this, 50 µL of IS (400.00 ng/mL) and 100 µL of plasma sample (respective concentration) were added into labeled polypropylene tubes and vortexed briefly after that 2.5 mL of methyl t-butyl ether was added and vortexed for approximately 10 min followed by centrifuged at 4000 rpm for approximately 5 min at 20 °C. Supernatant from each sample was transferred to labeled ria vial tube and evaporated at 40 °C until dryness. These samples were reconstituted with 500 µL of reconstitution solution [75% methanol in 10 mM ammonium acetate (pH 4.0)] and vortexed briefly, and then transferred the sample into autosampler vials for injection.
2.6. Method validation
2.6.1. Selectivity and specificity
The selectivity of the method was determined by six different rat blank plasma samples, which were pretreated and analyzed to test the potential interferences of endogenous compounds co-eluting with analyte and IS. Chromatographic peaks of analyte and IS were identified based on their retention times and MRM responses. The peak area of MC at the respective retention time in blank samples should not be more than 20% of the mean peak area of LOQ of MC. Similarly, the peak area of MCD10 at the respective retention time in blank samples should not be more than 5% of the mean peak area of LOQ of MCD10.
2.6.2. Matrix effect
To predict the variability of matrix effect in samples from individual subjects, matrix effect was quantified by determining the matrix factor, which was calculated as follows:
Six lots of blank biological matrices were spiked each in triplicates with the neat standard at the low quality control (LQC), high quality control (HQC) levels, and compared with neat standards of the same concentration in alternate injections. The overall precision of the matrix factor is expressed as coefficient of variation (CV %) and it should be ≤15% (CV %).
2.6.3. Linearity
The analytical curves were constructed using concentratons ranging from 1.00 to 400.00 ng/mL of MC in rat plasma. Calibration curves were obtained by weighted 1/conc.2 linear regression. The ratio of MC peak area to MCD10 peak area was plotted against the ratio of MC concentration in ng/mL. Calibration curve standard samples and quality control samples were prepared in replicates (n=6) for analysis. The correlation coefficient was obtained >0.9850 by using a simple linear regression model in the whole range of tested concentrations.
2.6.4. Precision and accuracy
Precision and accuracy for the back calculated concentrations of the calibration points, should be within ≤15% and ±15% of their nominal values. However, for lower limit of quantification (LLOQ) the precision and accuracy should be within ≤20% and ±20%.
2.6.5. Stability
LQC and HQC samples (n=6) were retrieved from the deep freezer after three freeze–thaw cycles according to the clinical protocols. Samples were frozen at −30 °C in three cycles of 24, 36 and 48 h. In addition, the long-term stability of MC in quality control samples was also evaluated by analysis after 55 days of storage at −30 °C. Autosampler stability was studied following 71.5 h storage period in the autosampler tray with control concentrations. Bench top stability was studied for 24.5 h period with control concentrations. Stability samples were processed and extracted along with the freshly spiked calibration curve standards. The precision and accuracy for the stability samples must be within ≤15% and ±15% respectively of their nominal concentrations.
2.6.6. Recovery
The extraction recovery of analyte and IS from rat plasma was determined by analyzing quality control samples. Recovery at three concentrations (3.00, 200.00, and 300.00 ng/mL) was determined by comparing peak areas obtained from the plasma sample with those from the standard solution spiked with the blank plasma residue. A recovery of more than 50% was considered adequate to obtain required sensitivity.
2.6.7. Limit of quantification (LOQ) and limit of detection (LOD)
The response (peak area) was determined in blank plasma samples (six replicates from different plasma), and spiked LOQ sample prepared from the same plasma was determined. The peak area of blank samples should not be more than 20% of the mean peak area of LOQ of MC and 5% of the mean peak area of MCD10. The precision and mean accuracy of the back calculated LOQ replicate concentrations must be ≤20% and ±20%, respectively.
