Abstract
Background & objectives:
Balaghat district in Central India is a highly malarious district where both Plasmodium falciparum and P. vivax are prevalent. In this district, the persistence of malaria was on an increase and not responsive to intervention measures even though there was no drug resistance. This study was undertaken by conducting mass screening to determine the prevalence of malaria among particularly vulnerable tribe of Balaghat, for developing evidence-based intervention measures for malaria control in hard to reach areas.
Methods:
This prospective study was carried out during 2013-2014 by conducting mass survey of the population in 10 villages of Birsa community health centre (CHC) and 12 villages of Baihar CHC. Finger-pricked blood smears were collected from all consenting individuals with or without fever for microscopic examination.
Results:
In the febrile group, the slide positivity rate (SPR) and slide falciparum rate (SFR) were 32.4 and 28.9 per cent, respectively, with 89.4 per cent P. falciparum, while in the afebrile individuals also, the SPR and SFR were high (29 and 26%, respectively), but these were significantly lower than that of febrile group. The gametocyte carriers were significantly higher (odds ratio 1.67, 95% confidence interval 1.25-2.25, P =0.0004) in afebrile patients when compared with febrile group. Vector incrimination showed the presence of four sporozoite-positive Anopheles culicifacies out of 1953 assayed.
Interpretation & conclusions:
Plasmodium falciparum malaria was high in young children (up to 8 years) as compared to the adult in both afebrile and febrile group in Balaghat district. High prevalence of gametocyte was observed in all age groups among the afebrile cases. The identification of afebrile malaria parasitaemia is an important challenge for the malaria elimination initiatives. A strong malaria surveillance system is fundamental to both programme design and implementation.
Keywords: Afebrile parasitaemia, Central India, forest, Plasmodium falciparum, Plasmodium vivax, tribal malaria
Malaria is a major health problem in tribal and forested areas of India. About eight per cent population of India residing in tribal and forested areas contributes to 46 per cent of total malaria cases, 70 per cent of Plasmodium falciparum and 47 per cent malaria deaths in the country1. The pathogenesis of malaria is complex and clinically P. falciparum can present with a wide spectrum of signs and symptoms ranging from a fatal disease to an apparently asymptomatic infection2. Much of the knowledge about malaria infections is based on the data generated from symptomatic infections. Studies on severe or complicated malaria have been extensively undertaken because it is a major cause of malaria attributable morbidity and mortality3,4,5. In contrast, diagnosing asymptomatic malaria is difficult due to lack of clinical manifestation6.
Madhya Pradesh (Central India) is a highly malarious State in India with a substantial population at risk that exhibits extreme variation in terms of transmission settings7. The prevalence of asymptomatic Plasmodium vivax and P. falciparum infections was recorded in one village of Gadchiroli, Maharashtra. However, the study was carried out only for two months and the sample size was very small8. There is a growing interest in defining the role of asymptomatic malaria infections as asymptomatic infections are found in both high-endemic regions of the world9 as also in low-endemic areas10. Moreover, it persists inter-seasonally in areas of seasonal transmission11. An epidemiological study on the dynamics of forest malaria in Balaghat district of Central India12 revealed that both P. falciparum and P. vivax were present throughout the year and passive surveillance (PCD) was missing large number of malaria infections13. Malaria risk is not equally distributed and variation in the risk of malaria within the population is a frequently described but poorly understood phenomenon14.
Malaria control is mainly based on two tools i.e. vector control by indoor residual spray (IRS) and/or insecticide-treated bed nets (ITNs) or long-lasting insecticide-treated nets (LLINs) and chemotherapy using chloroquine/artemisinin-based combination therapy (ACT) for the treatment of P. vivax and P. falciparum, respectively15. In Balaghat district, all these tools [IRS, ITNs and ACT (artesunate +sulphadoxine pyrimethamine)] were used, yet number of malaria cases showed no reduction even though there was no drug resistance against P. falciparum12. Numerous factors contribute to the persistence and spread of malaria transmission, of which afebrile parasitaemia is considered most important. Testing, treating and tracking of afebrile parasitaemia are a major challenge as these infections go undetected and untreated. Therefore, the present study was undertaken to determine the prevalence of afebrile parasitaemia due to P. falciparum/P. vivax in Balaghat district of Madhya Pradesh, India, for developing the evidence-based strategy for malaria control in difficult and hard to reach areas.
