Abstract
Background & objectives:
With improved survival of childhood cancer patients, the number of long-term cancer survivors is increasing. Some studies have assessed the long-term morbidity after childhood cancer treatment in the developing countries. This study was conducted to assess the spectrum of late effects of cancer treatment in paediatric cancer survivors.
Methods:
Evaluation of the first 300 patients who completed five years of follow up in the after treatment completion clinic was done. Details of primary diagnosis, treatment received and current clinical status were noted. The spectrum of late effects was ascertained by appropriate investigations.
Results:
Haematological malignancies comprised 25 per cent of total cases. Most common primary diagnosis comprised acute lymphoblastic leukaemia, retinoblastoma and Hodgkin's lymphoma. The median age at evaluation and follow up was 14 and 8.5 yr, respectively. Twenty three per cent (69) of the survivors had a minimal disability (growth retardation or underweight), 13 per cent (39) had moderate disabilities needing medical attention (hepatitis B surface antigen positive, myocardial dysfunction, azoospermia and hypothyroidism), while two per cent had major/life-threatening disabilities (mental retardation, liver disease and mortality). Eleven patients relapsed on follow up, of those five patients expired. Two second malignancies were recorded during the period of follow up.
Interpretation & conclusions:
Late effects were of concern; however, severe disability (Grade 3-5) was seen in only two per cent survivors. Lifelong follow up of childhood cancer survivors is required to assess cancer-related morbidity, occurrence of a secondary neoplasm, to facilitate timely diagnosis and to implement remedial or preventive interventions to optimize health outcomes. Awareness towards the existence of late effects of cancer therapy is required among parents, patients and health professionals.
Keywords: Cancer survivors, children, disability, late effects, second neoplasm, therapy
Advanced oncotherapy and better supportive care have led to increased survival of childhood malignancy patients over the past few decades. Over 80 per cent children with cancer survive more than five years from diagnosis in various centres and are cured of the disease1,2,3,4. In India, cancer survival is similar to the West only at tertiary care institutes. However, in other centres, the five-year overall survival for all childhood cancers combined was 37-40 per cent2. Better long-term survival is also accompanied with late or long-term effects of cancer treatment. It is believed that a third to half of childhood cancer survivors will experience a late/long-term effect of cancer therapy, of which up to half may be life-threatening5,6,7.
The complications of cancer therapy range from impaired growth and development, neurocognitive and psychosocial deficits, cardiopulmonary problems, endocrine organ dysfunction, gastrointestinal problems and secondary malignancies8,9,10,11. The majority of cancer survivors do not receive risk-based care. The Childhood Cancer Survivor Study (CCSS) reported 88.8 per cent of survivors receiving medical care, but only 31.5 per cent received cancer-focussed care6.
In studies from Central India8, scholastic problems were seen in 43 per cent of childhood cancer survivors (144/332); of them, 44 per cent (64/144) had received cranial irradiation. Psychosocial difficulties were seen in 57 per cent (188/332); of them, 32 per cent (60/188) received cranial irradiation. Other small studies have reported on nutritional problems, reproductive function, psychosocial issues and metabolic abnormalities12,13,14,15,16,17,18. In a study from south India19, male and female gonadal dysfunctions were found to be the most common late toxic effects. Oligospermia/azoospermia was seen in 89 per cent males and gonadal dysfunction, amenorrhoea or oligomenorrhoea was seen in 78 per cent females. All had received combination chemotherapy that included alkylating agents.
Studies addressing the entire spectrum of late effects and their impact on the health, social and professional life of cancer survivors are not available, particularly from developing countries. We report here data from a cohort of 300 childhood cancer survivors being followed in our Paediatric Cancer Survivor Clinic (PCSC) in the department of Pediatrics, All India Institute of Medical Sciences (AIIMS), New Delhi, India.
Material & Methods
The Paediatric Cancer Survivor Clinic (PCSC) of the department of Pediatrics, AIIMS is a weekly clinic where all children who have completed their treatment for the primary disease are followed. The primary objective of this clinic is to follow the patients for recurrence of primary disease, monitor growth, development and sexual maturation, to identify long/late effects of cancer treatment, and to follow these children for the occurrence of a second neoplasm.
The data of survivors of paediatric cancers who have been in remission for at least five years after treatment completion, were analysed. Ethics clearance to conduct this study was taken from the Institutional Ethics Committee.
