Abstract
A high performance liquid chromatography coupled with photodiode array detection method was developed for the identification and quantification of p-hydroxy benzoic acid and agnuside in the extracts of Vitex negundo and Vitex trifolia. The separation was achieved using acetonitrile and O-phosphoric acid–water (0.5%, v/v) as the mobile phase in an isocratic elution mode. Mean retention times of standard p-hydroxy benzoic acid and agnuside were 6.14 and 11.90 min respectively. The developed method was validated as per the ICH guidelines for limit of detection, limit of quantification, linearity, accuracy and precision. Good linearity (r2≥0.999) was observed for both the compounds in wide concentration range. Relative standard deviation values for intra-day and inter-day precision studies were less than 2%. The analytical recoveries of p-hydroxy benzoic acid and agnuside by the developed HPLC method were 93.07% and 106.11% respectively. Two compounds were identified and quantified in leaves and bar extracts of V. negundo and V. trifolia using the developed HPLC method.
Keywords: Vitex negundo, Vitex trifolia, HPLC-PDA, p-Hydroxy benzoic acid, Agnuside
1. Introduction
The genus Vitex belongs to the family Verbenacae. It includes 80 genera and about 800 species. Vitex is a genus of trees and shrubs widely distributed in the tropic and warm region. Vitex negundo Linn is a shrub quite prolific in India, occurring up to an altitude of about 1500 m. It is one of the common plants used in traditional medicine and reported to have a number of biological activities [1]. Flavonoids, iridoids, terpenes and steroids are the major class of compounds isolated from V. negundo[2]. Decoction of the leaves of V. negundo is used as a bath in the puerperal state of women in India. Also the dried leaves of V. negundo are smoked for the relief of headache and catarrh [3]. The leaf extract of V. negundo has been reported to exhibit a wide range of pharmacological activities. The methanolic extract of V. negundo exhibits very potent anti-feedent, anti-inflammatory, analgesic and anti-convulsant activities [4]. Vitex trifolia is a shrub or small tree, its leaves contain flavonoids, sterols and terpenoids. An external application of leaves of V. trifolia is useful for rheumatic pain, sprains, etc. Petroleum and ethanol extracts of leaves of V. trifolia exhibit moderate inhibiting activity against most of the tested Gram-positive and Gram-negative bacteria.
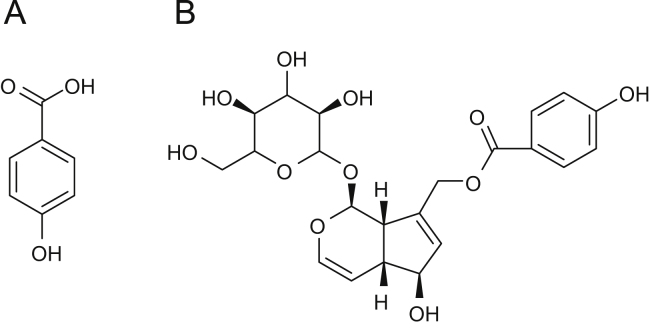
Agnuside (AGN, Fig. 1(B), an iridoid glycoside composed of aucubin) and p-hydroxy benzoic acid (PHBA, Fig. 1(A), a chemotaxonomic marker of the genus Vitex) were isolated from V. negundo, Vitex cymosa and Vitex agnus castus [4], [5], [6]. PHBA is another compound present in the Vitex species. AGN is reported to be linked with the occurrence of PHBA. PHBA has a high absorbance in UV region [7]. Although, a lot of research work have been reported for isolation, characterization and pharmacological activities of biologically active molecules from the Vitex species available in India and worldwide, only few validated HPLC methods are reported for the simultaneous identification and quantification of both PHBA and AGN in Vitex species.
Fig. 1.
Structures of (A) PHBA and (B) AGN.
Hoberg et al. [7] reported an HPLC method for the simultaneous determination of PHBA and AGN in eight drug samples of Agni-casti fructus with a total run time of 20 min using a gradient elution mode with acetonitrile and O-phosphoric acid (0.5%). Although, the gradient elution mode is employed to separate compounds differing in polarity, however, due to constant change in mobile phase compositions, the baseline might be drifted. Quantification of an iridiod negundoside in V. negundo by HPLC method was also reported [8], [9]. Lokhande and Verma [10] reported a TLC densitometric method for quantitative analysis of negundoside in V. negundo leaf powder. Hajimehdipoor et al. [11] also reported a validated HPTLC-densitometric method for assay of aucubin in Vitex-agnus-castus. Simultaneous quantification of major iridoids namely negundoside, agnuside and 6′-p-hydroxy benzoyl mussaenosidic acid by a validated HPTLC method was reported by Tiwari et al. [12].