Material & Methods
Baihar and Birsa community health centres (CHCs), a remote forested ecozone located at the border of Chhattisgarh State (CG), are primarily inhabited by Baiga, a particularly vulnerable tribe group. There are very few accredited social health activists in the villages. This prospective study was undertaken during 2013-2014 by conducting seasonal mass screening of the population in 10 villages of Birsa CHC (population 4978) and 12 villages of Baihar CHC (population 6218) of Balaghat district, Madhya Pradesh (MP), after obtaining written informed consent. These villages were selected randomly. From each selected village, every third house was visited using systematic random sampling technique. Within the selected household, all available individuals irrespective of their fever status who consented for the study were screened. The details of the study area have been reported earlier12,13. Precisely, study villages were located far from the roadside, highly scattered and interspersed with streams and their tributaries which provided numerous permanent breeding sites for mosquitoes.
Seasons were defined as spring (February-March), summer (April-June), monsoon (July-September), post-monsoon (October-November) and winter (December-January). Five mass surveys were conducted one in each season. Criteria for diagnosis of afebrile malaria were the presence of parasites in peripheral thick blood smears, an auxiliary temperature <37.5°C and absence of malaria-related symptoms. Informed written consent was obtained from each adult and from guardian of the children who were ready to participate in the study. Individuals who were malnourished or suffering from any other diseases or undertaking any kind of medical treatment were excluded. Individuals who were not residing in the study area were also excluded. The blood smears thus collected were stained with the Jaswant-Singh-Bhattacherji (JSB) stain as described earlier16. The microscopist examined 100 fields in thick smears under ×1000 magnification before declaring it negative. For quality control, 100 per cent of positive smears and 10 per cent of negative smears were blindly re-examined by a second expert. All parasite positive cases were given treatment as per the National Vector-Borne Disease Control Programme guidelines15. The Institutional Ethics Committee of the National Institute for Research in Tribal Health, Jabalpur, India, reviewed and approved the study protocol.
A. culicifacies was an efficient vector commonly prevalent in this region throughout the year, while Anopheles fluviatilis was found during post-monsoon and winter seasons. Morphologically identified specimens of A. culicifacies and A. fluviatilis were taken for the detection of malaria parasite using diagnostic polymerase chain reaction (PCR) technique (Thermal cycler ABI 2700, Applied Biosystems, Foster City, USA) at Molecular Parasitology Laboratory of National Institute for Research in Tribal Health, Jabalpur (MP). Genomic DNA of individual mosquitoes was isolated from head-thoracic and abdominal regions by phenol-chloroform method17 and eluted in 200 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) for further experimentation. PCR was performed using the oligonucleotide primers target 18s RNA gene18.
Statistical analysis: The data were double key-entered in MS Access 2003 (Microsoft Corporation, Redmond, Washington, USA) based data entry screen. Age (in yr) was re-coded as a categorical variable (<1, >1-4, >4-8, >8-14 and >14 above) before analysis. Data analysis was done using SPSS 17 for Windows (SPSS Inc., Chicago, USA). Odds ratios (OR) were computed to compare age group- and season-wise prevalence of febrile and afebrile cases using the 2×2 table. Gametocyte carriers were recorded only for P. falciparum. Slide-positive rate (SPR) was defined as the number of blood smears found positive for malaria parasites divided by the total number of blood smears examined. Slide falciparum rate (SFR) was defined as the total number of blood smears positive for P. falciparum divided by the total number of blood smears examined. P. falciparum percentage is defined as the number of P. falciparum positive cases divided by total malaria positive cases.
Results
A total of 6761 blood smears were collected from same number of individuals in mass surveys. Of these, 3748 were febrile, and 1213 of them were positive for malaria. The SPR and SFR were 32.4 and 28.9 per cent, respectively, with 89.4 per cent P. falciparum. Remaining 3013 blood smears were from afebrile individuals. The SPR and SFR were 29.2 per cent [881/3013, OR 0.86, 95% confidence interval (CI) 0.78-0.96, P =0.006] and 26.3 per cent (791/3013 OR 0.87, 95% CI 0.78-0.98 P =0.015), which were significantly lower than that of febrile individuals. The proportion of P. falciparum and P. vivax was 89.9 and 10.1 per cent, respectively, which was similar to infections observed in febrile cases (Table I). There was no difference in malaria prevalence between the two groups among males and females. However, gametocyte carriers were significantly higher (OR 1.67, 95% CI 1.25-2.25, P =0.0004) in afebrile infections (116/791) when compared with febrile patients (101/1084).