Evaluation: During evaluation, details of primary disease and treatment received, cumulative doses of various chemotherapies and radiation, and details of remission/relapse were recorded. Details of growth of these children were obtained from anthropometric recording using Centers for Disease Control (CDC) growth charts20. Growth was monitored at periodic intervals varying from 3 to 12 months depending on the schedule of a visit to the clinic which in turn depends on the duration of the disease-free interval. Myocardial function was assessed by conventional echocardiography in those children who had been treated with anthracycline/other cardiotoxic chemotherapy regimens/having received chest radiotherapy (RT). The cumulative dose of anthracyclines/other cardiotoxic drugs was recorded. Pulmonary function assessment was done by performing pulmonary function tests (PFTs) and spirometry. This was done in children who received chemotherapy that affected pulmonary function such as bleomycin or radiation to neck/chest.
For gonadal toxicity, history followed by physical examination was done. Endocrine assessment was done followed by investigations (hormone assessment). Hearing assessment was done by pure tone audiometry alone or in combination with brainstem-evoked response assessment. Vision testing was also done. Thyroid function was done where indicated. Transfusion-related hazards were assessed by evaluating viral markers. A detailed psychosocial evaluation and intelligence quotient (IQ) assessment were done for children who received central nervous system (CNS)-directed therapy in the form of chemotherapy/radiation. The IQ assessment was done using Malin's Intelligence Scale for Indian Children (MISIC)21. This is an Indian adaptation of the Wechsler Intelligence Scale for Children22. The Stanford–Binet test23 measures verbal and performance score based on basal and ceiling age. Child behaviour checklist with 113 items (Youth Self-Report Profile Boys & Girls) measures behavioural problems in children24. The minimum cut-off scores are 54 and 57 in boys and girls, respectively. The areas tested include features of withdrawal, somatic complaints, anxiety and depression, social problems, thought problems, attention problems, delinquent behaviour and aggressive behaviour.
Information on social adjustment into the home, school and society were recorded. Information regarding education and peer relationships was acquired from all children and grades of disability were ascertained. The disability score developed by the National Cancer Institute25 was used: mild (Grade 1), moderate (Grade 2, needing medical attention), severe (Grade 3 affecting daily/professional life) (Grade 4 life-threatening) and disabling or fatal (Grade 5). These children were followed for disease status and relapse if occurred. Occurrence of second malignant neoplasms (SMNs) was also noted.
Statistical analysis: Data were analyzed with SPSS 10.0 statistical package (SPSS, Chicago, Illinois, USA). Continuous and interval-related data are presented as the mean±SD, whereas categorical variables are presented as frequency distribution and percentages. Qualitative data were analyzed using the Chi-square test or Student's t test.
Results
The data were collected from 300 patients registered in the PCSC who had completed five years of follow up after treatment completion. The majority of the cancer survivors had acute lymphoblastic leukaemia (ALL), retinoblastoma and Hodgkin's lymphoma (HD) as the primary diagnosis (Table I). All 300 patients had received chemotherapy. Of these, 72 patients received RT. These included 60 ALL who received cranial RT, four Hodgkin lymphoma (site=neck and mediastinum), six retinoblastoma, one Ewing sarcoma, and one rhabdomyosarcoma.
Table I.
Demographic details of patients included in the study
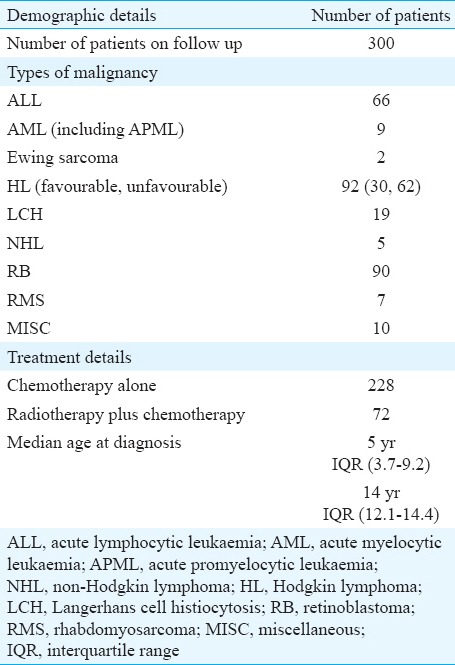
The median age at evaluation was 14 yr and the median follow up period was 8.5 years. All children had completed more than five years follow up. The longest follow up was 29 years. The male-to-female ratio was 4.6:1. The most common primary diagnosis was ALL.