To the best of information available in the literature, there is no other validated HPLC method reported for the simultaneous determination of PHBA and AGN in Vitex species. Keeping that in view, the present HPLC method was developed for simultaneous identification of PHBA and AGN in different extracts of V. negundo and V. trifolia.
2. Experimental
2.1. Plant materials and chemicals
Leaves and bark of V. negundo and V. trifolia were collected from the herbal garden of Directorate of Medicinal and Aromatic Plants Research, Boriavi, Anand, India during 2011. HPLC-grade solvents methanol, acetonitrile and analytical grade O-phosphoric acid were purchased from Merck, Mumbai, India. Deionized water used throughout the experiment was prepared using a Millipore water purification system (Millipore, gradient 0.22 µm). Standard AGN (purity≥95%) was purchased from Chromadex, USA. Standard PHBA was purchased from Sigma-Aldrich, Mumbai, India.
2.2. Preparation of standard solution and extract samples
Standard stock solutions of PHBA and AGN (500 μg/mL) were prepared by dissolving 5 mg of standard PHBA and AGN separately in 10 mL of methanol. Working solution of lower concentration was prepared by appropriate dilution of the stock solutions.
Leaves of V. negundo and V. trifolia were dried in shade. Dried leaves were finely powdered using an electric grinder and were used for preparation of the extract. Powdered samples (50 g) were extracted with methanol (250 mL × 6) for 24 h at room temperature. The methanol extract thus obtained was pooled together and concentrated under reduced pressure to obtain methanolic extract. Methanolic extract was suspended in water (200 mL) and extracted successively with hexane (100 mL ×6), chloroform (100 mL × 6) and ethyl acetate (100 mL × 6). The hexane extracts obtained were combined together, dried over anhydrous sodium sulfate and concentrated under reduced pressure to get a residue of hexane extract. Similar processing of chloroform and ethyl acetate extracts provided their concentrated extracts respectively. The concentrated extract samples obtained were further vacuum dried. Similarly, bark samples of V. negundo and V. trifolia were extracted with methanol to get the methanolic extract of the bark. Stock solutions of different extracts were prepared by dissolving extract in methanol (1 mg/mL) and filtered through a 0.45 µm membrane filter.
2.3. Chromatographic conditions of HPLC-PDA analysis
HPLC system for chromatographic analysis consisted of a separation module (Waters 600E) equipped with Empower software (Waters) and comprising of quaternary pump, an in-line vacuum degasser and a photodiode array detector (Waters 2996). The chromatographic separation was carried out on a RP-18 column (250 mm × 4 mm, 5 µm, Merck, India) using an isocratic elution. The mobile phase consisted of a mixture of solvent acetonitrile (15%, A) and 0.5% O-phosphoric acid-water (85%, B). The solvent flow rate was 1.0 mL/min. The injection volume was 10 µL and the column temperature was ambient. The photo diode array detector wavelength was set at 254 nm for the identification and quantification of PHBA and AGN in different extracts of V. negundo and V. trifolia. Chromatographic peaks were identified on the basis of retention time. The concentrations of PHBA and AGN were calculated by comparing the integrated peak areas of PHBA and AGN in the extract chromatograms with that of a standard curve prepared for the corresponding standard solution. For analytical recovery studies, sample extract was mixed with known amounts of standards and re- chromatographed to ascertain whether the peak of interest increased in height and area. Spiked peaks were also checked for peak symmetry and peak purity.
2.4. Preparation of calibration curve
Calibration curves for PHBA (5–100 µg/mL) and AGN (25–500 µg/ mL) were prepared by injecting different concentrations of standard samples, recording their peak areas and plotting peak areas vs concentration of the standard.
2.5. Accuracy and precision
Precision of the method was measured by determining repeatability of the standard concentration of PHBA and AGN. The repeatability of sample application and measurement of peak area were expressed in terms of the percentage of relative standard deviation (% RSD). The experiment was repeated thrice on the same day and additionally on three consecutive days to determine intra-day (repeatability) and inter-day (intermediate precision) variations. The intra-day and inter-day variations by developed HPLC method were carried out at three different concentration levels 5, 40, 100 and 25, 100, 250 µg/mL for PHBA and AGN, respectively.
