Abstract
Background & objectives:
It has been shown that joint damage due to subclinical synovitis progresses despite apparent clinical remission in rheumatoid arthritis (RA). Hence, finding more objective methods to investigate subclinical synovitis has become a current issue. Ultrasonography (US) has been among the most investigated methods. This study was conducted to detect whether there was subclinical inflammation in RA patients in clinical remission by power Doppler ultrasonography (PDUS) and to evaluate the effects of this inflammation on upper extremity function.
Methods:
Forty five RA patients fulfilled the remission criteria of disease activity score 28 using erythrocyte sedimentation rate (DAS28-ESR), were enrolled in the study. Bilateral wrist, 2nd and 3th metacarpophalangeal and proximal interphalangeal joints and 2nd and 5th metatarsophalangeal joints were examined by PDUS. Upper extremity function was assessed with Michigan Hand Outcomes Questionnaire (MHQ) and handgrip strength. The pain was evaluated by visual analogue scale (VAS).
Results:
In 29 of 45 RA patients in clinical remission, synovitis was detected by PDUS at least in one joint. VAS and DAS28-ESR scores were significantly lower and total MHQ, some subgroup scores of MHQ (overall hand function, activity of daily living and work performance) and grip strength of the dominant hand were higher in patients with PD signal negativity.
Interpretation & conclusions:
PDUS showed a crucial role in determining the subclinical synovitis. Subclinical synovitis negatively affects the upper extremity function. Ultrasound-defined remission may be considered for good functional status and real remission in patients with RA.
Keywords: Clinical remission, functional status, Michigan Hand Outcomes Questionnaire, power Doppler ultrasonography, rheumatoid arthritis, subclinical synovitis
Rheumatoid arthritis (RA) is a chronic, inflammatory disease leading to progressive joint destruction, functional disability and increased mortality1, so remission has become the primary therapeutic goal for preventing disability and maintaining quality of life2. The current methods for evaluating remission in RA are based on composite scores, which are combinations of clinical and laboratory indicators of inflammation. However, some studies suggest that joint destruction continues despite apparent clinical remission3,4,5, and conventional clinical approaches for detection of synovitis have inadequate sensitivity4.
In the last decade, musculoskeletal ultrasonography (US) has shown added value over clinical assessment. US can be used for direct visualization and objective quantification of synovial inflammation. It has been found to be more sensitive and reliable than clinical examination in the detection of synovial hypertrophy, effusion and inflammation6,7,8. Since it also helps to determine current disease activity, it is also suggested to be used in monitoring therapy9.
Synovitis may also affect functional status of patients with RA. There are a few studies on the relationship between functional status and power Doppler ultrasonography (PDUS)6,10,11,12,13, with varied findings. The aim of this study was to investigate whether there was subclinical inflammation in patients with RA in clinical remission with PDUS and to evaluate the effects of this probable inflammation on upper extremity functional status and physical function.
Material & Methods
This study was planned as a cross-sectional study at the Physical Medicine and Rehabilitation department and Rheumatology division of Cumhuriyet University, Medical Faculty Education and Research Hospital, in Sivas, Turkey. Forty five RA patients were classified according to criteria of 1987 American College of Rheumatology (ACR)14 and 2010 ACR-European League Against Rheumatism (EULAR)15 and fulfilled the remission criteria of disease activity score 28 using erythrocyte sedimentation rate (DAS28-ESR)16, who agreed to participate in this study, were selected from the outpatient rheumatology clinics between August 2013 and February 2014. The patients in clinical remission for at least for six months were included in the study17. Patients with juvenile idiopathic arthritis and those with problems which may affect the hand grip strength, such as fracture history, upper extremity peripheral nerve injury, entrapment neuropathy and other neurological diseases were excluded. History was taken for biological (rituximab, adalimumab, etanercept, infliximab, golimumab, abatacept, certolizumab pegol, tocilizumab) and non-biological (methotrexate, leflunomide, sulphasalazine, hydroxychloroquine) disease modifying anti-rheumatic drugs (DMARDs) intake. Variables such as age and gender characteristics were recorded.
