Abstract
A simple and selective ultra performance liquid chromatography–electrospray ionization tandem mass spectrometry (UPLC–ESI-MS/MS) assay was developed for the determination of the human plasma protein binding of four bioactive flavonoids (such as orientin and vitexin) in Polygonum orientale. Protein precipitation was used for sample preparation. Equilibrium dialysis technique was applied to determine the plasma protein binding under physiological conditions. The separation was achieved through a Waters C18 column with a mobile phase composed of 0.1% formic acid in acetonitrile and 0.1% aqueous formic acid using step gradient elution at a flow rate of 0.35 mL/min. A Waters ACQUITY™ TQD system was operated under the multiple reaction monitoring (MRM) mode of positive electrospray ionization. All of the recovery, precision, accuracy and stability of the method met the requirements. Good correlations (r>0.99) of the four compounds were found, which suggested that these compounds can be simultaneously determined with acceptable accuracy. Results showed that the plasma protein bindings of the four bioactive flavonoids were in the range of 74–89% over the six concentrations studied. The binding parameters containing protein binding affinity, protein binding dissociation constant, and protein binding site were studied. The maximum ability to bind with protein was also determined in the assay in order to understand the drug-protein binding of each compound better.
Keywords: Polygonum orientale, Plasma protein binding, Bioactive compounds, Equilibrium dialysis, UPLC–ESI-MS/MS
1. Introduction
Plasma-protein binding strongly affects the pharmacokinetic behavior of drugs with consequences in their overall pharmacological action [1]. The binding degree of a drug towards plasma proteins plays an important role in its distribution, elimination, and pharmacological effect.
Polygonum orientale, one of the well known Chinese medicines, has been widely used for the remedy of rheumatic arthritis, coronary disease, and hernia in Miao's medicine. Modern pharmacological studies showed that the aqueous extract of P. orientale possessed anti-myocardial ischemia and antioxidation activity [2], [3]. Chemical investigations have shown that among the flavonoids, lignans and saponins [4], [5] present as the major constituents in this herb, flavonoids are believed to be the active components in P. orientale [6]. Orientin, vitexin, cynaroside, and quercitrin (Fig. 1) are the main active constituents of P. orientale and can be considered as the marker compounds for the quality evaluation or standardization of P. orientale [7].
Fig. 1.
Chemical structures of orientin, vitexin, cynaroside, quercitrin and puerarin (I.S.).
Since the non-specific binding of a drug to plasma proteins modulates the availability of the drug to its intended target and the free fractions of a drug exert the main therapeutic effects occurring throughout the body, plasma protein binding is crucial for evaluating the extent and duration of the therapeutic action of a drug. Plasma protein binding data are therefore very useful in the design of optimal dose regimens and estimation of safety margins. As orientin, vitexin, cynaroside and quercitrin are the major active components of P. orientale and its several compound preparations have been under clinical evaluation [8], it is essential to develop a method to determine their plasma protein binding. Currently, many investigations [9], [10] have been conducted on the quantitative analysis of orientin, vitexin, cynaroside and quercitrin in the plant material and pharmaceutical preparations or biological fluids using High Performance Liquid Chromatography–Ultra Violet (HPLC–UV) and/or HPLC–MS/MS. However, as we know, there are still no reports about the interaction of these flavonoids and human proteins. In this investigation, a simple selective, and reliable UPLC–ESI-MS/MS assay that significantly reduced the matrix effects had been developed for the determination of the human plasma protein binding of four bioactive flavonoids (i.e., orientin, vitexin, cynaroside and quercitrin) in P. orientale.
2. Experimental
2.1. Materials and reagents
The reference standards of orientin, vitexin, cynaroside and quercitrin were isolated from P. orientale in our laboratory and their structures were confirmed by comparison of their MS, nuclear magnetic resonance (NMR) and specific rotation data with those reported in the literature. Their purities were all over 98% as determined by HPLC. The internal standard (I.S.) puerarin (≥ 99% purity) was purchased from National Institute for the Control of Pharmaceuticals and Biological Products (Beijing, China). Acetonitrile was of HPLC-grade from Merck Company (Darmstadt, Germany). HPLC-grade methanol was purchased from Tianjin Kermel Chemical Reagents Development Centre (Tianjin, China). All other reagents were of analytical grade.