Accuracy of the developed HPLC method was tested by performing recovery studies by spiking of 20, 40, 80 µg/mL of standard PHBA and 50, 200 and 400 µg/mL of standard AGN respectively to the blank extract (chloroform extract of V. negundo leaves, 500 µg/mL). The percent recovery was calculated based on the ratio of the peak area of the compound in the extract prepared via additional method to the area obtained by the direct injection of the corresponding standard solutions. The accuracy was determined to be the percent difference between the measured concentrations and the nominal concentrations obtained from the calibration curves.
3. Results and discussion
The Vitex species are used in the Indian system of medicine for the treatment of a range of diseases. V. negundo and V. trifolia are prescribed to be major herbal plants among Vitex species. Also, the genus Vitex is a rich source of iridoids which are well known for hepatoprotective activity. V. negundo is used in several hepatoprotective polyherbal commercial formulations and aqueous and ethanol extracts of V. trifolia also exhibit significant hepatoprotective activity against carbon tetracholoride induced liver damage in Wistar rats [13], [14], [15]. Therefore, analysis of major compounds present in Vitex species is of importance for the authentication of plant material, monitoring quality and also for research purposes. Although, occurrence of AGN is linked with PHBA, however, it is not proven that AGN is found only in the presence of PHBA [7]. Method validation demonstrated that the developed HPLC method is suitable for simultaneous qualitative and quantitative analysis of PHBA and AGN; the two compounds are related to each other.
3.1. Optimization of the chromatographic conditions
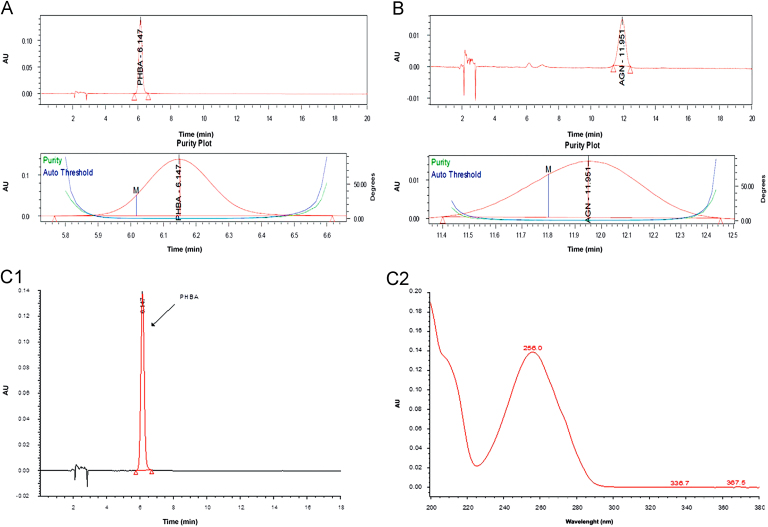
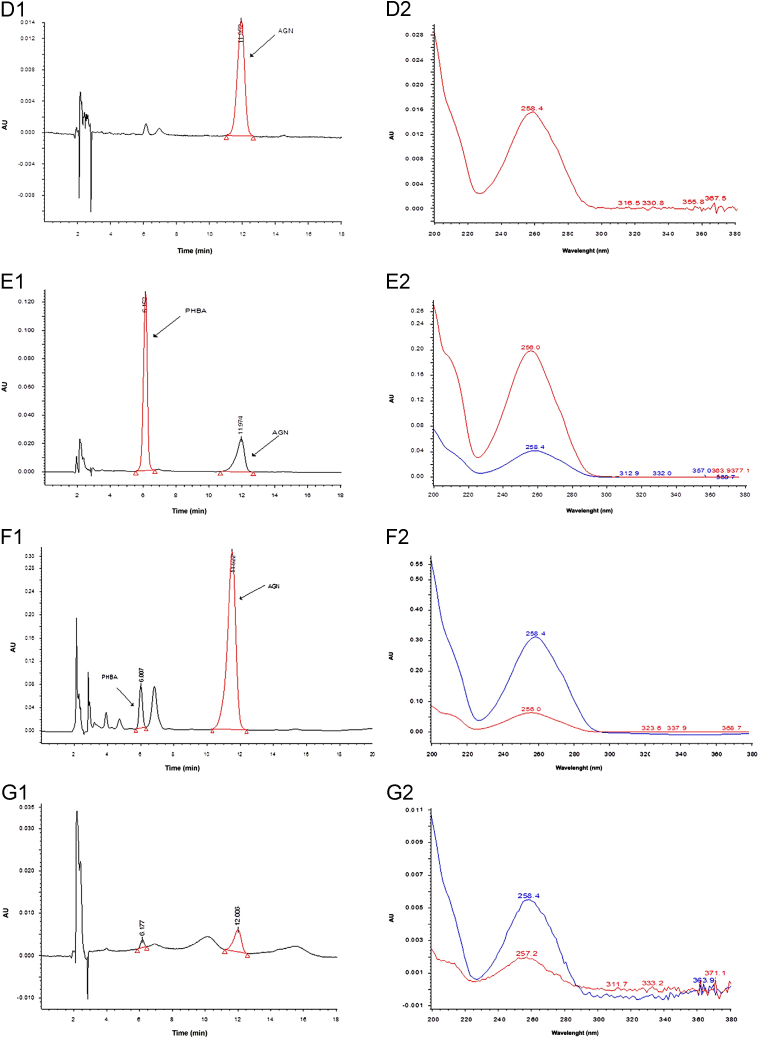
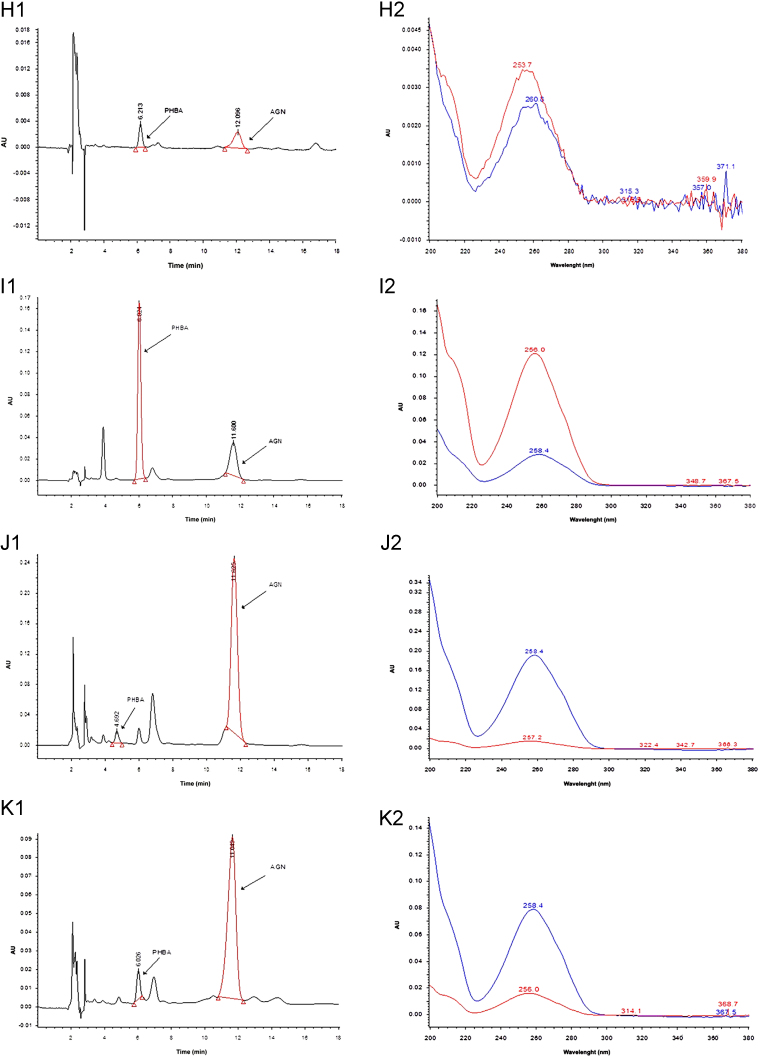
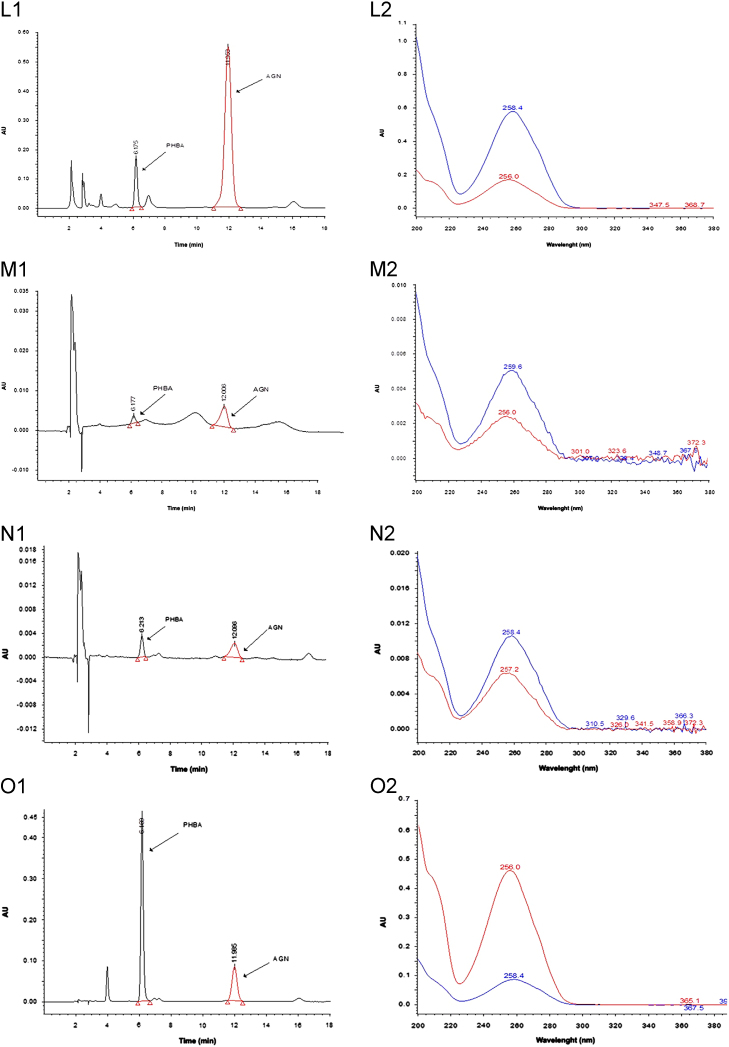
A reversed-phase C18 column was used and several different elution systems were tried. It was observed that the resolution of peaks of PHBA and AGN was unsatisfactory when either acetonitrile:water or methanol:water was used as a mobile phase. Several modifiers such as O-phosphoric acid, acetic acid and formic acid were used for optimization of chromatographic separation. The results suggested that the mobile phase consisting of a mixture of acetonitrile (15%, A): 0.5% O-phosphoric acid (85%, B) in an isocratic elution mode was ideal for chromatographic separation of PHBA and AGN. Interestingly, the elution order of PHBA and AGN was observed to be dependent on the ratio of solvent A to solvent B. Up to a mobile phase composition A:B (15:85, pH 2.61) PHBA was first eluted followed by AGN. However, at mobile phase composition A: B (20:80, pH 2.64) the elution order was reversed i.e., AGN was first eluted followed by PHBA. Finally, a mobile phase composition A:B (15:85) was selected for the method development. Under the optimized conditions two standards (PHBA and AGN) were well resolved with relatively high sensitivity at mean retention time of 6.14 and 11.90 min respectively when absorption was measured at 254 nm. At this wavelength, the best resolution between peaks as well as baseline was achieved and no interfering peaks were observed in the blank samples. The total run time was 20 min to ensure any late eluting peaks. Spectral purity of the peaks of standard PHBA, AGN and extract samples were verified in terms of the purity angle and purity threshold values (Fig. 2). Purity angle was found to be lower than the purity threshold value, depicting the homogeneity of the peak.
Fig. 2.