All patients underwent US examination within 30 min after the clinical evaluation. Disease activity was evaluated by DAS28-ESR and scores <2.6 were considered as remission16. Pain intensity was measured by visual analogue scale (10 cm VAS). Michigan Hand Outcomes Questionnaire (MHQ)18 was used to assess upper extremity function and disability. Hand grip strength was measured bilaterally with hydraulic hand dynamometer (Model SH5001, Saehan Corporation, Masan, Korea) after the US examination on the same day.
US Assessment: US examination was performed with LOGIQ P5 (General Electric Medical System, Korea) with a linear probe at 7-12 MHz and a Doppler frequency of 5-6.7 MHz. The wrist, 2nd and 3rd metacarpophalangeal (MCP) joints, 2nd and 3rd proximal interphalangeal (PIP) joints and the 2nd and 5th metatarsophalangeal (MTP) joints were examined bilaterally by US9 according to the EULAR19 guidelines. The wrist was examined for synovitis from the dorsal aspect; the probe was parallel to the extensor digitorum tendons (dorsomedian). Second and 3rd MCP and PIP joints were evaluated for synovitis from the palmar and dorsal aspects and 2nd and 5th MTP joints were examined only from the dorsal aspect.
Power Doppler (PD) imaging was obtained by selecting a region of interest that included the bony margins, articular space and a variable view of surrounding tissues. Pulse repetition frequency was adjusted to the lowest available value to increase sensitivity, which ranged from 500 to 750 Hz. The colour gain was set just below the level that any signal should not be visualized at the underlying bony surface, ranging between 15 and 30 dB. After the synovial tissue was detected by grey scale US in the region of interest, the degree of activation was determined with PD. The intra-articular PD signal was subjectively graded on a semiquantitative scale from 0 to 3 (0=absence, no intra-articular flow; 1=mild, single vessel signal; 2=moderate, confluent signals in less than half of the synovial area; 3=marked, signals in more than half of the synovial area)6. The patients with at least one joint involvement according to PD signal positivity were accepted as active regarding US evaluation.
The local ethics committee approved the study protocol. Informed written consent was obtained from each participant.
Power Doppler ultrasonography (PDUS) intra-observer reliability: Intra-observer reliability of PDUS assessment was evaluated by recording representative images from the full baseline examination of 45 patients in this study. The stored images were blindly read and scored for PD signal by the same expert who performed all US examinations a minimum of three months after the corresponding real time scanning6.
Statistical analysis: The Statistical Package for the Social Sciences (SPSS) version 22.0 for Windows (SPSS, IBM Corp., Armonk, NY, USA) was used for the statistical analysis. The normality of distribution of parameters was assessed by the Kolmogorov–Smirnov test. Differences between groups with respect to non-normally distributed variables were compared using the Mann–Whitney U-test and normally distributed variables were compared by Student's t test. Relationships between parameters were analyzed using Spearman's or Pearson's correlation coefficient, depending on the variable distribution. Chi-square difference test was performed for categorical data. Intra-observer agreement in US findings was calculated by the intraclass correlation coefficient (ICC) and Kappa (κ) statistics. Receiver operating characteristic (ROC) curve analysis was done to determine a new cut-off value for DAS28-ESR (instead of 2.6). Optimal cut-off values were obtained by the maximum value of sensitivity plus ‘1-specificity’.
Results
Demographic and clinical features of the 45 RA patients who fulfilled remission according to DAS28-ESR are given in Table I. Twenty one (46.7%) patients were on non-biological DMARDs (methotrexate, hydroxychloroquine, sulphasalazine, leflunomide). Four (8.9%) patients were on biological DMARDs (infliximab, etanercept, adalimumab, golimumab). Twenty (44.4%) patients were using both non-biological and biological DMARDs.
Table I.