Sixty nine patients (23%) had a Grade 1 disability (growth impairment with height or weight less than expected for age), 39 (13%) had Grade 2 disability needing medical attention [majority due to hepatitis B surface antigen (HBsAg) positivity], one had Grade 3 (mental retardation) and one died of hepatic failure and four expired from relapse (Grade 5). Eleven patients had a relapse on follow up: of these, five patients expired. Two second malignancies were recorded (Hodgkin lymphoma in one child with a primary diagnosis of retinoblastoma and acute myeloid leukaemia in another with a primary diagnosis of retinoblastoma) (Table II).
Table II.
Data on adverse effects of therapy (general)
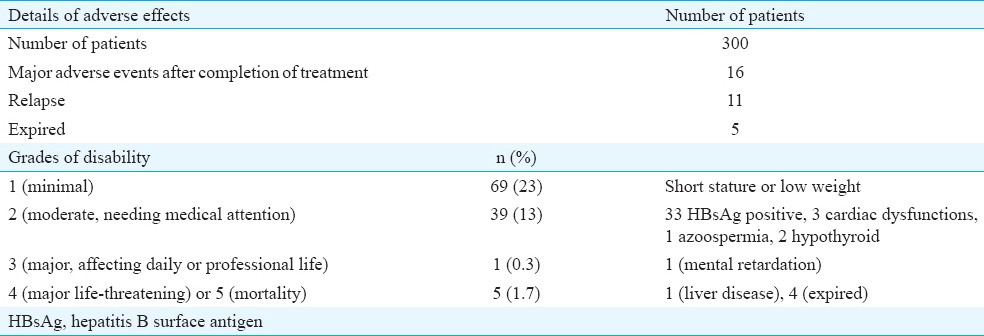
Organ-specific side effects (Table III)
Table III.
Adverse effects of therapy, organ specific
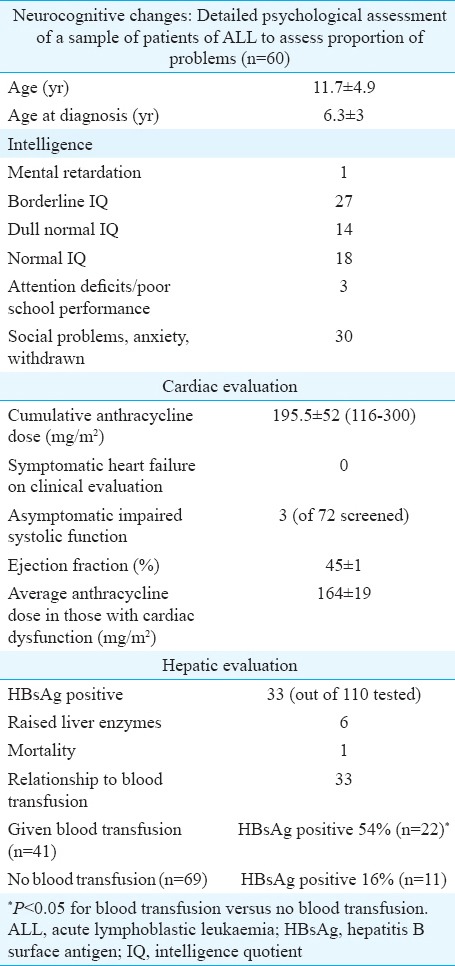
Growth: Growth data were available in 300 patients, of whom 26 per cent (n=78) had a height less than the third centile for that age according to the standard CDC growth charts. Height velocity was also assessed in patients who had received RT. Data for 181 of the 228 patients who did not receive RT were available for current height from the case records. Amongst these, 20 per cent had their height less than the third centile. Seventy two patients received RT and 26 per cent patients had their height velocity less than the third centile. The difference between those with and without RT was not significantly different. Of the 298 patients who had their weight assessments 71 (24%) had a weight less than the third centile. Three patients were found to be obese.