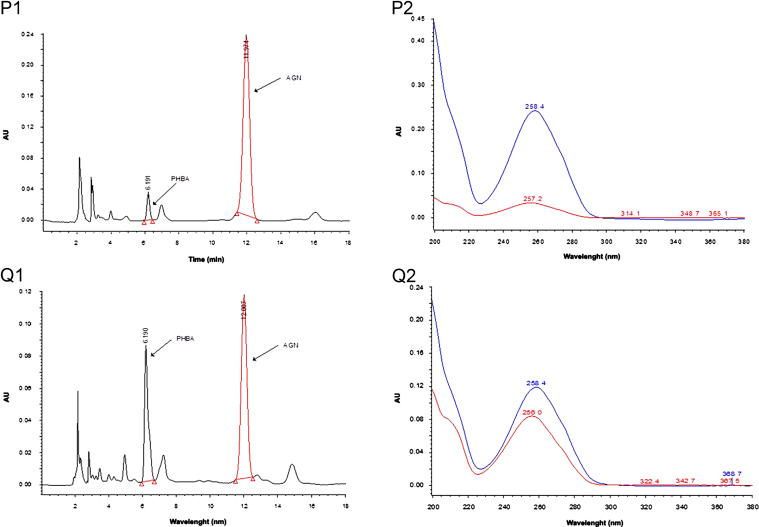
Peak purity plot of (A) standard PHBA and (B) standard AGN, HPLC chromatograms (1) and corresponding PDA spectrum(2) of (C) standard PHBA, (D) standard AGN, (E) standard mixture of PHBA and AGN, (F) methanol extract of Vitex negundo leaves, (G) hexane extract of V. negundo leaves, (H) chloroform extract of V. negundo leaves, (I) ethyl acetate extract of V. negundo leaves, (J) aqueous extract of V. negundo leaves, (K) methanol extract of V. negundo bark, (L) methanol extract of Vitex trifolia leaves, (M) hexane extract of V. trifolia leaves, (N) chloroform extract of V. trifolia leaves, (O)ethyl acetate extract of V. trifolia leaves, (P) aqueous extract of V. trifolia leaves, (Q) methanol extract of V. trifolia bark.
3.2. Method validation
3.2.1. Limit of detection (LOD) and limit of quantification (LOQ)
LOD of an individual analytical procedure is the lowest amount of an analyte in a sample that can be detected but not necessarily quantified. LOQ of an individual analytical procedure is the lowest amount of analyte in a sample that can be determined with suitable precision and accuracy. LOD was found to be 2.5 and 10 μg/mL for PHBA and AGN respectively. LOQ was found to be 5 and 25 μg/mL for PHBA and AGN respectively.
3.2.2. Linearity
Calibration curves were constructed as a function of the concentrations of standard analytes (X) vs. their peak areas (Y). Calibration curves were linear over a large concentration range of 5–100 µg/mL for PHBA and 25–500 µg/mL for AGN. Calibration curves exhibited good linear regressions (Y=107000X–27900, r2=0.999 for PHBA and Y=29100X+194000, r2=0.999 for AGN).
3.2.3. Precision and accuracy
The results of the intra-day and inter-day precision experiments are shown in Table 1. The developed method was found to be precise as the RSD values for repeatability of intra-day and inter-day precision studies were below 2.5%, which is under limit as per recommendations of ICH guidelines [16]. The overall recovery percentages were in the range of 100.41–109.41 for PHBA and 85.30–98.23 for AGN (Table 2). These results demonstrated that the developed method is reproducible with good accuracy.
Table 1.
Intra-day and inter-day precision data of developed HPLC method.
| Standard (µg/mL) | Intra-day (RSD %) | Inter-day (RSD %) |
|---|---|---|
| PHBA | ||
| 5 | 0.28 | 0.30 |
| 40 | 0.45 | 2.27 |
| 100 | 0.43 | 1.74 |
| AGN | ||
| 25 | 0.07 | 0.41 |
| 100 | 0.03 | 0.44 |
| 250 | 0.05 | 0.26 |
Table 2.
Analytical recovery of PHBA and AGN by developed HPLC method (n=3).
| Standard | Added concentration (µg/mL) | Measured concentration (µg/mL) | Recovery (%) | RSD (%) | Mean recovery (%) |
|---|---|---|---|---|---|
| PHBA | 20 | 21.88 | 109.41 | 2.43 | |
| 40 | 40.16 | 100.41 | 1.90 | 106.11 | |
| 80 | 86.91 | 108.53 | 0.54 | ||
| AGN | 50 | 42.65 | 85.30 | 0.29 | |
| 200 | 196.46 | 98.23 | 0.54 | 93.07 | |
| 400 | 382.74 | 95.68 | 0.18 |
3.2.4. Robustness
For verification of robustness of the HPLC method, chromatographic conditions which may affect the performance of the method such as flow rate, organic content in mobile phase, wavelength of detection were deliberately changed. Also, the robustness of the developed HPLC method was verified on other HPLC system (Shimazdu SPD-10A). Very low value (less than 5%) of overall RSD (%) between the data at each variable condition indicated robustness of the developed HPLC method.
