Abstract
A simple and rapid gas chromatography/mass spectrometry (GC/MS) analysis method was developed for the determination of fat-soluble parts of sinapis semina. Four major compounds were chosen as marker compounds to evaluate the method. Various extraction techniques were evaluated and the greatest efficiency was observed with sonication extraction using diethyl ether. The method was valuated as follows: acceptable apparatus suitability was obtained by testing the resolutions, tailing factors and theoretical plate number of the marker compounds; the precision and reproducibility, expressed as relative standard deviation (RSD), fell within the prescribed limits. Eight samples of sinapis semina collected from markets in Xi'an were monitored by using the method. The fingerprints of those samples were analyzed by hierarchical cluster analysis (HCA) similarity analysis. The result indicated that the combination of fingerprint and HCA could be used to analyze sinapis semina from different habitats.
Keywords: Sinapis semina, Sonication extraction, GC/MS, Fingerprint, HCA
1. Introduction
Sinapis semina are dry seeds of Sinapis alba L. This traditional Chinese medicine has been known to have the following pharmacological activities: anti-tumor, the analgesic and the antiviral activities [1]. The fat-soluble compounds are important parts of sinapis semina. Thus, it is important to isolate and identify the fat-soluble compounds for the research of sinapis semina. For the analysis of fat-soluble compounds in herbal medicines, gas chromatography and gas chromatography/mass spectrometry(GC–MS) have been widely used [2], [3]. GC/MS has particularly served as a suitable and reliable method for the determination of fat-soluble compounds of herb medicine, due to its efficient separation and identification abilities.
Quality control is an important means for insuring the efficacy of herbal medicines, and this process often closely monitors the concentrations of chemical constituents in these medicines [4], [5]. However, the chemical composition of herbal medicines is complex and the quantification of compounds is dependent on environmental conditions, harvest period, storage time and processing method. Sinapis semina have been grown in many parts of the country. The fat-soluble parts of sinapis semina from different regions are related to its effect.
Many methods have been used for the quantitative extraction of fat-soluble compounds form herb medicine, including steam distillation [6], solvent immersion extraction [7] and solid-phase extraction [8]. However, these methods are laborious and time consuming. Sonication extraction has been used for rapid extraction of fat-soluble compounds from herbal medicine. It is useful due to its easy operation as well as its use of less organic solvent [9], [10], [11], [12].
To evaluate the quality of sinapis semina, a simple quantification of one or even several compounds in herb medicine is not an adequate approach. Chinese medicine chromatographic fingerprint technique is a kind of comprehensive, quantifiable chromatographic identification method. The technology is based on the systematic study of the Chinese herbal medicine chemical composition. Since the technology embodies the holistic and systemic Chinese traditional medicine characteristics, the chromatographic fingerprint analysis of herbal medicines has gained rising attention in recent years [13], [14], [15], [16]. Moreover, hierarchical cluster analysis (HCA) has been used to sort samples into groups by measuring similarity between samples [16], [17], [18], [19], [20]. The similarity and dissimilarity between samples can be represented in dendrogram for easy interpretation.
In this work, a GC/MS method, combined with sonication extraction, can be used as a rapid and an effective method for the characterization of fat-soluble compounds for the quality control of sinapis semina. A total of eight sinapis semina samples were collected from the market and analyzed using the validated analytical method. The chromatographic fingerprints of those sources were established and analyzed by hierarchical cluster analysis (HCA), similarity analysis. The combination of fingerprint and HCA can be used to analyze sinapis semina from different habitats.
2. Materials and methods
2.1. Reagents
Acetic ether (chromatographic grade) was purchased from Fisher Scientific (Pittsburgh, PA, USA). Petroleum ether and diethyl ether (analytical pure) were purchased from Hongyan reagent factory (Tianjin, China).
Analytical samples of sinapis semina were purchased from Xi'an medicinal materials market (China) and identified by an expert discriminator of herbal medicine. Prior to use, the sinapis semina samples were dried and pulverized, then filtered using a standard sieve with a mesh size of 18.