Semi-permeable membranes with a molecular cut-off of 10 kDa were obtained from Solarbio Biological Product Company (Beijing, China). The membranes were first washed with distilled water, and then soaked in phosphate buffer (pH=7.4) before being placed into the plasma for analysis. The human blood was offered by the blood centre of Guizhou Province (licensing agreement: 07001).
2.2. Chromatography
LC analyses were performed on a Waters ACQUITY™ UPLC with the power of UPLC (Waters, Milford, MA, USA). Chromatographic separation was achieved on a Waters BEH C18 column (2.1 mm×50 mm, 1.7 µm, Waters, Milford, MA, USA) and a C18 -guard column Waters Van Guard BEH (2.1 mm×5 mm, 1.7 µm, Waters, Milford, MA, USA) with a step gradient of 0.1% formic acid in acetonitrile (A) and 0.1% aqueous formic acid (B) [0–0.5 min, A (10%); 0.5–2 min, A (10–30%); 2–2.5 min, A (90%); 2.5–3.5 min, A (10%)] at a flow rate of 0.35 mL/min. The column was maintained at 45 °C, the auto-sampler was at 15 °C and the injection volume was 1 µL.
2.3. Mass spectrometry
All the mass experiments were carried out using a Waters ACQUITY TQD (triple quadrupole mass spectrometer) equipped with a Z-spray ESI source. The cone voltage was set at 3 kV. Nitrogen was used as nebulizing gas with a source temperature of 120 °C. Desolvation gas (nitrogen) was heated to 350 °C and delivered at a flow rate of 650 L/h. Argon was used as collision gas at a flow rate of 0.16 mL/min. All the data were collected by the MassLynx™ V4.1 workstation (Micromass, Manchester, UK). Quantification was performed using positive MRM mode with m/z 449.2→329.2 for orientin, m/z 433.2→313.0 for vitexin, m/z 449.2→287.1 for cynaroside, m/z 449.2→303.4 for quercitrin, and m/z 417.0→267.0 for puerarin. The cone voltages for orientin, vitexin, cynaroside, quercitrin and puerarin were 35, 35, 35, 35, and 30 V, respectively. The optimized collision energies of 30, 30, 25, 30, and 15 eV were used for these components.
2.4. Preparation of standard solutions and quality control samples
Stock solutions of standards were prepared by dissolving orientin, vitexin, cynaroside and quercitrin in methanol to yield their concentrations of 1.006, 0.996, 0.982, 1.010 µg/mL, respectively. Working standard solutions were freshly prepared by mixing and diluting the standard solution with methanol to give six concentrations in the plasma and phosphate buffer. The concentration of puerarin (I.S., 9 µg/mL) was made through the diluting in methanol for use. All the solutions were stored at −20 °C and warmed to the room temperature before use.
To prepare the standard calibration samples, the mixed standard solutions for six concentrations (50 µL) were spiked into the blank plasmas (200 µL) or phosphate buffer (1 mL). The samples were then treated as the following sample preparation procedure. The quality control (QC) samples, which were used in the validation, were prepared in the same way as the standard calibration samples.
2.5. Sample preparation
2.5.1. Preparation of the extract of P. orientale
The dried P. orientale was extracted three times by refluxing with boiling water (1:10, w/v) for 1 h, and the extraction solutions were combined to be filtered, concentrated under reduced pressure and then precipitated with ethanol. The ethanol was removed under reduced pressure with discarding of precipitate. The concentration of 1 g/mL of extract was produced by dissolving residue in water then extracted with n-butanol (2:1, v/v) for four times. The n-butanol was removed under reduced pressure, and the residue was dissolved with 80% ethanol, and then loaded on the polyamide column, elution with 80% ethanol will be proceeded. Therefore, the extract of P. orientale was attained under reduced pressure to dryness.
2.5.2. The plasma samples (fluid within the dialysis bags)
To 200 µL of plasma, 50 µL of methanol, 20 µL of the IS solution (9 µg/mL), and 50 µL of formic acid (3 M) were added. The mixture was vortex-mixed for 1 min. Addition of 800 µL of methanol to the mixture followed by vortex-mixing (1 min) and sonication (5 min) gave a suspension, centrifugation (36,670×g, 10 min, 4 °C) of which allowed the precipitation of the proteins. The upper organic phase was transferred into clean tubes. The process of evaporation of all the upper samples under a gentle stream of nitrogen at 48 °C gave rise to a series of residues, each of which was redissolved in 500 µL of methanol. The mixture was then vortex-mixed (1 min), sonicated (5 min), and centrifuged (36,670g, 10 min, 4 °C). 1 µL of the supernatant was injected into the UPLC–MS/MS system for analysis.