The LOD is a parameter that provides the lowest concentration in a sample that can be detected from background noise but not quantitated. LOD was determined using the signal-to-noise ratio (S/N) of 3:1 by comparing test results from samples with known concentrations of analyte with those from the blank samples.
2.7. Application to pharmacokinetic study of MC in rat plasma
The validated method has been successfully used to quantify MC concentrations in rat plasma. The study was conducted according to current GCP guidelines and approved by an authorized animal ethics committee. Male Sprague-Dawley rats were obtained from Bioneeds, Bangalore. After i.v. administration of drug (0.9mg/200g body weight of rats) through left femoral vein, 0.2 mL of blood samples were collected via the right femoral vein at specific time intervals for 39 h. There was a total of 14 blood collection time points including the predose sample (0.3, 0.6, 1, 1.5, 2, 4, 7, 11, 15, 21, 27, 33, 39 h). The blood samples were collected in separate vacutainers containing K2EDTA as an anticoagulant. The plasma from these samples was separated by centrifugation at 3000 rpm within the temperature range of 2–8 °C. The plasma samples thus obtained were stored at –30 °C until analysis. After analysis the pharmacokinetic parameters were computed using WinNonlin® software version 5.2.
3. Results and discussion
3.1. Method development
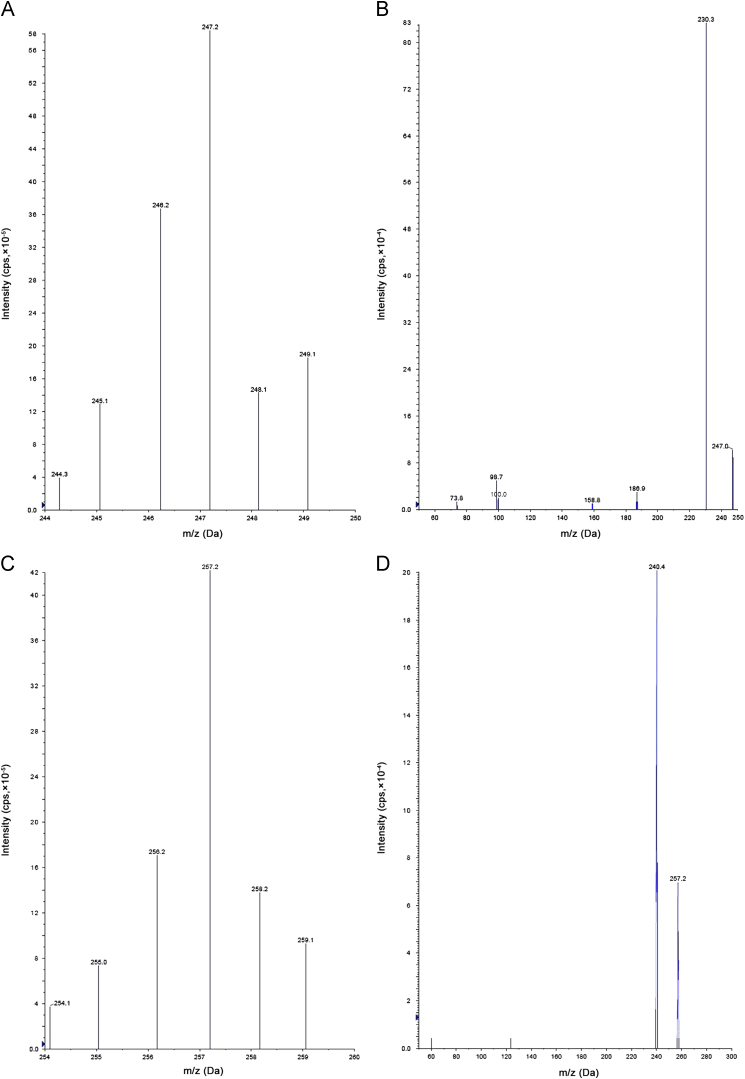
LC–MS/MS has been used as one of the most powerful analytical tools in clinical pharmacokinetics for its selectivity, sensitivity and reproducibility. The goal of this work was to develop and validate a simple, rapid and sensitive assay method for the quantitative