2.2. Sample preparation
Dried sinapis semina were pulverized and 10.0 g was added into 40 mL diethyl ether in a volumetric flask. For solvent immersion extraction, the sample mixture was allowed to stand for 24 h at room temperature in a volumetric flask. The mixture was extracted for 30 min in an utrasonic bath at room temperature. After extraction, the extract was filtered under reduced pressure. The filtrate was collected and solvents were recovered to obtain oil substance. The oil substance was diluted 100 times by using acetic ether and vortexed for 1 min. Finally, 1 μL of the diluted sample was injected into the GC/MS system.
2.3. GC/MS assay
A capillary gas chromatography–mass spectrometry (GCMS-QP2010PLUS Shimadzu, Kyoto, Japan) with a Rtx-5MS capillary column (30 m×0.25 mm i.d.; 0.25 μm film thickness, Restek, CA, USA) was used. Helium (purity 99.999%) was the carrier gas with a constant flow of 1.00 mL/min. The inlet temperature was maintained at 300 °C. The initial temperature was 200 °C, ramped at 10 °C/min up to 280 °C and held for 15 min, and finally ramped at 20 °C/min up to 300 °C, held for 5 min. The mass spectrometer was operated in electron ionization mode at 70 eV. The mass range was scanned from 50m/z to 600m/z for full-scan mode. Data were collected using the GC/MS analysis software, and analyzed using a NIST library (Shimadzu, Kyoto, Japan).
Under these conditions, the volatile compounds of eight sinapis semina samples were analyzed by GC/MS.
2.4. Data analysis
HCA is a multivariate analysis technique that is used to sort samples into groups. Here, different samples of sinapis semina were analyzed by using SPSS 16.0 software, and the results were subjected to HCA. Similarity tests were performed on the basis of the relative retention time (RRT) and relative peak area (RPA) (angle cosine method to calculate the relative peak area), using the professional software named Similarity Evaluation System for Chromatographic Fingerprint (2004).
3. Results and discussion
3.1. Extraction procedure
The herbal sample is homogenized and extracted with a suitable solvent to reduce the bulk of the sample matrix and to extract fat-soluble compounds into the solvent. Both the selection of solvent and the extraction method can be critical in obtaining a satisfactory extraction of fat-soluble compounds from herbal drugs. In this study, the traditional steam distillation and the ultrasonic extraction were compared. The result shows that the extraction rate is 8.96% by using the ultrasonic extraction method, while the traditional steam distillation is only 6.48%. The traditional steam distillation consumed too much time and it was difficult to control the temperature. So the ultrasonic extraction was selected to extract fat-soluble parts from sinapis semina. Petroleum ether, ethyl acetate, and diethyl ether were used as extraction solvents. Total ion chromatograms of the extracts obtained from sinapis semina are shown in Fig. 1. As seen from Fig. 1, ultrasonic extraction with diethyl ether can help obtain more compounds. The extraction efficiency of ultrasonic extraction with diethyl ether, ethyl acetate, and petroleum ether was 8.96%, 7.63% and 7.54% respectively. Finally, diethyl ether was chosen due to its high extraction efficiency.
Fig. 1.
Total ion chromatograms of sinapis semina: (A) sonication extraction with ethyl acetate; (B) sonication extraction with diethyl ether; and (C) sonication extraction with petroleum ethyl.
The extraction yield, the rapidity, and the convenience of sample preparation were taken into consideration; the sonication extraction method using diethyl ether was deemed most suitable.