2.5.3. The phosphate buffer samples (fluid outside the dialysis bags)
Unlike the plasma samples, the phosphate buffer samples do not require the step of precipitation the proteins. The method was specially established to the samples.
To 1 mL of phosphate buffer, 50 µL of methanol, 20 µL of the I.S. solution (9 µg/mL) and 50 µL of formic acid (3 M) were added and the mixture was vortex-mixed for 1 min. All other sample processing (except for precipitation of the proteins) were the same as those in Section 2.5.1.
2.6. Method validation
Because of the matrix effects in the detection with LC–ESI-MS/MS, two different media (i.e., human plasma and phosphate buffer) involved in this report had been distributed with the differently quantitative methods which will be verified using different method validations [11], [12]. For the large distance of concentrations between the fluid inside and outside the dialysis bags, the errors would rose when the analytes have been quantitated with the same calibration curves; while when the mode of electrospray ionization was used, the different media may affect the MS signal [13]. With the separated methods that were described in this paper, these effects could be avoided.
2.6.1. Selectivity
The specificity of the method was determined by comparing the chromatograms of blank plasma or phosphate buffer with the corresponding spiked plasma or phosphate buffer. Blank samples of all matrixes were extracted to certify the absence of interfering peaks.
2.6.2. Linearity of calibration and the lower limit of detection (LLOD)
Calibration curves were constructed by performing a linear regression analysis of the peak area ratios (y) of each compound to the internal standard vs. their respective concentrations (x) by the 1/x2 weighted least-square linear regression. The LLOD was the concentration of a compound that produced a signal three times the analytical background noise.
2.6.3. Precision, accuracy and recovery
The intra- and inter-day precisions were obtained by analyzing the QC samples of orientin, vitexin, cynaroside and quercitrin at three levels in six replicates within one day and for three consecutive days. The accuracy was determined as the percentage difference between the mean detected concentrations and the nominal concentrations. The recovery of each compound was assessed by comparing the peak height of a sample containing a known amount of a compound with the peak height obtained from direct injections of a standard solution containing the same concentration of the compound.
2.6.4. Analyte stability
The stability of the four analytes in human plasma and phosphate buffer was evaluated at three concentration levels of processed QC samples and in three replicates. The samples were either stored for 6 h at ambient temperature or freeze (−20 °C)–thaw (room temperature) for three times before being stored under auto-sampler conditions (15 °C) for 24 h. Stability was assessed by comparing the mean concentration of the stored QC samples with the mean concentration of freshly prepared QC samples.
The stability of the drug in human plasma and phosphate buffer was also evaluated. The samples were stored for 96 h at 4 °C. Stability was assessed by comparing the same concentration of the drug which is prepared freshly.
2.7. Equilibrium dialysis
The extract was made the concentrations of 1.2, 3, 6, 15, 30, 60 µg/mL by dissolving the extracts from P. orientale in the phosphate buffer. The blank human plasma was added into the semi-permeable membrane. The dialysis bag was placed in a flask containing a volume of phosphate buffer. In prior to analysis, the dialysis system was incubated at 4 °C for 72 h to achieve equilibrium between plasma and phosphate buffer. The fluid outside and inside the dialysis bags was collected after the incubation. Concentrations within the dialysis bags which contain the compounds as protein-bound residues and in free form were determined as the total compound concentration, the concentrations of fluid outside the dialysis bags were determined as the concentrations of free form.
The binding degrees of the four compounds in the equilibrium dialysis experiments can be expressed as
where Dt is the total compound concentration in the plasma compartment and Df is the concentration of the compound in free form in the phosphate buffer compartment.