determination of MC from plasma samples. A simple extraction technique was utilized for the extraction of MC and MCD10 from the plasma samples. Chromatographic conditions, especially the composition and nature of the mobile phase, were optimized through several trials to achieve better resolution and increase the signal of MC and MCD10. The MS optimization was performed by direct infusion of solutions of both MC and MCD10 into the ESI source of the mass spectrometer. Other parameters, such as the nebulizer and the desolvation gases, were optimized to obtain a better spray shape, resulting in better ionization and droplet drying to form. In our case, the protonated ionic MC and MCD10 (IS) molecules. A product ion spectrum for MC and MCD10 yielded high-abundance fragment ions of m/z 247.2/230.3 (Fig. 2A and B) and m/z 257.2/240.4 (Fig. 2C and D). After the MRM channels were tuned, the mobile phase was changed from an organic phase to a more aqueous phase to obtain a fast and selective LC method. A good separation and elution were achieved using 10 mM ammonium acetate (pH 4.0): methanol 25:75(v/v) as the mobile phase, at a flow rate of 0.7 mL/min and injection volume of 10 µL.
Fig. 2.
Mass spectra of (A) milnacipran parent ion, (B) milnacipran product ion, (C) milnacipran-d10 parent ion and (D) milnacipran-d10 product ion.
3.2. Method validation
The developed method was validated over a linear concentration range of 1.00–400.0 ng/mL. The validation parameters, including selectivity and specificity, matrix effect, linearity, precision and accuracy, stability (freeze–thaw, auto sampler, bench top, long term), recovery, LOQ and LOD, were evaluated under validation section as per standard guidelines [15], [16].