Clinical and demographic characteristics of the rheumatoid arthritis patients in clinical remission (n=45)
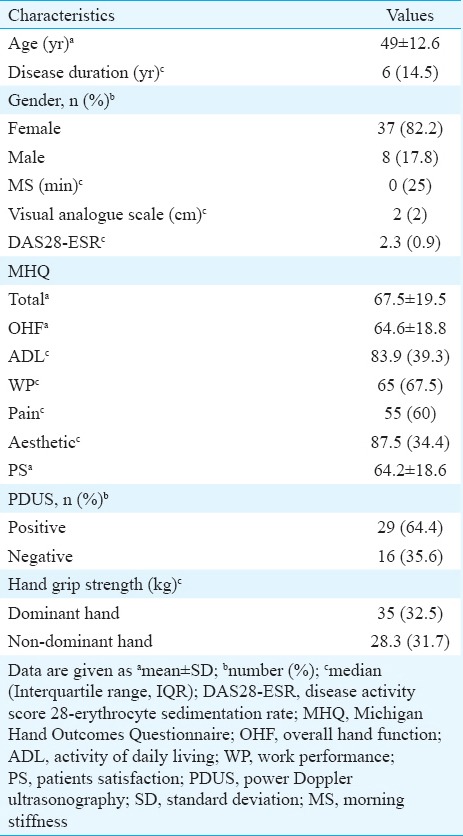
None of the patients had any active synovitis on clinical examination. Dominant hand was right in all patients. Ninety wrist, 180 MCP, 180 PIP and 180 MTP joints were evaluated by US. Synovial PD signal was detected in joints of 29 patients (64.4%) in remission according to DAS28-ESR. There was PD signal positivity in 63 joints. The most commonly affected joints were the right (n=17, 27%) and left (n=17, 27%) wrists. The least involved joints were the left (n=1, 1.6%) 3rd MCP, right (n=1, 1.6%) and left (n=1, 1.6%) 2nd PIP and the left (n=1, 1.6%) 2nd MTP. There was grade 2 PD signal positivity in only three joints (a right wrist, a left wrist and 5th MTP). The PD signal positivity was grade 1 in other active joints.
PD signal positivity in MTP joints was seen in two patients without involvement of the wrist or hand joints. The data of these two patients were not taken into account in comparing the MHQ scores in patients with or without synovitis (n=43; positive PD signal n=27, negative PD signal n=16). Four RA patients had deformity in the hand. In comparing the grip strength of patients with or without synovitis, data of these four patients with hand deformities (three with positive PD signal) and two patients with only MTP joint synovitis were not included (n=39; positive PD signal n=24, negative PD signal n=15).
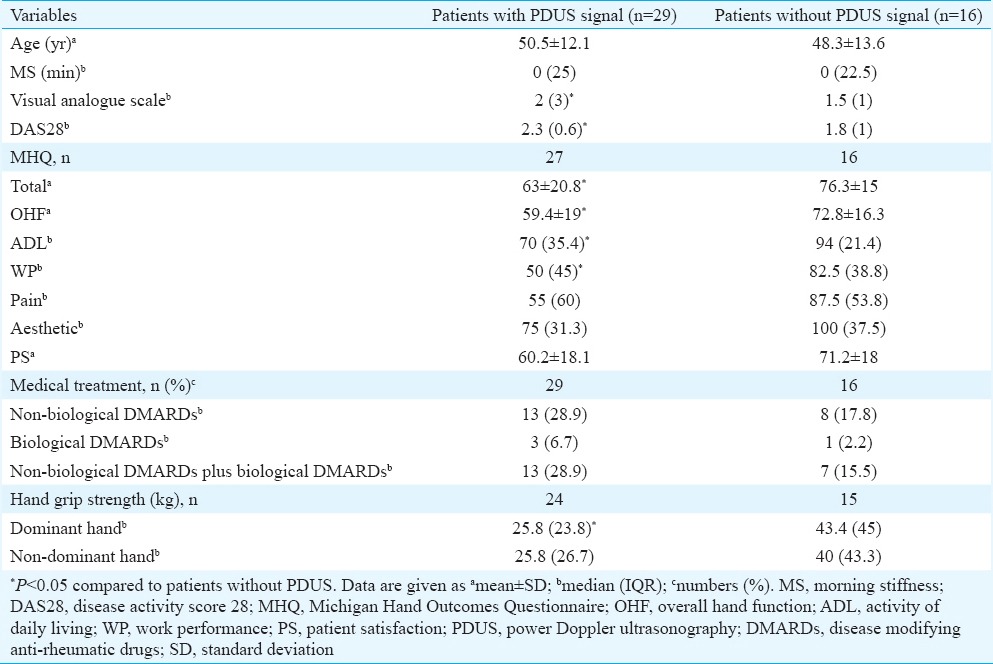