3. Results and discussion
3.1. Selectivity
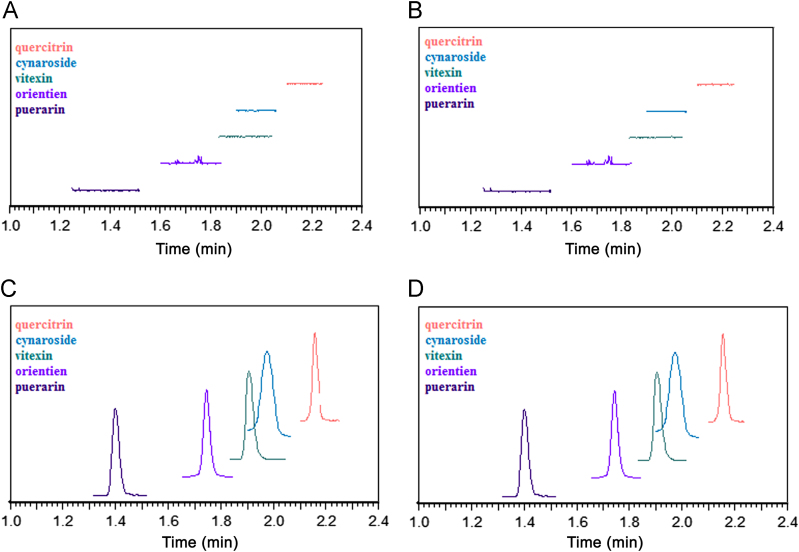
Typical chromatograms of blank samples, spiked samples, plasma and phosphate buffer containing extract of P. orientale in the drug–protein binding study are shown in Fig. 2. The results showed that the four flavonoids and the internal standard were well separated and no significant interferences were observed at the retention times of the analytes and the internal standard.
Fig. 2.
UPLC chromatograms of plasma and phosphate buffer solution with puerarin, orientin, vitexin, cynaroside and quercitrin. (A) Blank plasma; (B) blank buffer solution; (C) the plasma sample; and (D) the phosphate buffer solution sample.
3.2. Linearity of calibration and LLOD
Under current chromatographic conditions, all the four compounds performed good linearity (r>0.996). The ranges of the calibration curves were specified as approximately 80–120% of the test concentration of the samples. These ranges covered the amounts of the analytes of all samples and provided suitable levels of linearity, accuracy and precision. The results are listed in Table 1.
Table 1.
Linear relation between peak area and concentration in the two media.
| Media | Analyte | Regression equation | r | Linear range (µg/mL) | LLOD (µg/mL) |
|---|---|---|---|---|---|
| Plasma | Orientin | y=0.472 x−0.027 | 0.998 | 0.330–80.2 | 0.0146 |
| Vitexin | y=0.651 x−0.030 | 0.999 | 0.108–26.2 | 0.0023 | |
| Cynaroside | y=2.76 x−0.013 | 0.998 | 0.0152–3.68 | 0.0018 | |
| Quercitrin | y=0.538 x−0.044 | 0.997 | 0.0831–20.2 | 0.036 | |
| Phosphate buffer | Orientin | y=2.84 x−0.059 | 0.998 | 0.0274–6.01 | 0.0028 |
| Vitexin | y=3.46 x−0.066 | 0.998 | 0.0246–5.98 | 0.0105 | |
| Cynaroside | y=130.5 x−0.025 | 0.997 | 0.00303–0.737 | 0.0006 | |
| Quercitrin | y=2.67 x−0.027 | 0.996 | 0.0104–2.53 | 0.0051 | |
3.3. Precision, accuracy and extraction recovery
The data of intra- and inter-day precisions, accuracy and recovery for these analytes in the two media from the QC samples (Table 2) showed that the intra- and inter-day precisions (RSDs) were less than 12.1% and 14.1%, and the accuracy was within 113.7%, while the mean recoveries of the four analytes at three different concentrations were ranged from 81.8% to 108.4% in plasma and 82.3%–104.1% in phosphate buffer.
Table 2.
Summary of precision, accuracy and recovery from QC samples (n=3 days and six replicates per day).