Table I.
Age group-wise prevalence of malaria in febrile and afebrile individuals in district Balaghat (2013-2014)
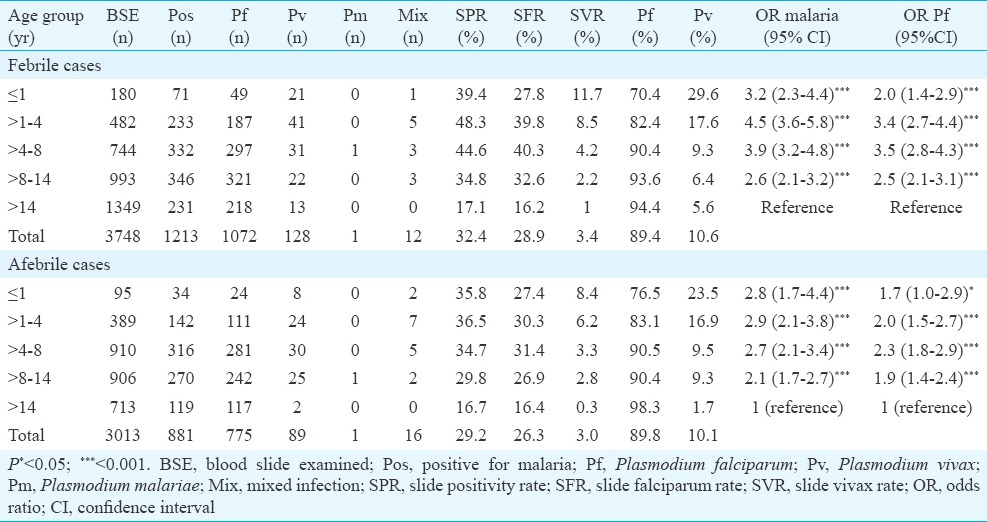
Age group-wise analysis of afebrile parasite carriers revealed (Table I) that slide vivax rate (SVR) was highest among infants (8.4%) and showed a steady declining trend with increasing age (P <0.001). SFR was higher in 1-8 yr old children and lower among greater than eight years children and this difference was significant (OR 1.55, 95% CI 1.32-1.84, P <0.001). Gametocytes prevalence was high among all age groups and peaked in 1-4 yr old young children. The difference was significant when compared with adults (OR 3.0, 95% CI 1.4-6.7, P <0.001).
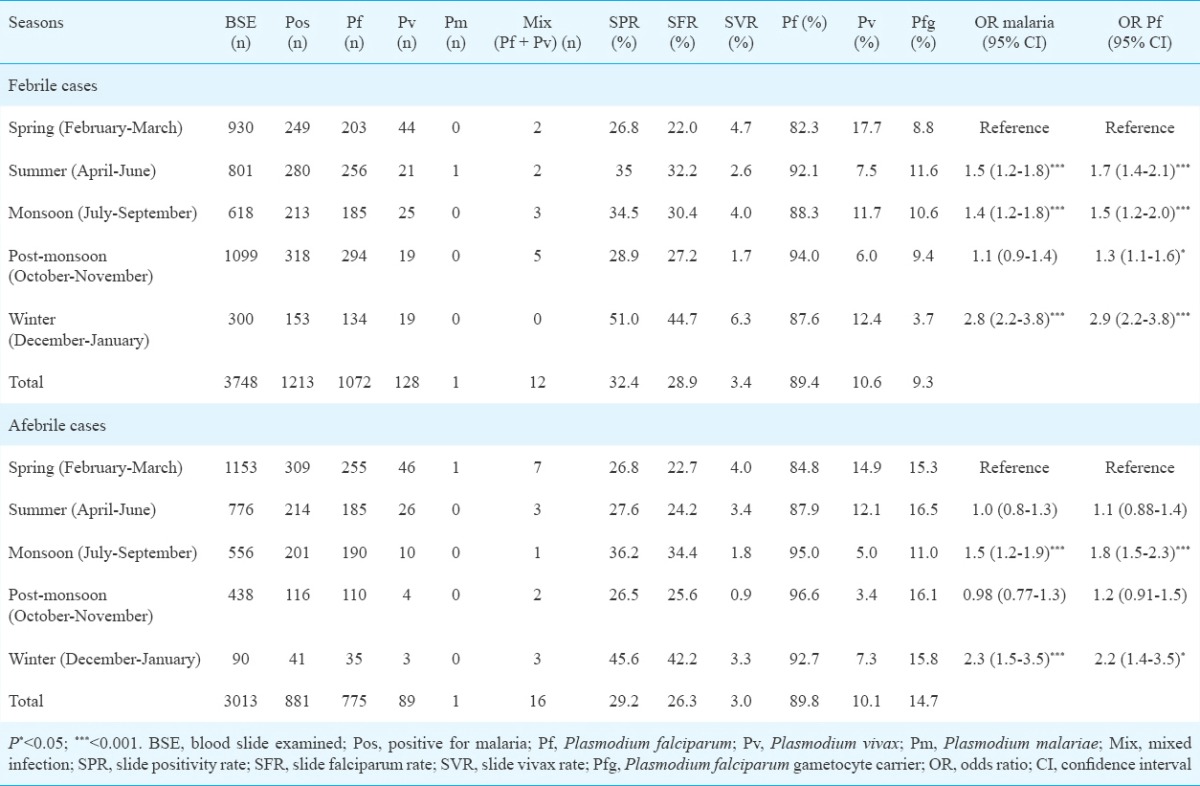
Further analysis of the distribution of afebrile malaria cases by seasons revealed that highest SPR (45.6%) was found in the winter season and lowest (26.5%) in the post-monsoon (OR 2.3, 95% CI 1.4-3.7) (Table II). While highest SVR was in the spring season (4.0%) and lowest in the post-monsoon season (0.9%), SFR was highest in winter (42.2%) and lowest in spring (22.7%). This difference was significant (OR 2.5, 95% CI 1.6-3.9, P <0.001) and the almost similar trend was shown by febrile patients (Table II). Parasite densities ranged from 207 parasites/μl to 9240 parasites/μl. The gametocyte prevalence was high in all the seasons.
Table II.
Season-wise prevalence of malaria in febrile and afebrile individuals in district Balaghat (2013-2014)
Vector incrimination showed the presence of four sporozoite-positive (2 P. vivax, 2 P. falciparum) A. culicifacies out of 1953 assayed by PCR. These sporozoite-positive vectors for P. vivax were found in March while sporozoite-positive vectors for P. falciparum were found in October and November.
Discussion