There was significant difference between the 29 patients with positive PD signals and the 16 patients with negative PD signals regarding VAS score and DAS28-ESR score (Table II). There was no difference regarding medical treatment in patients with or without synovitis according to PDUS (Table II). Total MHQ and some subgroup scores of MHQ [overall hand function (OHF), activity of daily living (ADL) and work performance (WP)] were significantly higher in patients with PD signal negativity (Table II). Grip strength of the dominant hand was found to be higher in patients with PD signal negativity (n=15). There was a significant correlation between the joint count detected by PDUS and VAS (P =0.039, r=0.309), total MHQ (P =0.002, r=−0.460) and subgroups of MHQ, namely, OHF (P =0.008, r=−0.399), ADL (P =0.008, r=−0.400), WP (P =0.009, r=−0.395) and patient satisfaction (PS) (P =0.004, r=−0.427).
Table II.
Data of the rheumatoid arthritis patients with and without power Doppler ultrasonography signal
The intra-observer ICC for the number of joints with a PD signal was 0.96 (95% CI 0.92, 0.98; P <0.001) and κ-correlation coefficient (CC) was 0.76 (P <0.001). The intra-observer ICC for the cumulative flow score (CFS) was 0.94 (95% CI 0.90, 0.97; P <0.001) and κ-CC was 0.70 (P <0.001).
ROC curve analysis was performed to determine a new cut-off value for DAS28-ESR (instead of 2.6) for evaluating activation in patients with PD signal positivity. The new cut-off value of DAS28-ESR for activation was determined to be 2.08 with a sensitivity of 0.724 and specificity of 0.625 (P =0.041, area=0.685) (Figure).
Figure.
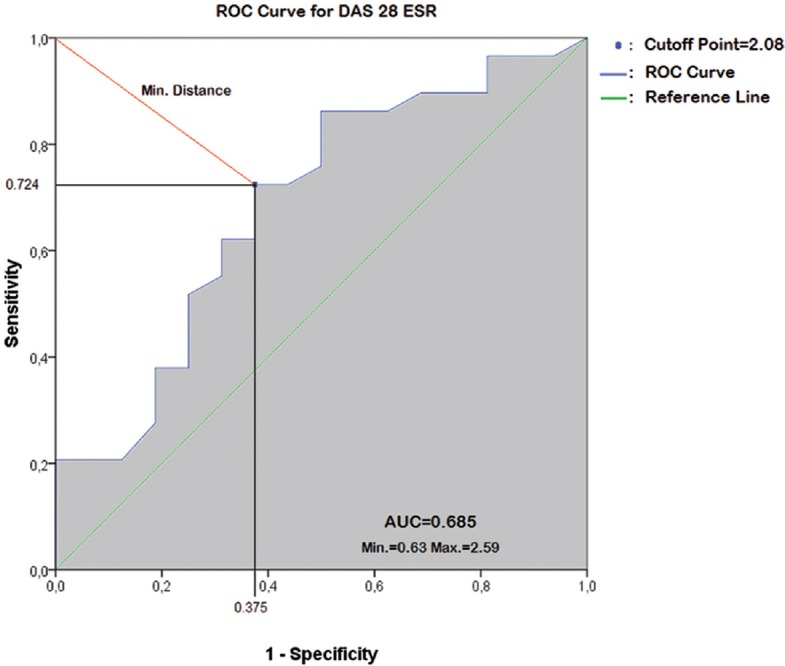
Analysis of new cut-off point value of clinical remission for disease activity score 28 using erythrocyte sedimentation rate (DAS28-ESR) in rheumatoid arthritis patients with active disease according to ultrasonography. ROC, receiver operating characteristic; Min, minimum; Max, maximum; AUC, area under curve.