3.2. Optimization of GC/MC condition
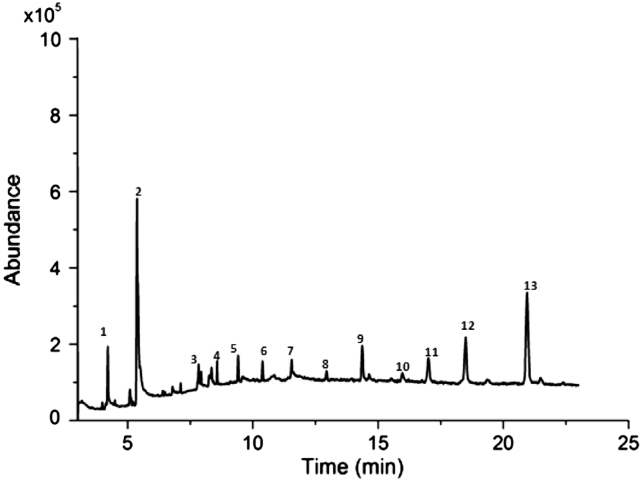
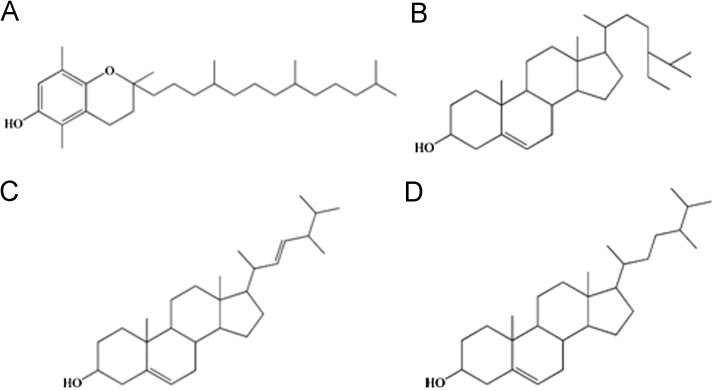
In order to let the analytes be separated within a short time, the temperature program was adopted. A total of 13 compounds were identified in this work. The compounds whose similarity and content (%) are above 80% and 0.40%, respectively, are listed in Table 1 and the chromatogram is shown in Fig. 2. Peaks that existed in all batches of samples were considered as “marker compounds” for methodology study. Four common peaks were chosen, whose structures are shown in Fig. 3.
Table 1.
The constituents and relative contents in the ester-solubility fractions of sinapis semina.
| Number | Compound | Similarity (%) | Molecular formula | Relative content (%) |
|---|---|---|---|---|
| 1 | Ethylbenzene | 89 | C8H10 | 0.42 |
| 2 | o-Xylene | 95 | C8H10 | 1.70 |
| 3 | 2,6-Dihexadecanoate,l-(+)-Ascorbic acid | 91 | C38H68O8 | 3.71 |
| 4 | (Z,Z)-9,12-Octadecadienoic acid | 93 | C18H32O2 | 45.56 |
| 5 | Olealdehyde | 87 | C18H34O | 2.20 |
| 6 | Z,Z-8,10-Hexadecadien-1-ol | 85 | C16H30O | 4.72 |
| 7 | Oleic acid | 88 | C18H34O2 | 2.39 |
| 8 | (Z)- 7-Hexadecenal | 82 | C16H30O | 1.97 |
| 9 | (8Z)-14-Methyl-8-hexadecenal | 81 | C17H32O | 4.51 |
| 10 | beta-Tocopherol | 88 | C28H48O2 | 3.56 |
| 11 | Brassicasterin | 80 | C28H46O | 4.02 |
| 12 | Campesterol | 86 | C28H48O | 7.35 |
| 13 | gamma-Sitosterol | 89 | C29H50O | 17.89 |
Fig. 2.
The total ion chromatograms of sinapis semina extracted by sonication extraction with ethyl acetate.
Fig. 3.
The structures of marker compounds in sinapis semina: (A) beta-tocopherol; (B) brassicasterin; (C) campesterol and (D) gamma-sitosterol.
3.3. Method validation
To precisely evaluate the quality control of sinapis semina, the method validation was performed to determine the marker compounds in herbal plant. In this study, apparatus suitability, precision, repeatability, and sample's stability were tested for analytical method validation.
3.3.1. Apparatus suitability
In this work, the resolutions, tailing factors and theoretical plate number of the marker compounds were calculated, which are shown in Table 2. As shown in Table 2, the theoretical plate numbers of the maker compounds are within the range of 141,911–199,388 and the RSDs of the theoretical plate numbers decreased to 1.555–9.770%. The tailing factors were in the range 0.956–1.372. In addition, resolutions of four marker compounds were within the range of 8.409–17.139. The result indicated that the method was system applicable.
Table 2.