Neuropsychological effects: A sequential group of 60 survivors of acute lymphocytic leukaemia underwent an IQ assessment and psychosocial assessment. The age at evaluation was 11.7±4.9 yr; and age at diagnosis was 6.3±3 years. Only one patient had mental retardation. The child with mental retardation was an ALL survivor who was diagnosed at 3.5 yr of age and evaluated five years after therapy. He had received prophylactic cranial radiation in addition to intrathecal methotrexate. Behavioural abnormalities were identified. All patients were poor in arithmetic skills, memory and comprehension. Complaints from school were frequent. Thirty children were socially withdrawn, had somatic complaints such as headache, had social problems and were found to be anxious and depressed. Three children had deficits in attention and tended to day dreaming with poor school performance. Aggression was also noted in these patients and they were found to be stubborn with a tendency towards temper tantrums and frequent fighting.
Endocrine function: The gonadal function was assessed in 22 patients (HD =18, ALL=2, Langerhans cell histiocytosis =1, yolk sac tumour=1). All those tested were males who had either pubertal delay or were contemplating marriage and had received chemotherapy/radiation that could affect reproductive function. The mean age was 13.7±4.3 years. Follicle-stimulating hormone and luteinizing hormone were high in nine patients. Five of them had low testosterone suggestive of hypogonadism. All five had received cyclophosphamide for Hodgkin's lymphoma. Azoospermia was seen in one of the two tested. This patient had Hodgkin's lymphoma and was treated with MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) regimen.
Thyroid function was assessed in 110 patients. Hypothyroidism (high thyroid-stimulating hormone with normal T4/T3) was seen in only two (1.8%) patients, indicative of subclinical hypothyroidism.
Hepatic function: Of the 300 patients, six had raised liver enzymes and one patient died of liver failure. A total of 110 patients received some form of blood component therapy, and of these, 41 patients were tested for hepatitis B antigen and 22 were found to be positive. Among patients with no history of transfusion, 69 patients were tested for hepatitis B antigen and only 11 were found to be positive. The presence of HBsAg positivity was significantly associated (P <0.001) with blood transfusion.
Cardiac evaluation: No patient had symptomatic heart failure on follow up. Myocardial function was assessed in 72 patients treated with anthracycline-based regimens (cumulative anthracycline dose was 195.5±52 mg/m2). Three patients, all males were found to have mild myocardial dysfunction which was asymptomatic. Median age was 8.5 yr (7-11 yr). They had no other cause for left ventricular dysfunction (valvular heart disease/hypertension).
Second malignant neoplasm (SMN): Of the 300 patients, SMN was seen in two patients; one patient had retinoblastoma and developed Hodgkin lymphoma and the other child was a known case of retinoblastoma and he developed acute myeloid leukaemia after five years of treatment completion.
Pulmonary function testing (PFT): PFT was done in Hodgkin lymphoma survivors (n=92) at the completion of therapy and yearly intervals thereof (all of whom had an exposure to bleomycin) and a restrictive pattern was observed in six per cent (n=6) patients.
Discussion
This study provided data of 300 childhood cancer survivors. Haematological malignancies comprised almost 25 per cent of cases. The proportion of haematolymphoid malignancy to other childhood cancer was 172:128. The male-to-female ratio observed in our study was 4.4:1. The proportion of similar malignancies in another Indian study was 326:2998 with a male:female ratio of 2.7:1 (457:168). Two hundred and twenty eight patients received chemotherapy alone while 72 received chemotherapy and radiotherapy.
Twenty three per cent of the survivors had a minimal disability (growth retardation or underweight), 13 per cent had moderate disabilities needing medical attention while two per cent had major/life-threatening disabilities. This was similar to the Swiss Pediatric Oncology Group (SPOG) data with 16.5 per cent having a moderate or more disability11. It was less than another European series where 42.4 per cent had moderate or more disability, but this study had a much longer follow up of about 30 yr after diagnosis4 (Table IV).
Table IV.