Discussion
The results revealed that there was inflammation in the joints detected by PDUS in 64.4 per cent of the RA patients in clinical remission. The upper extremity functional status, physical functions, VAS and DAS28-ESR scores were also better in patients without subclinical inflammation compared to those with inflammation.
RA treatment targets suppressing inflammation for eliminating synovitis, preventing further joint destruction and disability and maintaining the quality of life2. For this purpose, clinical remission as defined by the DAS28-ESR has been commonly used as an ideal therapeutic target. DAS28-ESR <2.6 has been recommended by the EULAR and ACR as a measure of remission20. Several studies have reported that progressive radiographic damage occurs in patients who are in remission according to the DAS28-ESR4,21. The progression of radiological destruction despite the clinical remission indicates that the existing scales are not enough to evaluate the remission successfully. More objective methods are required to evaluate the synovitis and joint destruction. US is considered to be an inexpensive, harmless and easily accessible method which also enables evaluation of many joints at the same time22,23.
A considerable number of patients in clinical remission according to various clinical criteria show inflammation on US4,10,17,21,24. Geng et al24 reported that it would be better to use more than one composite remission index at the same time to eliminate the subclinical synovitis.
In the present study, synovitis by PD was detected in 64.4 per cent patients with RA. Our results indicate that subclinical synovitis that is responsible for bone damage is ongoing in majority of RA patients achieving clinical remission. It may be suggested that not only clinical remission but also remission by imaging should be targeted. It has also been suggested to compose assessment criteria by involving US evaluations along with clinical scores such as US-DAS25.
In our study, VAS and DAS28-ESR scores were found to be significantly higher in patients with synovitis according to PDUS. Balsa et al26 accepted remission according to PD signal negativity and found SDAI scores significantly higher in active patients whereas there was no difference in DAS28 scores and reported SDAI to be superior to DAS28 in defining clinical remission. In another study where both clinically active and inactive patients with RA were involved, a correlation between PDUS and VAS scores was observed but relation between PDUS and activity of the disease was not mentioned6. Our study also showed a correlation between VAS and inflamed joint count detected by PDUS but we did not find a correlation between DAS28-ESR and inflamed joint count.
A new cut-off value for DAS28-ESR was determined as 2.08 for remission in patients according to PDUS signal activity. When this cut-off was taken into consideration instead of 2.6, there were still PDUS positivity in eight (47.05%) of the 17 patients accepted to be in remission. In a study27 where more stringent DAS28 criteria were used, 32 patients had a DAS28 <1.17 but eight (25%) had still significant PD activity. This shows that no matter how strict the available remission criteria are, these are not good enough to eliminate subclinical inflammation.
Achieving and maintaining a good functional status is an essential part of remission in RA patients2,21. In a study by Ozgocmen et al11, a significant correlation of CFS was reported with Duruoz's Hand Index, Hand Function Test and Health Assessment Questionnaire (HAQ). However, they included both clinically active and inactive patients in their study whereas only clinically inactive (according to DAS 28-ESR) patients were included in the present study. Some studies found no relationship of PDUS with functional status6,10,12. One study revealed a negative13 whereas another study revealed a positive correlation28 between PDUS parameters and HAQ.
In the present study, we evaluated the functions of upper extremity which is more frequently involved and has an important role in activities of daily living by MHQ and grip strength. Upper extremity functional status was found to be better in patients satisfying remission according both to DAS28-ESR and PDUS evaluation. There was also a correlation between the total MHQ score, the subgroups of MHQ, namely, the OHF, ADL, WP and PS scores with the active joint count by PDUS. Taking these results into consideration, it may be suggested that functional status is also related to subclinical synovitis and can be an indicator of it. Accordingly, cut-off values to determine the subclinical synovitis for MHQ and grip strength may also be defined in studies with more number of patients in remission according to PDUS. These cut-off values may be used in clinics instead of US where it is not available. While determining remission in patients with RA, tests evaluating functional condition and physical functions are required.