The system applicability of four marker compounds in sinapis semina.
| Marker compounds | Theoretical plate numbers |
Tailing factors |
Resolution |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | CV (%) | 1 | 2 | 3 | CV (%) | 1 | 2 | 3 | CV (%) | |
| A | 198,341 | 199,612 | 193,779 | 1.56 | 1.37 | 1.21 | 1.19 | 7.92 | 14.13 | 16.90 | 15.40 | 8.96 |
| B | 141,911 | 169,565 | 168,349 | 9.77 | 1.02 | 1.01 | 0.96 | 3.25 | 17.14 | 18.06 | 17.91 | 2.79 |
| C | 184,364 | 189,917 | 199,388 | 3.97 | 1.09 | 1.01 | 1.05 | 3.88 | 8.41 | 8.87 | 8.98 | 3.47 |
| D | 180,040 | 178,770 | 194,639 | 4.78 | 1.04 | 1.03 | 1.13 | 5.37 | 13.38 | 13.47 | 13.87 | 1.93 |
3.3.2. Repeatability, precision and stability
The repeatability was assessed by analyzing six independently prepared samples of sinapis semina. Intra-day precision was determined by analyzing five replicates during a single day. However, the inter-day precision was determined by analyzing five replicates on five consecutive days. The relative standard deviation (RSD) was taken as a measure of repeatability and precision. The stability of sample solutions was tested at room temperature. The sample solution was analyzed at 0, 12, 24, 48, and 72 h. The results are shown in Table 3. From the table, we can see that this method was stable and reliable, as the RSDs were less than 5%.
Table 3.
Precision, repeatability and stability of four marker compounds in sinapis semina.
| Marker compounds | Precision |
Repeatability | Stability | ||
|---|---|---|---|---|---|
| Inter-day precision RSD% | Intra-day precision RSD% | ||||
| A | RRT | 0.123 | 0.047 | 0.144 | 0.212 |
| RPA | 3.806 | 0.657 | 3.560 | 1.392 | |
| B | RRT | 0.107 | 0.039 | 0.117 | 0.229 |
| RPA | 3.309 | 0.207 | 3.420 | 1.752 | |
| C | RRT | 0.121 | 0.045 | 0.145 | 0.243 |
| RPA | 2.618 | 0.849 | 2.826 | 4.405 | |
| D | RRT | 0.106 | 0.034 | 0.601 | 0.236 |
| RPA | 2.405 | 0.352 | 3.584 | 1.752 | |
RRT: relative retention time.RPA: relative peak area.
3.4. Establishment of fingerprint
3.4.1. Selection of standard samples
To gain the standardized fingerprint, the standard samples with good quality were selected to establish the mean chromatogram. Eight sinapis semina samples (see Table 4) which met the requirement of the Pharmacopoeia of People's Republic of China (2010 Edition) were selected as the standard samples. All standard samples were analyzed with the developed method.
Table 4.
The information and correlation coefficients of samples.
| Sample | Production | Similarity |
|---|---|---|
| S1 | Anhui | 0.973 |
| S2 | Guangxi | 0.988 |
| S3 | Hebei | 0.992 |
| S4 | Hennan | 0.993 |
| S5 | Jilin | 0.911 |
| S6 | Shandong | 0.904 |
| S7 | Shanxi | 0.991 |
| S8 | Szechwan | 0.945 |
The similarity was calculated by similarity analysis; each fingerprint (from Fig. 4) was compared to the consensus chromatogram “R”. All samples showed a similarity of at least >0.904%.
3.4.2. Selection of reference substance
There are two kinds of reference substances: one is an internal reference substance to which the common peaks belong to and the other is an external reference substance which is added to the sample. In this study, peak no. 3 was chosen as the internal reference substance because this peak, which was present in the middle of the chromatogram with maximum content, existed in all chromatograms.
3.4.3. Fingerprint
The fingerprint analysis was carried out by using the software “Similarity Evaluation System for Chromatographic Fingerprint of TCM”. The mean chromatograms and correlation coefficients of the samples are shown in Fig. 4 and Table 4. In comparison to the consensus fingerprint, all sinapis semina samples showed a similarity of at least 0.904% (shown in Table 4). Sinapis semina from Shanxi, Henan, Hebei, and Guangxi provinces, China have higher similarity of 98.8–99.5%, followed by those from Anhui, Shandong, Jilin and Sichuan provinces, which were between 90.4% and 97.3%.