3.2.5. System suitability
HPLC method was also validated for its system suitability (Fig. 2). System suitability parameters for PHBA (USP plate count=3131, USP tailing=0.90) and AGN (USP plate count=2849, USP tailing=0.78, resolution=9.07 and selectivity=2.13) demonstrated that developed method is suitable for determining two compounds in Vitex extracts. Purity angle and purity threshold values corresponding to peak of PHBA in different extracts samples of V. negundo were methanol (0.36, 0.4), hexane (6.18, 6.83), chloroform (3.46, 3.58), ethyl acetate (0.18, 0.32), aqueous (1.06, 1.07), bark methanol (2.39, 3.45). Similarly, purity angle and purity threshold values corresponding to peak of AGN in different extracts samples of V. negundo were methanol (0.26, 0.27), hexane (2.42, 2.59), chloroform (4.66, 5.05), ethyl acetate (0.58, 0.65), aqueous (0.260, 0.28), bark methanol (0.77, 2.0).
Purity angle and purity threshold values corresponding to peak of PHBA in different extracts samples of V. trifolia were methanol (0.23, 0.29), hexane (5.70, 5.73), chloroform (1.93, 2.16). ethyl acetate (0.07, 0.27), aqueous (0.72, 1.46), bark methanol (0.46, 0.48). Similarly, purity angle and purity threshold values corresponding to peak of AGN in different extracts samples of V. trifolia were methanol (0.28, 0.37), hexane (2.38, 2.57), chloroform (1. 08, 1.30), ethyl acetate (0.24, 0.33), aqueous (0.40, 0.55), bark methanol (0.31, 1.89).
3.3. Determination of PHBA and AGN in the extracts of V. negundo and V. trifolia
The developed method was applied for the determination of PHBA and AGN contents in the different extracts of V. negundo and V. trifolia. Representative HPLC-PDA chromatograms of extracts are shown in Fig. 2. Contents of PHBA and AGN in different extracts are summarized in Table 3. PHBA was detected only in methanol and ethyl extracts of leaves of V. negundo and methanol extract of bark of V. negundo. Although peaks of PHBA were detected in other extracts also but as its content was below LOQ, therefore, it was not quantified. Its content was higher in extract of V. trifolia. AGN was detected in methanol, ethyl acetate and aqueous extracts of V. negundo and V. trifolia. Methanolic extract of bark of V. negundo and V. trifolia also had lower AGN concentration than the methanolic extract of leaves. AGN concentration was maximum in the methanol extract followed by aqueous and ethyl acetate extracts. AGN concentration in methanolic extract of bark of V. trifolia was higher than that in methanolic extract of bark of V. negundo. The above evidences validate the use of Vitex species in herbal preparations as in herbal preparations water is used as the principal solvent.
Table 3.
Applicability of the developed method for the determination of PHBA and AGN in V. negundo and V. trifolia extractb samples.
| Extract | V. negundo | V. trifolia | ||
|---|---|---|---|---|
| PHBAa | AGNa | PHBAa | AGNa | |
| Leaves | ||||
| Methanol | 1.55 | 49.86 | 37.61 | 48.10 |
| Hexane | BQL | BQL | BQL | BQL |
| Chloroform | BQL | BQL | BQL | BQL |
| Ethyl acetate | 3.25 | 3 .43 | 3.45 | 10.07 |
| Aqueous | BQL | 30.92 | 7.49 | 38.07 |
| Bark | ||||
| Methanol | BQL | 15.35 | BQL | 16.81 |
BQL=below quantification limit (LOQ).
Mean percentage (n=6).
500 μg/mL dissolved in methanol.
This is the first report of a validated HPLC method for rapid identification and quantification of PHBA and AGN in the extracts of leaves and barks of V. negundo and V. trifolia. Present studies also substantiate that occurrence of AGN is linked with PHBA. The salient advantages of the developed HPLC method are (1) sample preparation is very simple and does not require use of solid phase extraction cartridges and (2) using this method quantitative analysis and quality control of large number of extracts as well as commercial samples of Vitex species can be carried out.