Our study had several limitations. First, we assessed intra-observer reliability on recorded images in place of real time evaluation of the same patients. Second, there were some limitations in the use of PDUS such as the absence of technical standardization in PDUS parameters, the utilization of semiquantitative scoring method, impressionability by pathologies in periarticular structures (tenosynovitis, bursitis) and patients and performers movements. The training and experience of the user and the quality of the US machine are also important for PDUS measurements29,30.
In conclusion, PDUS showed a crucial role in determining the subclinical synovitisin RA patients. This subclinical synovitis also negatively affects the upper extremity functions. Hence, tests which evaluate upper extremity functional status and physical functions should be considered into daily practice. Ultrasound-defined remission may be considered for good functional status and real remission in patients with RA.
Footnotes
Conflicts of Interest: None.
References
- 1.Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–72. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, Dijkmans BA. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi: 10.1002/art.11481. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimi R, Hama M, Takase K, Ihata A, Kishimoto D, Terauchi K, et al. Ultrasonography is a potent tool for the prediction of progressive joint destruction during clinical remission of rheumatoid arthritis. Mod Rheumatol. 2013;23:456–65. doi: 10.1007/s10165-012-0690-1. [DOI] [PubMed] [Google Scholar]
- 5.Mulherin D, Fitzgerald O, Bresnihan B. Clinical improvement and radiological deterioration in rheumatoid arthritis: Evidence that the pathogenesis of synovial inflammation and articular erosion may differ. Br J Rheumatol. 1996;35:1263–8. doi: 10.1093/rheumatology/35.12.1263. [DOI] [PubMed] [Google Scholar]
- 6.Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A. Assessment of inflammatory activity in rheumatoid arthritis: A comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis. 2005;64:375–81. doi: 10.1136/ard.2004.023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung PP, Dougados M, Gossec L. Reliability of ultrasonography to detect synovitis in rheumatoid arthritis: A systematic literature review of 35 studies (1,415 patients) Arthritis Care Res (Hoboken) 2010;62:323–34. doi: 10.1002/acr.20102. [DOI] [PubMed] [Google Scholar]
- 8.Kane D, Balint PV, Sturrock RD. Ultrasonography is superior to clinical examination in the detection and localization of knee joint effusion in rheumatoid arthritis. J Rheumatol. 2003;30:966–71. [PubMed] [Google Scholar]
- 9.Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: A pilot project. Arthritis Rheum. 2009;61:1194–201. doi: 10.1002/art.24646. [DOI] [PubMed] [Google Scholar]
- 10.Naredo E, Valor L, De la Torre I, Martínez-Barrio J, Hinojosa M, Aramburu F, et al. Ultrasound joint inflammation in rheumatoid arthritis in clinical remission: How many and which joints should be assessed? Arthritis Care Res (Hoboken) 2013;65:512–7. doi: 10.1002/acr.21869. [DOI] [PubMed] [Google Scholar]
- 11.Ozgocmen S, Ozdemir H, Kiris A, Bozgeyik Z, Ardicoglu O. Clinical evaluation and power Doppler sonography in rheumatoid arthritis: Evidence for ongoing synovial inflammation in clinical remission. South Med J. 2008;101:240–5. doi: 10.1097/SMJ.0b013e318164e16a. [DOI] [PubMed] [Google Scholar]