| Media | Analyte | Added conc. (µg/mL) | Found conc. (µg/mL) | Precision |
Accuracy (%) | Extraction recovery (%) | |
|---|---|---|---|---|---|---|---|
| Intra-day (RSD, %) | Inter-day (RSD, %) | ||||||
| Plasma | Orientin | 0.99 | 0.93±0.07 | 3.6 | 3.5 | 94.0 | 100.0 |
| 8.91 | 9.80±0.45 | 3.4 | 3.0 | 110.0 | 106.5 | ||
| 80.16 | 86.01±1.01 | 3.6 | 5.5 | 107.3 | 106.7 | ||
| Vitexin | 0.32 | 0.27±0.03 | 4.2 | 2.4 | 82.7 | 81.8 | |
| 2.91 | 2.81±0.17 | 5.8 | 1.1 | 96.9 | 103.1 | ||
| 26.15 | 30.40±0.34 | 3.3 | 3.6 | 112.7 | 103.8 | ||
| Cynaroside | 0.045 | 0.041±0.005 | 8.0 | 4.5 | 91.1 | 98.0 | |
| 0.41 | 0.40±0.02 | 5.3 | 1.9 | 98.0 | 103.2 | ||
| 3.68 | 3.94±0.10 | 2.5 | 4.5 | 106.5 | 106.0 | ||
| Quercitrin | 0.25 | 0.24±0.01 | 4.8 | 5.4 | 94.9 | 106.6 | |
| 2.24 | 1.93±0.10 | 4.6 | 7.5 | 86.1 | 108.4 | ||
| 20.20 | 22.19±0.43 | 2.2 | 8.3 | 110.3 | 100.8 | ||
| Posphate buffer | Orientin | 0.074 | 0.067±0.004 | 5.4 | 4.8 | 90.3 | 83.7 |
| 0.67 | 0.63±0.04 | 7.0 | 7.6 | 94.7 | 91.7 | ||
| 6.01 | 6.58±0.20 | 3.0 | 3.6 | 109.5 | 99.2 | ||
| Vitexin | 0.074 | 0.069±0.004 | 5.9 | 4.5 | 93.0 | 82.3 | |
| 0.66 | 0.64±0.03 | 4.0 | 7.1 | 96.6 | 89.7 | ||
| 5.98 | 7.13±0.17 | 2.4 | 3.1 | 109.3 | 104.1 | ||
| Cynaroside | 0.009 | 0.007±0.001 | 12.1 | 14.1 | 82.2 | 92.2 | |
| 0.082 | 0.083±0.007 | 8.4 | 7.0 | 101.0 | 91.7 | ||
| 0.74 | 0.85±0.03 | 3.9 | 3.4 | 113.7 | 98.6 | ||
| Quercitrin | 0.031 | 0.025±0.002 | 7.5 | 5.8 | 80.7 | 85.9 | |
| 0.28 | 0.24±0.03 | 10.4 | 4.9 | 85.6 | 93.3 | ||
| 2.53 | 2.47±0.07 | 2.9 | 7.2 | 97.9 | 84.9 | ||
3.4. Analyte stability
The stability of orientin, vitexin, cynaroside and quercitrin during sample processing and of the QC samples were investigated. The results showed that the four analytes were stable for at least 6 h at ambient temperatures in QC samples and 24 h in auto-sampler conditions. While in QC samples, three freeze–thaw cycles showed a reduction of the concentration below change of 10%.
The results of the analytes from the drug showed that the drug was stabilized in plasma and phosphate buffer samples at least 96 h within the reduce of 3%.
3.5. Plasma protein binding
Because the free and bound amounts of the drug are not affected in the experiment, equilibrium dialysis has been widely used for protein binding measurements. This technique has been successfully applied to quantify the concentration of each compound in human plasma in the present compound–protein binding study. The binding degree of each compound to the human plasma is shown in Fig. 3. The results showed that the binding degrees of orientin, vitexin, cynaroside and quercitrin were in the range of 74–89% covering all concentrations studied.
Fig. 3.
The protein binding of orientin, vitexin, cynaroside and quercitrin in the extract of P. orientale as determined in human plasma.
For better understanding of the binding of these analytes to plasma proteins, the plasma protein binding affinity (K) and dissociation constant (Kdp), the number of binding sites (n), and the maximum binding ability (β) of these compounds were derived based on the modified Scatchard equation. These parameters could be expressed as follows [14], [15]:
where r is calculated as [Db]/[Pt], Df, Db, and Dt are expressed as the free, bound, and total-drug concentration, respectively; Pt (71.28 g/L) is the total protein in human plasma which is calculated by the Lowry method; W is the plasma water content (99.25%). The concrete results are presented in Table 3. After calculation of Kdp and β, the compound–protein binding in any concentration can be calculated by the following equation:
Table 3.
Plasma protein binding parameters of the four bioactive compounds in the drug–protein binding.
| Analyte | K×104 (L/mol) | n (×10−4) | β (μmol/g) | Kdp (μmol/L) |
|---|---|---|---|---|
| Orientin | 3.973 | 9.659 | 2.685 | 26.55 |
| Vitexin | 5.825 | 5.128 | 1.650 | 19.72 |
| Cynaroside | 22.90 | 0.9820 | 0.3660 | 5.512 |
| Quercitrin | 19.11 | 2.285 | 0.6420 | 5.288 |
The concentrations of the extract of P. orientale were ranged from 1.2 to 60 μg/mL.