In this study, >50 per cent of the infections were reported in children less than eight years in both febrile and afebrile groups. The number of malaria cases and age group indicated that the risk for the age group under eight years was relatively higher than in adults (2.6 times in febrile group and 2.1 times in afebrile group), suggesting a considerable degree of immunity in the adults. Gametocyte rate was particularly high in children below five years as reported earlier from Africa19. A review of age pattern of malaria revealed that as transmission increased, there was a shift of malaria towards younger age groups regardless of seasonality20. In a study carried out in the Purulia district, West Bengal21, the prevalence of asymptomatic infection was >12 per cent in children less than four years which was comparable to the results of this study (20% in children up to four years), while >45 per cent asymptomatic Plasmodium species were recorded in children up to four years of age in native Amazonian populations22. These studies taken together indicate that individuals become immune to malaria as a function of the number of exposures. As A. culicifacies is an efficient endophilic vector which feed and rest indoors, hence, more children were perhaps exposed to A. culicifacies.
Comparison of malaria data between febrile and afebrile group revealed a similar trend in age- and season-wise prevalence. A study carried out in Zambia has revealed that asymptomatic malaria parasite carriers and symptomatic malaria cases are found together23. However, in most malaria endemic settings, asymptomatic infections outnumber symptomatic infections24. Thus, the success of elimination strategies relies on the techniques of finding and treating asymptomatic reservoir. The clinical consequences of asymptomatic malaria may vary across different epidemiological settings and are not fully understood10. In endemic areas, asymptomatic parasitaemia is involved in the development of partial immunity and may protect against clinical severity from new infections25. Malaria control programmes in India are primarily based on passive surveillance systems to test and treat symptomatic individuals who visit health facilities13. It is known that in areas of high transmission, large numbers of infections are afebrile, which may act as a reservoir of Plasmodium infection for malaria mosquitoes, thereby limiting malaria control measures26.