Fig. 4.
The mean chromatograms of sinapis semina samples: (S1) Anhui; (S2) Guangxi; (S3) Hebei; (S4) Henan; (S5) Jilin; (S6) Shandong; (S7) Shanxi and (S8) Sichuan.
3.5. Quality assessment by HCA
To differentiate the sinapis semina samples collected from the market, HCA was applied to the chromatographic data that were obtained from all the samples. Four marker compounds were selected for this analysis and peak area ratios of the marker compounds were obtained by calculating the peak area ratio of analytes to internal standard.
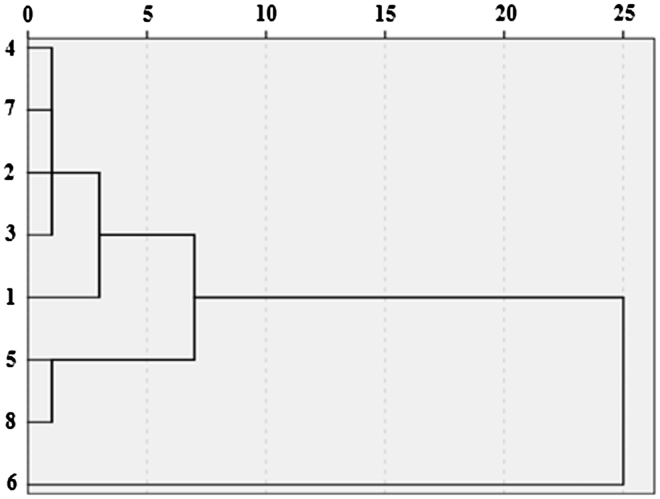
Fig. 5 shows the dendrogram of HCA, demonstrating that the similarity characteristics of eight sinapis semina could be revealed more clearly. Samples are divided into four main clusters. Four batches of sinapis semina samples from Shanxi, Henan, Hebei and Guangxi were clustered into one group, with that of Anhui clustered into one group. Samples from Jilin, Sichuan were clustered into one group, with that of Anhui clustered into a large class. Samples from Shandong showed the highest difference from the others. It can be seen that the cluster analysis result is similar to that of the fingerprint analysis.
Fig. 5.
Dendrogram of sinapis semina generated by the weighted pair group method based on hierarchical cluster analysis.
4. Conclusion
In this study, we described a simple and rapid GC/MS method for quantifying volatile compounds in sinapis semina. The sonication method was shown to be rapid and effective for the extraction of volatiles from herbal medicine. Our validation data indicate that the developed analytical method is suitable for the quantification of the chemical standardization of herbal medicines obtained from sinapis semina. In addition, HCA can provide important information on the differentiation of herbal plants from different regions. This developed GC/MS method, combined with multivariate statistical analysis, is a promising prospective methodology for analyzing sinapis semina from different habitats.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 81230079), the Program for New Century Excellent Talents in University (NCET-08-0437), and the Fundamental Research Funds for Central Universities of China.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Agnihotri V.K., Agarwal S.G., Dhar P.L. Essential oil composition of Mentha pulegium L. growing wild in the north-western Himalayas India. Flavour Fragrance J. 2005;20:607–610. [Google Scholar]
- 2.Zhang Y., Wang Z.Z. Comparative analysis of essential oil components of three Phlomis species in Qinling Mountains of China. J. Pharm. Biomed. Anal. 2008;47:213–217. doi: 10.1016/j.jpba.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Dubey N.K., Kumar R., Tripathi P. Global promotion of herbal medicine: India's opportunity. Curr. Sci. 2004;86:37–41. [Google Scholar]
- 4.Hendriks M.M.W.B., Juarez L.C., Bont D.D. Preprocessing and exploratory analysis of chromatographic profiles of plant extracts. Anal. Chim. Acta. 2005;545:53–64. [Google Scholar]
- 5.Benzo M., Gilardoni G., Gandini C. Determination of the threshold odor concentration of main odorants in essential oils using gas chromatography–olfactometry incremental dilution technique. J. Chromatogr. A. 2007;1150:131–135. doi: 10.1016/j.chroma.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Gherman C., Culea M., Cozar O. Comparative analysis of some active principles of herb plants by GC/MS. Talanta. 2000;53:253–262. doi: 10.1016/s0039-9140(00)00458-6. [DOI] [PubMed] [Google Scholar]