3.2.1. Selectivity and specificity
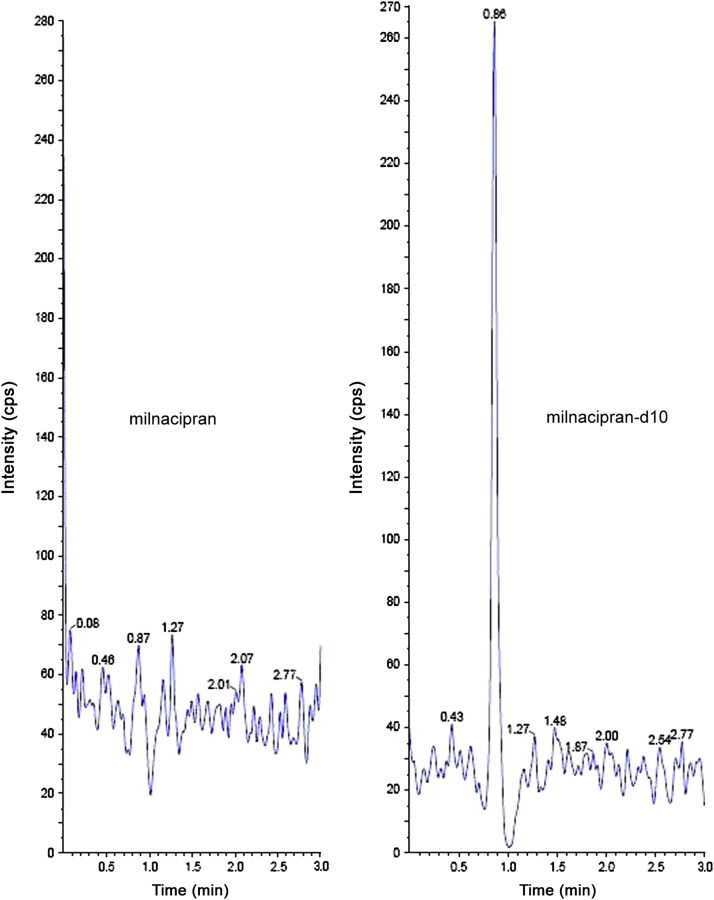
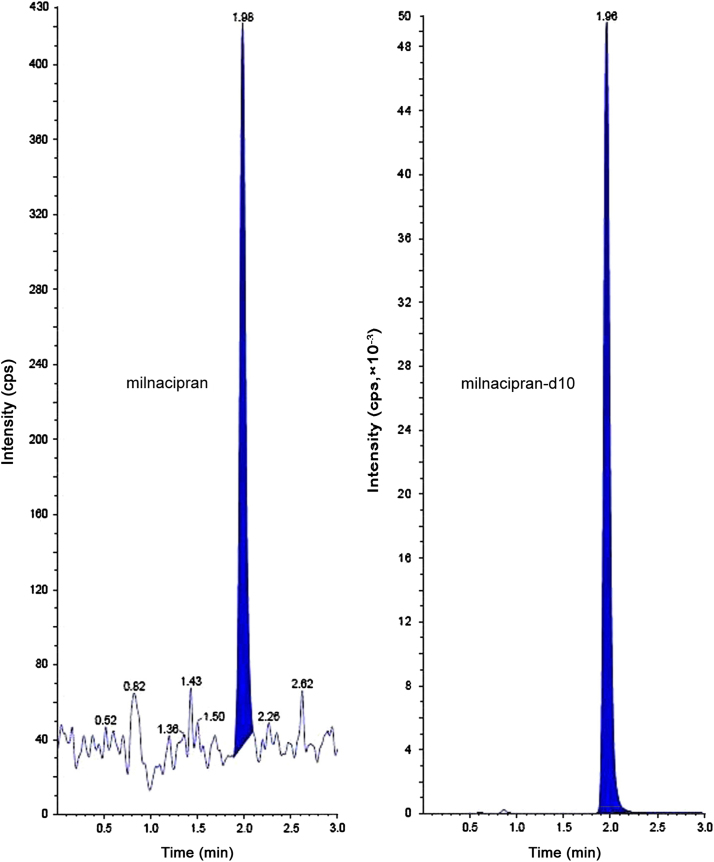
The analysis of MC and MCD10 using MRM function was highly selective with no interfering compounds (Fig. 3). Specificity was performed by using six different lots of rat plasma. Here only one blank plasma interference is shown (Fig. 3). Chromatograms obtained from plasma spiked with MC (1.00 ng/mL) and MCD10 (200.00 ng/mL) are shown in Fig. 4.
Fig. 3.
Chromatogram of blank rat plasma.
Fig. 4.
LLOQ chromatograms of milnacipran and milnacipran-d10.
3.2.2. Matrix effect
The overall precision of the matrix factor was determined to be 6.68 at the low concentration and 4.36 at the high concentration for MC.
3.2.3. Linearity
Calibration curves were plotted as the peak area ratio (MC/MCD10) versus (MC) concentration. Calibration was found to be linear over the concentration range of 1.00–400.00 ng/mL. The RSDs was less than 5% and the accuracy ranged from 93.52% to 104.77%. The determination coefficients (r2) were greater than 0.9850 for all curves (Table 1).
Table 1.
Calibration curves details of MC from one batch of validation.
| Spiked plasma concentration (ng/mL) | Mean concentration (ng/mL)±SD | RSDa (%) (n=5) | Accuracy (%) |
|---|---|---|---|
| 1.00 | 1.01±0.03 | 2.48 | 101.40 |
| 2.00 | 1.99±0.09 | 4.57 | 99.60 |
| 5.00 | 4.68±0.24 | 5.11 | 93.52 |
| 20.00 | 19.72±0.65 | 3.31 | 98.59 |
| 40.00 | 40.59±1.11 | 2.73 | 101.49 |
| 80.00 | 79.73±2.85 | 3.58 | 99.66 |
| 160.00 | 167.64±2.67 | 1.59 | 104.77 |
| 240.00 | 245.64±10.01 | 4.07 | 102.35 |
| 320.00 | 318.50±18.34 | 5.76 | 99.53 |
| 400.00 | 395.79±25.36 | 6.41 | 98.95 |
(Standard deviation/mean concentration measured)×100.