The treatment of the infective parasite reservoir of afebrile individuals may be an important intervention strategy to interrupt the transmission in highly endemic inaccessible areas. This will have direct benefits not just for the individuals but also at the community level. Without treatment, they will continue to serve as a source of malaria reservoir and enhance transmission by transmitting the malaria parasites to a large number of mosquitoes19. Intermittent preventive treatment (IPT), administration of a full course of an antimalarial treatment to a population at risk of specified time points regardless of the infection status of individuals, has been suggested as a method of treatment for asymptomatic individuals to reduce transmission of malaria6. A document by Malaria Eradication Research Agenda Consultative Group suggests that any parasite-positive subject even with low parasite density has the potential to initiate malaria transmission and thus a threat to malaria elimination efforts27.
It has been reported that asymptomatic P. falciparum infection of varying duration is cleared without treatment28. Thus, longitudinal follow up is particularly important in difficult and hard to reach areas in India to differentiate between infections that appear asymptomatic at the time of detection but may become symptomatic after the initial detection10. Screening of afebrile malaria parasite carriers is a valid, reliable and easily interpreted index for evaluating the utilization and implementation of the malaria vector control programme29.
The strength of this study was that estimates of afebrile malaria parasitaemia were based on microscopic examination of blood smears unlike many studies where afebrile infections were diagnosed by rapid diagnostic test (RDT) and PCR23,30. One major limitation was that this study was carried out in highly malarious areas of one district. Therefore, further information from areas of different transmission dynamics is required as the size of this hidden disease reservoir may vary according to transmission intensities.
In conclusion, younger age group (< 8 yr) was at a higher risk of P. falciparum malaria infection compared to adults. The treatment of the infective parasite reservoir of afebrile individuals may be an important intervention strategy to interrupt the transmission in highly endemic inaccessible areas. The strong surveillance system and appropriate use of antimalarial drug treatment in combination with adequate vector control interventions may perhaps be the most suitable methods for controlling malaria in this endemic area.
Acknowledgment
Authors acknowledge all patients for their cooperation in mass screening.
Footnotes
Conflicts of Interest: None.
References
- 1.Sharma RK, Thakor HG, Saha KB, Sonal GS, Dhariwal AC, Singh N, et al. Malaria situation in India with special reference to tribal areas. Indian J Med Res. 2015;141:537–45. doi: 10.4103/0971-5916.159510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloland PB, Ruebush TK, McCormick JB, Ayisi J, Boriga DA, Oloo AJ, et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission I. Description of study site, general methodology, and study population. Am J Trop Med Hyg. 1999;60:635–40. doi: 10.4269/ajtmh.1999.60.635. [DOI] [PubMed] [Google Scholar]
- 3.Jain V, Nagpal AC, Joel PK, Shukla M, Singh MP, Gupta RB, et al. Burden of cerebral malaria in central India (2004-2007) Am J Trop Med Hyg. 2008;79:636–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Jain V, Basak S, Bhandari S, Bharti PK, Thomas T, Singh MP, et al. Burden of complicated malaria in a densely forested Bastar region of Chhattisgarh State (Central India) PLoS One. 2014;9:e115266. doi: 10.1371/journal.pone.0115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochar DK, Shubhakaran B, Joshi A, Thanvi I, Kumawat BL. Neurological manifestations of falciparum malaria. J Assoc Physicians India. 1997;45:898–9. [PubMed] [Google Scholar]
- 6.Laishram DD, Sutton PL, Nanda N, Sharma VL, Sobti RC, Carlton JM, et al. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J. 2012;11:29. doi: 10.1186/1475-2875-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla M, Singh N, Singh MP. Spleen rates and infant parasite rates as surveillance tool for malaria control in remote hard to reach areas of central India. Malar J. 2011;10:381. doi: 10.1186/1475-2875-10-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlekar SR, Deshpande MM, Andrew RJ. Prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in tribal population of a village in Gadchiroli district of Maharashtra state, India. Biol Forum Int J. 2012;4:42–4. [Google Scholar]