4. Conclusions
A sensitive, accurate and precise method based on equilibrium dialysis and UPLC–ESI-MS/MS has been developed and validated for simultaneous determination of the binding degrees of orientin, vitexin, cynaroside, and quercitrin in P. orientale within 3.5 min. The results showed that the four bioactive flavonoids had high binding affinities in the physiological conditions. Equilibrium dialysis may still be a suitable method for investigating simultaneously the binding characteristics (e.g., n and K) of several compounds to plasma proteins.
Acknowledgments
This work was supported by the National Science Foundation of China (No. 30860366) and Guizhou Province Municipal Science and Technology Project (No. 2007-6010).
Footnotes
Peer review under responsibility of Xi′an Jiaotong University.
References
- 1.Chrysanthakopoulos M., Giaginis C., Tsantili-Kakoulidou A. Retention of structurally diverse drugs in human serum albumin chromatography and its potential to simulate plasma protein binding. J. Chromatogr. A. 2010;1217:5761–5768. doi: 10.1016/j.chroma.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S.X., Guan Q.X. Orientin chemical constituents and pharmacology research. Heilongjiang Med. J. 2008;21:97. [Google Scholar]
- 3.Wang A.M., Wang Y.L., Lan Y.Y. HPLC fingerprint analysis of Herba Polygoni Orientalis. Chin. Traditional Patent Med. 2006;28:5–7. [Google Scholar]
- 4.Li Y.J., He X., Liu L.N. Studies on chemical constituents in herb of Polygonum orientale. J. Chin. Mater. Med. 2005;30:444–446. [PubMed] [Google Scholar]
- 5.Zheng S.Z., Wang D.Y., Meng J.C. Studies on the lignans of Polygonum orientale. Acta Bot. Sin. 1998;5:466–469. [Google Scholar]
- 6.Zheng S.Z., Wang D.Y., Liu W.X. The flavonoids of Polygonum orientale L. J. NW. Norm. Univ. 1999;35:37–41. [Google Scholar]
- 7.Li Y.J., He X., Liu Z.B. Chemical constituents of flowers from Polygonum orientale. J. Chin. Mater. Med. 2009;34:2613–2614. [PubMed] [Google Scholar]
- 8.Zhang G.H., Ma C. Advances in studies on pharmacokinetics of flavonoids. Chin. Traditional Herbal Drugs. 2004;35:582–585. [Google Scholar]
- 9.Li X.Q., Huo T.G., Qin F. Determination and pharmacokinetics of orentin in rabbit plasma by liquid chromatography after intravenous administration of orentin and Trollius chinensis Bunge extract. J. Chromatogr. B. 2007;853:221–226. doi: 10.1016/j.jchromb.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Chen L.H., Zou J.B., Liu N.B. Determination of isoorientin and orientin of Passiflora leaves in various specimens by RP-HPLC. Chin. Pharm. 2009;12:874–875. [Google Scholar]
- 11.Li X.N., Wang Q., Zhang X.W. HPLC study of pharmacokinetics and tissue distribution of morroniside in rats. J. Pharm. Biomed. Anal. 2007;45:349–355. doi: 10.1016/j.jpba.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Zhao L., Wei Y.M., Zhong X.D. PK and tissue distribution of docetaxel in rabbits after i.v. administration of liposomal and injectable formulations. J. Pharm. Biomed. Anal. 2009;49:989–996. doi: 10.1016/j.jpba.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Souverain S., Rudaz S., Veuthey J.L. Matrix effect in LC–ESI-MS and LC–APCI-MS with off-line and on-line extraction procedures. J. Chromatogr. A. 2004;1058:61–66. [PubMed] [Google Scholar]
- 14.Si L.Q., LI G., Chen Y. High performance liquid chromatographic determination MELE concentration and the binding rate of MELE to human plasma proteins. J. Chin. Hosp. Pharm. 2006;3:253–255. [Google Scholar]
- 15.Wang H., Chen J.I., Zhang Q.M. High-performance liquid chromatographic determination of baicalin in human plasma. J. Shengyang Pharm. Univ. 2000;17:107–109. [Google Scholar]