3.2.4. Precision and accuracy
Precision and accuracy for this method was controlled by calculating the intra- and inter-batch variations at four concentrations (1.00, 3.00, 200.00 and 300.00 ng/mL) of QC samples in six replicates. As shown in Table 2, the intra-day RSD was less than 10.85% and the accuracy ranged from 97.00% to 104.20%. Inter-day RSD was less than 8.29% and the accuracy ranged from 101.64% to 106.23%. These results indicate the adequate reliability and reproducibility of this method within the analytical curve range.
Table 2.
Precision and accuracy of MC from one batch of validation.
| Spiked plasma conc. (ng/mL) | Intra-day |
Inter-day |
||||
|---|---|---|---|---|---|---|
| Concentration measured (n=6) (ng/mL) (mean±SD) | RSDa (%) | Accuracy (%) | Concentration measured (n=30) (ng/mL) (mean±SD) | RSDa (%) | Accuracy (%) | |
| 1.00 | 1.03±0.11 | 10.85 | 103.17 | 1.06±0.09 | 8.29 | 106.23 |
| 3.00 | 2.91±0.20 | 6.72 | 97.00 | 3.06±0.19 | 6.20 | 102.01 |
| 200.00 | 208.40±11.25 | 5.40 | 104.20 | 203.48±8.95 | 4.40 | 101.74 |
| 300.00 | 311.65±19.23 | 6.17 | 103.88 | 304.93±13.77 | 4.52 | 101.64 |
(Standard deviation/mean concentration measured)×100.
3.2.5. Stability
Quantification of MC in plasma subjected to three freeze–thaw (−30 °C to room temperature) cycles showed the stability of the analyte. No significant degradation of MC was observed even after 71.5 h storage period in the autosampler tray, and the final concentrations of MC was between 102.67% and 103.49% of the theoretical values. In addition, the long-term stability of MC in QC samples after 55 days of storage at −30 °C was also evaluated. The concentrations ranged from 98.47% to 101.11% of the theoretical values. These results confirmed the stability of MC in rat plasma for at least 55 days at −30 °C (Table 3).
Table 3.
Stability of MC in plasma samples.
| Stability | Spiked plasma concentration (ng/mL) | Concentration measured (ng/mL) (mean±SD; n=6) | RSDa (%) (n=6) |
|---|---|---|---|
| Room temperature stability (24.5 h) | 3.00 | 2.85±0.26 | 9.14 |
| 300.00 | 298.07±13.36 | 4.48 | |
| Processed sample stability (71.5 h) | 3.00 | 3.08±0.09 | 2.84 |
| 300.00 | 310.46±8.09 | 2.60 | |
| Long term stability (55 days) | 3.00 | 3.03±0.09 | 2.95 |
| 300.00 | 295.41±3.38 | 1.15 | |
| Freeze–thaw stability (cycle 3, 48 h) | 3.00 | 2.86±0.20 | 7.10 |
| 300.00 | 297.11±7.46 | 2.51 |
(Standard deviation/mean concentration measured)×100.
3.2.6. Recovery
The recovery following the sample preparation using the liquid–liquid extraction method with methyl-t-butyl ether was calculated by comparing the peak area ratios of MC in plasma samples with the peak area ratios of solvent samples and estimated at control levels of MC. The recovery of MC was determined at three different concentrations 3.00, 200.00 and 300.00 ng/mL and found to be 103.45%, 104.00% and 112.48%, respectively. The overall average recovery of MC and MCD10 was found to be 106.64% and 102.62% respectively.
3.2.7. LOQ andLOD
The LOQ for this method was proven as the lowest concentration of the calibration curve which was proven as 1.00 ng/mL.
The LOD was determined using aqueous solutions. For MC 10 μL of a 5.0 pg/mL solution was injected to give an on-column mass of 0.05 pg.