- 7.Bertoli A., Pistelli L., Morelli I. Volatile constituents of different parts (roots, stems and leaves) of Smyrnium olusatrum L. Flavour Fragrance J. 2004;19:522–525. [Google Scholar]
- 8.Lee M.K., Ahn Y.M., Lee K.R. Development of a validated liquid chromatographic method for the quality control of Prunellae Spica: determination of triterpenic acids. Anal. Chim. Acta. 2009;633:271–277. doi: 10.1016/j.aca.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Lee M.K., Ling J.H., Chun M.H. Simultaneous determination of biological marker compounds in Ostericum koreanum by HPLC method and discrimination by principal component analysis. Bull. Korean Chem. Soc. 2008;29:2465–2470. [Google Scholar]
- 10.Seo J., Kim H.Y., Chung B.C. Simultaneous determination of anabolic steroids and synthetic hormones in meat by freezing-lipid filtration, solid-phase extraction and gas chromatography–mass spectrometry. J. Chromatogr. A. 2005;1067(1–2):303–309. doi: 10.1016/j.chroma.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 11.Hong J., Kim H.Y., Kim D.G. Rapid determination of chlorinated pesticides in fish by freezing-lipid filtration, solid-phase extraction and gas chromatography–mass spectrometry. J. Chromatogr. A. 2004;1038:27–35. doi: 10.1016/j.chroma.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Kim M.S., Kang T.W., Pyo H. Determination of organochlorine pesticides in sediment using graphitized carbon black solidphase extraction and gas chromatography/mass spectrometry. J. Chromatogr. A. 2008;1208:25–33. doi: 10.1016/j.chroma.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira L.S., Rodrigues F. de M., de Oliveira F.S. Headspace solid phase microextraction/gas chromatography–mass spectrometry combined to chemometric analysis for volatile organic compounds determination in canine hair: a new tool to detect dog contamination by visceral leishmaniasis. J. Chromatogr. B. 2008;875:392–398. doi: 10.1016/j.jchromb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Martens H., Naes T. Multivariate Calibration. second ed. Wiley; New York: 1991. [Google Scholar]
- 15.Oh S.Y., Ko J.W., Jeong S.Y. Application and exploration of fast gas chromatography-surface acoustic wave sensor to the analysis of thymus species. J. Chromatogr. A. 2008;1205:117–127. doi: 10.1016/j.chroma.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 16.Zeng Z.D., Liang Y.Z., Chau F.T. Mass spectralprofiling: an effective tool for quality control of herbal medicines. Anal. Chim. Acta. 2007;604:89–98. doi: 10.1016/j.aca.2007.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B.Y., Hu Y., Liang Y.Z. Quality evaluation of fingerprints of herbal medicine with chromatographic data. Anal. Chim. Acta. 2004;514:69–77. [Google Scholar]
- 18.Yan S., Luo G., Wang Y. Simultaneous determination of nine components in Qungkailing injection by HPLC/ELSD/DAD and its application to the quality control. J. Pharm. Biomed. Anal. 2006;40:889–895. doi: 10.1016/j.jpba.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Lee I.K., Kim M.A., Lee S.Y. Phytochemical constituents of Schizonepeta tenuifolia Briquet. Nat. Prod. Sci. 2008;14:100–106. [Google Scholar]
- 20.Lin Z.J., Ji W., Desai-Krieger D. Simultaneous determination of pioglitaz-one and its two active metabolites in plasma by GC–MS. J. Pharm. Biomed. Anal. 2003;33:101–108. doi: 10.1016/s0731-7085(03)00344-3. [DOI] [PubMed] [Google Scholar]