4. Conclusion
A simple and efficient reverse-phase HPLC method was developed for simultaneous identification and quantification of PHBA and AGN in different extracts prepared from leaves and bark of V. negundo and V. trifolia. The developed HPLC method was validated as per ICH guidelines. The developed method is simple, precise and accurate and it can be used for bio-prospecting for other Vitex species available in India as well as quality control of herbal formulation containing the above two compounds and also for pharmacokinetic studies of related extracts and drugs. The contents of PHBA and AGN were lower in bark samples than in leaves samples, thereby implementing that this part need not be used so that Vitex species could be better protected and utilized continually.
Acknowledgment
Authors express their sincere thanks to the Director, DMAPR, Anand for permitting the research work. We also acknowledge the help rendered by Shri B.K. Mishra for assistance in plant material collection and its processing.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Cushine T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar M., Rawat P., Dixit P. Anti-osteoporotic constituents from Indian medicinal plants. Phytomedicine. 2010;17:993–999. doi: 10.1016/j.phymed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 3.K.R. Kitrikar, B.D. Basu, Indian Medicinal Plants, vol. III. 2nd edition, Bishen Singh Mahendrapal Singh, Dehra Dun, India,1994, pp.1936–1938.
- 4.Sharma R.L., Prabhakar A., Dhar K.L. A new iridoid glycoside from Vitex negundo Linn (Verbenacae) Nat. Prod. Res. 2009;23(13):1201–1209. doi: 10.1080/14786410802696494. [DOI] [PubMed] [Google Scholar]
- 5.Hansel R., Leukert C.H., Rimpler H. Chemotaxonomische untersuchungen in der gattung Vitex L. Phytochemistry. 1965;4:19–27. [Google Scholar]
- 6.Boros C.A., Stermitz F.R. Iridoids, an updated review, part I. J. Nat. Prod. 1990;53:1055–1147. [Google Scholar]
- 7.Hoberg E., Meier B., Sticher O. An analytical high performance liquid chromatographic method for the determination of agnuside and p-hydroxy benzoic acid contents in Agni-casti fructus. Phytochem. Anal. 2000;11:327–329. [Google Scholar]
- 8.Sing A.P., Ryali S., Varadhacharyulu P. Negundoside from leaves of Vitex negundo. Int. J. Chem. Anal. Sci. 2011;2:1197–1198. [Google Scholar]
- 9.Lokhande P.J., Verma J.K. Quantification of negundoside in Vitex negundo Linn. leaf powder by high performance liquid chromatography. Acta Chromatogr. 2010;22:591–597. [Google Scholar]
- 10.Lokhande P.J., Verma J.K. Quantification of negundoside in Vitex negundo Linn. leaf powder by high performance thin –layer chromatography. J. Planar Chromatogr. 2010;22:225–228. [Google Scholar]
- 11.Hajimehdipoor H., Shekarch M., Hamedani M.P. A validated HPTLC-densitometric method for assay of aucubin in Vitex agnus-castus L. Iranian J. Pharm. Res. 2011;10:705–710. [PMC free article] [PubMed] [Google Scholar]
- 12.Tiwari N., Luqman S., Masood N. Validated high performance thin layer chromatographic method for simultaneous quantification of major iridoids in Vitex trifolia and their antioxidant studies. J. Pharm.l Biomed. Anal. 2012;61:207–214. doi: 10.1016/j.jpba.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan K., Senthilkumar A., Chandrasekaran M. Differential larvicidal efficacy of four species of Vitex against Culex quinquefasciatus larvae. Parasitol. Res. 2007;101:721–723. doi: 10.1007/s00436-007-0714-5. [DOI] [PubMed] [Google Scholar]
- 14.Najmi A.K., Pillai K.K., Pai S.N. Free radical scavenging and hepato protective potential of jigrine post treatment against thioacetamide induced hepatic damage. J. Ethnopharmacol. 2005;97:521–525. [Google Scholar]
- 15.Manjunatha B.K., Vidya S.M. Hepatoprotective of Vitex trifolia against carbon tetra chloride induced hepatic damage. Ind. J. Pharm. Sci. 2008;70:241–245. doi: 10.4103/0250-474X.41466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ICH guidelines, Technical Requirements for Registration of Pharmaceuticals for Human Use: Harmonized Triplicate Guidelines on Validation of Analytical Procedures: Methodology, Recommended for Adoption at Step 4 of the ICH Process on November 1996 by the ICH Steering Committee, IFPMA, Switzerland, 1996