3.3. Pharmacokinetics and statistical analysis
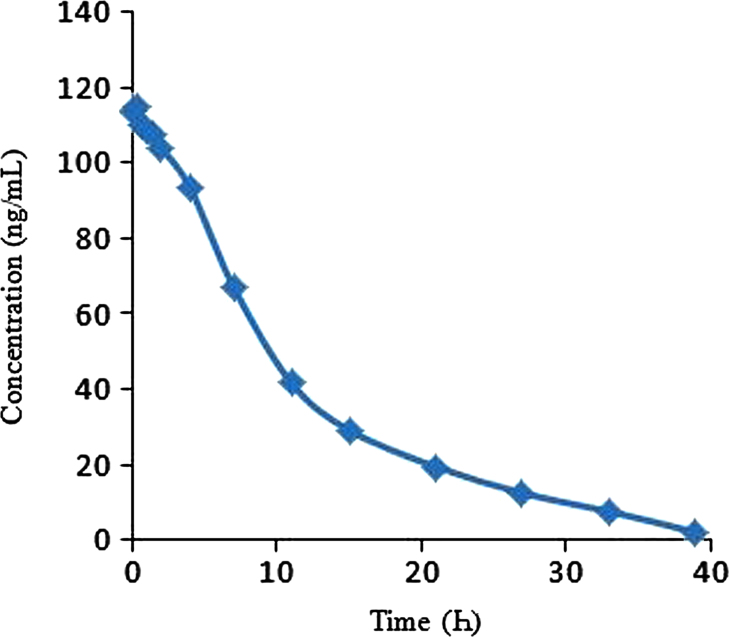
The validated method has been successfully applied to quantify MC concentrations in rat plasma. The pharmacokinetic parameters evaluated were Cmax (maximum observed drug concentration during the study), AUC0-39(area under the plasma concentration–time curve measured 39 h, using the trapezoidal rule), Tmax (time to observe maximum drug concentration), Kel (apparent first order terminal rate constant calculated from a semi-log plot of the plasma concentration versus time curve, using the method of least square regression) and T1/2 (terminal half-life as determined by quotient 0.693/Kel)[16], [17]. Pharmacokinetic details are shown in Table 4. The mean concentration versus time profile of MC in rat plasma is shown in Fig. 5.
Table 4.
Mean pharmacokinetic parameters of milnacipran in rat plasma after intravenous administration of 0.9 mg/200 g male rat.
| Pharmacokinetic parameter | Milnacipran values |
|---|---|
| AUC0–t (ng h/mL) | 1345 |
| Cmax (ng/mL) | 115 |
| AUC0−∞ (ng h/mL) | 1365 |
| Kel (h_1) | 0.10303 |
| Tmax (h) | 0.3 |
| T1/2 (h) | 6.7 |
AUC0–∞: area under the curve extrapolated to infinity.
AUC0–t: area under the curve up to the last sampling time.
Cmax: the maximum plasma concentration.
Tmax: the time to reach peak concentration.
Kel: the apparent elimination rate constant.
Fig. 5.
Mean plasma concentrations versus time graph of milnacipran after intravenous administration of 0.9 mg/200 g in male rats.
4. Conclusions
The developed method is rapid, sensitive, rugged and reproducible with high recovery. Each sample requires less than 3 min of analysis time. Drug and IS were extracted with the simplest liquid–liquid extraction method with less matrix effect. The developed method was successfully applied in the pharmacokinetic study to evaluate plasma concentration of MC in healthy rats.