- 12.Dejaco C, Duftner C, Wipfler-Freissmuth E, Weiss H, Graninger WB, Schirmer M. Ultrasound-defined remission and active disease in rheumatoid arthritis: Association with clinical and serologic parameters. Semin Arthritis Rheum. 2012;41:761–7. doi: 10.1016/j.semarthrit.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Chen J, Li F, Xie X, Du J, Mao N, et al. Grey scale and power Doppler ultrasonographic assessment of bone erosion and disease activity in early rheumatoid arthritis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38:1270–4. doi: 10.3969/j.issn.1672-7347.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 16.Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: Agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford) 2004;43:1252–5. doi: 10.1093/rheumatology/keh297. [DOI] [PubMed] [Google Scholar]
- 17.Spinella A, Sandri G, Carpenito G, Belletti L, Mascia MT. The discrepancy between clinical and ultrasonographic remission in rheumatoid arthritis is not related to therapy or autoantibody status. Rheumatol Int. 2012;32:3917–21. doi: 10.1007/s00296-011-2259-2. [DOI] [PubMed] [Google Scholar]
- 18.Öksüz Ç, Akel BS, Oskay D, Leblebicioǧlu G, Hayran KM. Cross-cultural adaptation, validation, and reliability process of the Michigan Hand Outcomes Questionnaire in a Turkish population. J Hand Surg Am. 2011;36:486–92. doi: 10.1016/j.jhsa.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–9. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aletaha D, Landewe R, Karonitsch T, Bathon J, Boers M, Bombardier C, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Rheum. 2008;59:1371–7. doi: 10.1002/art.24123. [DOI] [PubMed] [Google Scholar]
- 21.Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 22.Hoving JL, Buchbinder R, Hall S, Lawler G, Coombs P, McNealy S, et al. A comparison of magnetic resonance imaging, sonography, and radiography of the hand in patients with early rheumatoid arthritis. J Rheumatol. 2004;31:663–75. [PubMed] [Google Scholar]
- 23.Szkudlarek M, Narvestad E, Klarlund M, Court-Payen M, Thomsen HS, Østergaard M. Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: Comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum. 2004;50:2103–12. doi: 10.1002/art.20333. [DOI] [PubMed] [Google Scholar]
- 24.Geng Y, Han J, Deng X, Zhang Z. Presence of power Doppler synovitis in rheumatoid arthritis patients with synthetic and/or biological disease-modifying anti-rheumatic drug-induced clinical remission: Experience from a Chinese cohort. Clin Rheumatol. 2014;33:1061–6. doi: 10.1007/s10067-014-2634-y. [DOI] [PubMed] [Google Scholar]
- 25.Damjanov N, Radunovic G, Prodanovic S, Vukovic V, Milic V, Simic Pasalic K, et al. Construct validity and reliability of ultrasound disease activity score in assessing joint inflammation in RA: Comparison with DAS-28. Rheumatology (Oxford) 2012;51:120–8. doi: 10.1093/rheumatology/ker255. [DOI] [PubMed] [Google Scholar]
- 26.Balsa A, de Miguel E, Castillo C, Peiteado D, Martín-Mola E. Superiority of SDAI over DAS-28 in assessment of remission in rheumatoid arthritis patients using power Doppler ultrasonography as a gold standard. Rheumatology (Oxford) 2010;49:683–90. doi: 10.1093/rheumatology/kep442. [DOI] [PubMed] [Google Scholar]
- 27.Saleem B, Brown AK, Keen H, Nizam S, Freeston J, Wakefield R, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis. 2011;70:792–8. doi: 10.1136/ard.2010.134445. [DOI] [PubMed] [Google Scholar]
- 28.Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: Predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57:116–24. doi: 10.1002/art.22461. [DOI] [PubMed] [Google Scholar]
- 29.Wakefield RJ, Brown AK, O’Connor PJ, Emery P. Power Doppler sonography: Improving disease activity assessment in inflammatory musculoskeletal disease. Arthritis Rheum. 2003;48:285–8. doi: 10.1002/art.10818. [DOI] [PubMed] [Google Scholar]
- 30.Bhasin S, Cheung PP. The role of power Doppler ultrasonography as disease activity marker in rheumatoid arthritis. Dis Markers. 2015;2015:325909. doi: 10.1155/2015/325909. [DOI] [PMC free article] [PubMed] [Google Scholar]