Comparative table showing various series of cancer survivors
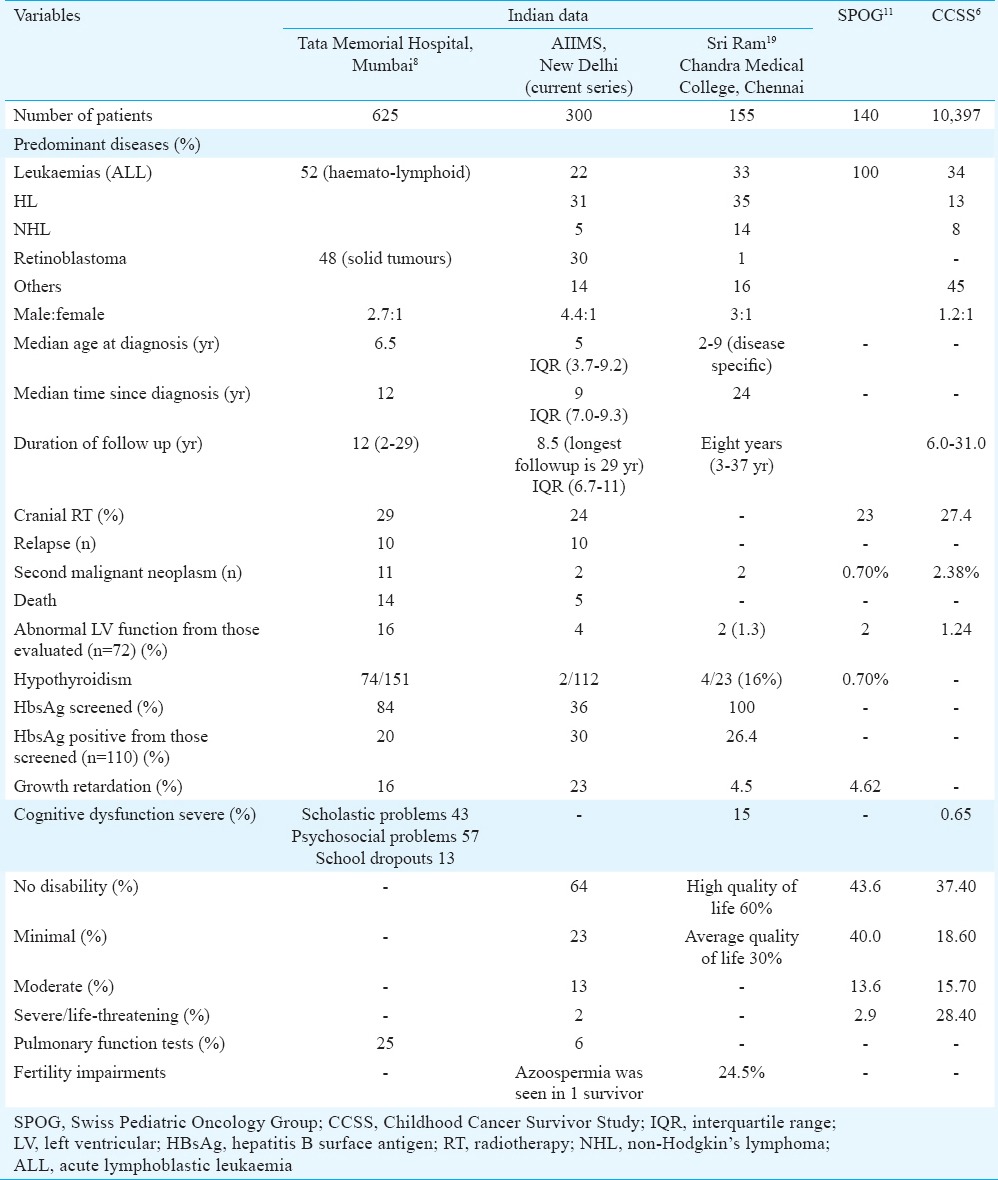
Radiation was not associated with significant hypothyroidism in our study. Subclinical hypothyroidism was seen in only 1.8 per cent patients and none of them received RT. About 50 per cent of patients in the Tata Memorial hospital study8 had hypothyroidism. In the SPOG study11, hypothyroidism was seen in 0.7 per cent. Subclinical hypothyroid in the general population in Indian children in this age group is less than two per cent26.
Long-term hepatotoxic effects following chemotherapy are not common27,28. Viral infections such as hepatitis A virus, HBV and Cytomegalovirus may be acquired during the treatment for cancer either due to immunosuppression or related to transfusions which may lead to chronic active hepatitis, fibrosis and cirrhosis. These patients may have an increased risk of developing hepatocellular carcinoma. Certain drugs such as methotrexate, actinomycin D, 6 mercaptopurine and thioguanine are also implicated as causes for chronic liver disease27,28.