Acknowledgments
Authors wish to thank the support received (for providing Literature survey) from IICT (Indian Institute of Chemical Technology) Hyderabad India, Manipal accunova, Manipal, India to carry out this research work.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Puozzo C., Pozet N., Deprez D. Pharmacokinetics of milnacipran in renal impairment. Eur. J. Drug Metab. Pharmacokinet. 1998;23(2):280–286. doi: 10.1007/BF03189352. [DOI] [PubMed] [Google Scholar]
- 2.Bantsiele G.B., Bentue-Ferrer D., Saïkali S. Behavioral effects of four antidepressants on an ischemic rat model of emotional disturbances. Behav. Brain Res. 2009;201(2):265–271. doi: 10.1016/j.bbr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi H., Yoshida K., Takahashi H. Milnacipran plasma levels and antidepressant response in Japanese major depressive patients. Hum. Psychopharmacol. 2003;18(4):255–259. doi: 10.1002/hup.484. [DOI] [PubMed] [Google Scholar]
- 4.Rop P.P., Sournac M.H., Burle J. Blood concentration of milnacipran in a case of a fatal automobile accident. J. Anal. Toxicol. 2002;26(2):123–126. doi: 10.1093/jat/26.2.123. [DOI] [PubMed] [Google Scholar]
- 5.Mandrioli R., Raggi M.A. Advances in the enantioseparation of second-generation antidepressant drugs by electrodriven methods. Electrophoresis. 2006;27(1):213–221. doi: 10.1002/elps.200500297. [DOI] [PubMed] [Google Scholar]
- 6.Labat L., Deveaux M., Dallet P. Separation of new antidepressants and their metabolites by micellar electrokinetic capillary chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;773(1):17–23. doi: 10.1016/s1570-0232(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 7.Lecoeur-Lorin M., Delepee R., Ribet J.P. Chiral analysis of milnacipran by a nonchiral HPLC—circular dichroism: improvement of the linearity of dichroic response by temperature control. J. Sep. Sci. 2008;31(16–17):3009–3014. doi: 10.1002/jssc.200800291. [DOI] [PubMed] [Google Scholar]
- 8.Patti A., Pedotti S., Sanfilippo C. Chiral HPLC analysis of milnacipran and its FMOC-derivative on cellulose-based stationary phases. Chirality. 2008;20(2):63–68. doi: 10.1002/chir.20496. [DOI] [PubMed] [Google Scholar]
- 9.Puozzo C., Filaquier C., Zorza G. Determination of milnacipran, a serotonin and noradrenaline reuptake inhibitor, in human plasma using liquid chromatography with spectrofluorimetric detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;806(2):221–228. doi: 10.1016/j.jchromb.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 10.Tournel G., Houdret N., Hédouin V. High-performance liquid chromatographic method to screen and quantitate seven selective serotonin reuptake inhibitors in human serum. J. Chromatogr. B Biomed. Sci. Appl. 2001;761(2):147–158. doi: 10.1016/s0378-4347(01)00305-x. [DOI] [PubMed] [Google Scholar]
- 11.Shinozuka T., Terada M., Tanaka E. Solid-phase extraction and analysis of 20 antidepressant drugs in human plasma by LC/MS with SSI method. Forensic. Sci. Int. 2006;162(1–3):108–112. doi: 10.1016/j.forsciint.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Konda R., Chandu B.R., Challa B.R. Bio-analytical method development and validation of Rasagiline by high performance liquid chromatography tandem mass spectrometry detection and its application to pharmacokinetic study. J. Pharm. Anal. 2012;2(5):342–349. doi: 10.1016/j.jpha.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponnuru V.S., Challa B.R., Nadendla R. Quantification of sibutramine and its two metabolites in human plasma by LC–ESI-MS/MS and its application in a bioequivalence study. J. Pharm. Anal. 2012;2(4):249–257. doi: 10.1016/j.jpha.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponnuru V.S., Challa B.R., Nadendla R. Quantification of desloratadine in human plasma by LC–ESI-MS/MS and application to a pharmacokinetic study. J. Pharm. Anal. 2012;2(3):180–187. doi: 10.1016/j.jpha.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidance for Industry: Bioanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), May 2001.
- 16.Guidance for Industry Food-effect Bio Availability and Fed Bio Equivalence Studies, U.S Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), December 2002.
- 17.Guidance for Industry Bio Availability and Fed Bio Equivalence Studies for Orally Administered Drug Products-General Considerations, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), March 2003.