- 9.Eke RA, Chigbu LN, Nwachukwu W. High prevalence of asymptomatic Plasmodium infection in a Suburb of Aba Town, Nigeria. Ann Afr Med. 2006;5:42–5. [Google Scholar]
- 10.Cucunubá ZM, Guerra AP, Rahirant SJ, Rivera JA, Cortés LJ, Nicholls RS, et al. Asymptomatic Plasmodium spp.infection in Tierralta, Colombia. Mem Inst Oswaldo Cruz. 2008;103:668–73. doi: 10.1590/s0074-02762008000700007. [DOI] [PubMed] [Google Scholar]
- 11.Babiker HA. Unstable malaria in Sudan: The influence of the dry season. Plasmodium falciparum population in the unstable malaria area of Eastern Sudan is stable and genetically complex. Trans R Soc Trop Med Hyg. 1998;92:585–9. doi: 10.1016/s0035-9203(98)90774-x. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Chand SK, Bharti PK, Singh MP, Chand G, Mishra AK, et al. Dynamics of forest malaria transmission in Balaghat district, Madhya Pradesh, India. PLoS One. 2013;8:e73730. doi: 10.1371/journal.pone.0073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh N, Bharti PK, Kumre NS. Active V. Passive surveillance for malaria in remote tribal belt of central India: Implications for malaria elimination. Pathog Glob Health. 2016;110:178–84. doi: 10.1080/20477724.2016.1223920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, et al. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201:1764–74. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- 15.National Vector Borne Disease Control Programme. National Drug Policy on Malaria. 2013. [accessed on Jan 15, 2016]. Available from: http://nvbdcp.gov.in/Doc/National-Drug-Policy-2013.pdf .
- 16.Singh J, Bhattacharji LM. Rapid staining of malarial parasites by a water soluble stain. Indian Med Gaz. 1944;79:102–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Coen E, Strachan T, Dover G. Dynamics of concerted evolution of ribosomal DNA and histone gene families in the melanogaster species subgroup of Drosophila. J Mol Biol. 1982;158:17–35. doi: 10.1016/0022-2836(82)90448-x. [DOI] [PubMed] [Google Scholar]
- 18.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 19.Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in Western Kenya. Malar J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carneiro I, Roca-Feltrer A, Griffin JT, Smith L, Tanner M, Schellenberg JA, et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: A systematic review and pooled analysis. PLoS One. 2010;5:e8988. doi: 10.1371/journal.pone.0008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguly S, Saha P, Guha SK, Biswas A, Das S, Kundu PK, et al. High prevalence of asymptomatic malaria in a tribal population in Eastern India. J Clin Microbiol. 2013;51:1439–44. doi: 10.1128/JCM.03437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP, et al. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66:641–8. doi: 10.4269/ajtmh.2002.66.641. [DOI] [PubMed] [Google Scholar]
- 23.Stresman GH, Kamanga A, Moono P, Hamapumbu H, Mharakurwa S, Kobayashi T, et al. A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in Southern Province, Zambia. Malar J. 2010;9:265. doi: 10.1186/1475-2875-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: Asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–39. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 25.Staalsoe T, Hviid L. The role of variant-specific immunity in asymptomatic malaria infections: Maintaining a fine balance. Parasitol Today. 1998;14:177–8. doi: 10.1016/s0169-4758(98)01228-9. [DOI] [PubMed] [Google Scholar]
- 26.Andrade BB, Reis-Filho A, Barros AM, Souza-Neto SM, Nogueira LL, Fukutani KF, et al. Towards a precise test for malaria diagnosis in the Brazilian Amazon: Comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malar J. 2010;9:117. doi: 10.1186/1475-2875-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The malERA Consultative Group on Diagnoses and Diagnostics. A research agenda for malaria eradication: Diagnoses and diagnostics. PLoS Med. 2011;8:e1000396. doi: 10.1371/journal.pmed.1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Missinou MA, Lell B, Kremsner PG. Uncommon asymptomatic Plasmodium falciparum infections in Gabonese children. Clin Infect Dis. 2003;36:1198–202. doi: 10.1086/374555. [DOI] [PubMed] [Google Scholar]
- 29.Orogade AA, Ogala WN, Aikhionbare H. Asymptomatic malaria parasitaemia - A suitable index for evaluation of Malaria vector control measures. Niger J Paediatr. 2002;29:23–6. [Google Scholar]
- 30.Singh N, Bharti PK, Singh MP, Singh R, Yeboah-Antwi K, Desai M, et al. What is the burden of submicroscopic malaria in pregnancy in central India? Pathog Glob Health. 2015;109:30–8. doi: 10.1179/2047773215Y.0000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]