The cumulative risk for SMNs at 20 yr post-treatment varies from 3-10 per cent and is 3-20 times greater when compared with the general population. In our study, two patients developed a second neoplasm. SMNs were seen in 1.9 per cent paediatric cancers in another Indian study8. Similar results were seen in other studies that showed SMNs in 0.7 and 2.38 per cent patients, respectively2,6. In a study by Hijiya et al10, 2169 patients with ALL treated for a period of 36 yr were evaluated for the occurrence of secondary neoplasms. The cumulative incidence of secondary neoplasms was 4.17 per cent at 15 yr and increased substantially after 20 yr reaching 10.85 per cent at 30 yr. Lifelong follow up of childhood cancer survivors is needed to ascertain the full impact of treatment and other related factors on the occurrence of a second neoplasm.
Neurocognitive late effects are commonly seen in cancers that require CNS-directed therapies such as cranial irradiation or intrathecal or systemic chemotherapy29. Children with CNS tumours, ALL and head and neck sarcomas are particularly vulnerable. Observed deficits include deficits in general intelligence, age appropriate developmental progress, academic achievement, visual and perceptual motor skills, memory, language and attention. In the SPOG11 study, the mean global, verbal and non-verbal IQs as a group were comparable with those found in the general population. The results of the comparison between children having and those not having received prophylactic cranial irradiation showed significantly higher scores in chemotherapy-only treated patients both for global and verbal performances6. In our study, 60 patients underwent a detailed cognitive and psychosocial evaluation. Global IQ was well preserved. Only one child had mental retardation. All patients were poor in arithmetic skills, memory and comprehension. In the study by Kurkure et al8, scholastic problems were seen in 43 per cent of childhood cancer survivors; of these, 44 per cent had received cranial irradiation. Psychosocial difficulties were seen in 57 per cent. Of these, 32 per cent received cranial irradiation.
In a study from south India19, male and female gonadal dysfunctions were seen as the common late toxic effects. Oligospermia/azoospermia was seen in 89 per cent males, and gonadal dysfunction, amenorrhea or oligomenorrhoea were seen in 78 per cent females. All had received combination chemotherapy that included alkylating agents. Dental abnormalities (caries) were seen in 16 per cent survivors. Salivary gland irradiation leading to qualitative/quantitative changes in the salivary flow was a possible cause for dental caries which was given as treatment for head and neck tumours or Hodgkin lymphoma19.
Short period of follow up was the major limitation of our study. More adult survivors of childhood cancer with longer periods of follow up will reflect the spectrum of late effects and second neoplasm in a meaningful way. Further, this was a retrospective analysis where the data were limited and all late effects might not have been covered. Chemotherapy and RT protocols for various cancers have changed over the time.
Long-term morbidity in childhood cancer survivors is an emerging concern: the challenge being to improve survival while reducing the incidence and severity of treatment-related late/long effects. Our study showed that the late effects occurred were of concern; however, severe disability (grade 3-5) was seen in only two per cent survivors. Mild growth retardation was seen in almost one-fourth and about 13 per cent needed some medical attention for other problems such as HBsAg positivity. Most of the late effects could be tackled, and the quality of life of childhood cancer survivors was optimum. Awareness towards the existence of late effects of cancer therapy is required among parents, patients and health professionals. Lifelong follow up of childhood cancer survivors is recommended to define accurately cancer-related morbidity, to facilitate timely diagnosis of secondary disease and to implement remedial or preventive interventions that optimize health outcomes.
Footnotes
Conflicts of Interest: None.
References
- 1.Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653–63. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora RS, Eden TO, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer. 2009;46:264–73. doi: 10.4103/0019-509X.55546. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al., editors. SEER Cancer Statistics Review, 1975-2003. Bethesda, MD: National Cancer Institute; 2006. Based on November 2005 SEER data submission, posted to the SEER website, 2006. Available from: http://www.seer.cancer.gov/csr/1975_2003/ [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Lackner H, Benesch M, Schagerl S, Kerbl R, Schwinger W, Urban C. Prospective evaluation of late effects after childhood cancer therapy with a follow-up over 9 years. Eur J Pediatr. 2000;159:750–8. doi: 10.1007/pl00008340. [DOI] [PubMed] [Google Scholar]
- 6.Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, et al. Medical care in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4401–9. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KD, Pandey M, Jain R, Mehta R. Cancer survivorship and models of survivorship care: A review. Am J Clin Oncol. 2015;38:627–33. doi: 10.1097/COC.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 8.Kurkure P, Achrekar S, Dalvi N, Goswami S. Childhood cancer survivors – Living beyond cure. Indian J Pediatr. 2003;70:825–8. doi: 10.1007/BF02723807. [DOI] [PubMed] [Google Scholar]
- 9.King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162–76. doi: 10.1634/theoncologist.6-2-162. [DOI] [PubMed] [Google Scholar]
- 10.Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–15. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 11.von der Weid N. Swiss Pediatric Oncology Group (SPOG). Late effects in long-term survivors of ALL in childhood: Experiences from the SPOG late effects study. Swiss Med Wkly. 2001;131:180–7. doi: 10.4414/smw.2001.09671. [DOI] [PubMed] [Google Scholar]
- 12.Nandakumar A, Anantha N, Appaji L, Swamy K, Mukherjee G, Venugopal T, et al. Descriptive epidemiology of childhood cancers in Bangalore, India. Cancer Causes Control. 1996;7:405–10. doi: 10.1007/BF00052665. [DOI] [PubMed] [Google Scholar]
- 13.Arora PR, Misra R, Mehrotra S, Mittal C, Sharma S, Bagai P, et al. Pilot initiative in India to explore the gonadal function and fertility outcomes of a cohort of childhood cancer survivors. J Hum Reprod Sci. 2016;9:90–3. doi: 10.4103/0974-1208.183508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal H, Kumar P. Late effects of treatment in survivors of retinoblastoma in India: Are we on the road to recovery? South Asian J Cancer. 2016;5:22. doi: 10.4103/2278-330X.179688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia S, Armenian SH, Armstrong GT, van Dulmen-den Broeder E, Hawkins MM, Kremer LC, et al. Collaborative research in childhood cancer survivorship: The current landscape. J Clin Oncol. 2015;33:3055–64. doi: 10.1200/JCO.2014.59.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurkure P, Prasad M, Dhamankar V, Bakshi G. Very small embryonic-like stem cells (VSELs) detected in azoospermic testicular biopsies of adult survivors of childhood cancer. Reprod Biol Endocrinol. 2015;13:122. doi: 10.1186/s12958-015-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhartiya D, Anand S, Parte S. VSELs may obviate cryobanking of gonadal tissue in cancer patients for fertility preservation. J Ovarian Res. 2015;8:75. doi: 10.1186/s13048-015-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr RD. Nutritional status in children with cancer: Before, during and after therapy. Indian J Cancer. 2015;52:173–5. doi: 10.4103/0019-509X.175827. [DOI] [PubMed] [Google Scholar]
- 19.Rajendranath R, Veeraiah S, Ramesh A, Sagar TG. Late effects of treatment in survivors of childhood cancer from a tertiary cancer center in South India. South Asian J Cancer. 2014;3:60–5. doi: 10.4103/2278-330X.126529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 21.Mallhi P, Singhi P. Screening young children for delayed development. Indian Pediatr. 1999;36:569–77. [PubMed] [Google Scholar]
- 22.Malin AJ. Malin's intelligence scale for children. Indian J Ment Retard. 1971;4:15–25. [Google Scholar]
- 23.Caruso JC. Reliable component analysis of the Stanford-Binet: Fourth edition for 2- to 6-year-olds. Psychol Assess. 2001;13:261–6. [PubMed] [Google Scholar]
- 24.Achenbach TM, Rescorla LA. Burlington, VT: University of Vermont Department of Psychiatry; 2000. Manual for the ASEBA preschool forms and profiles. [Google Scholar]
- 25.Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 26.Seshadri KG. Subclinical hypothyroidism in children. Indian J Endocr Metab. 2012;16(Suppl S2):156–8. doi: 10.4103/2230-8210.104028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sodhi JS, Wani N, Jeelani S, Geelani S, Akhtar F, Javid G, et al. Occult hepatitis B virus infection as a cause of posttransfusion hepatitis in patients with cancers. Indian J Gastroenterol. 2013;32:291–6. doi: 10.1007/s12664-013-0323-4. [DOI] [PubMed] [Google Scholar]
- 28.Power JP, El Chaar M, Temple J, Thomas M, Spillane D, Candotti D, et al. HBV reactivation after fludarabine chemotherapy identified on investigation of suspected transfusion-transmitted Hepatitis B virus. J Hepatol. 2010;53:780–7. doi: 10.1016/j.jhep.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, et al. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]


