Abstract
Background & objectives:
Japanese encephalitis (JE) is a major public health problem in India because of high mortality rate and residual neuropsychiatric damage in the survivors. The present study was undertaken to investigate JE positivity amongst patients admitted with acute encephalitis syndrome (AES) in upper Assam districts and different parameters with their changing trends related to it.
Methods:
It was a hospital-based prospective cross-sectional study conducted from January 2012 to December 2014. A total of 1707 consecutive non-repetitive hospitalized patients, satisfying the clinical case definition of AES as per the WHO guidelines, were included in the study. Cerebrospinal fluid (CSF) and serum samples were tested for JEV-specific IgM antibodies.
Results:
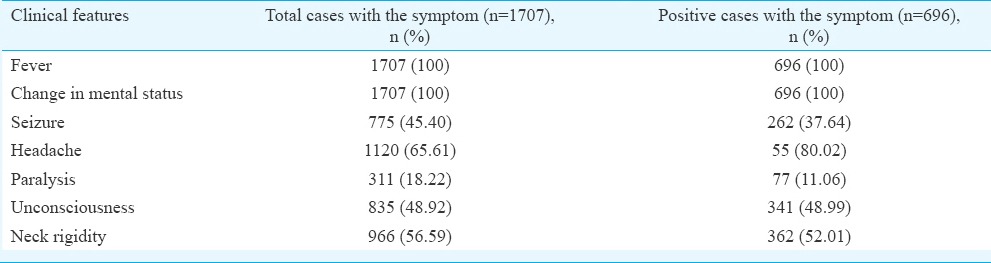
Of the 1707 patients admitted, 696 (40.77 %) were diagnosed as JE with male-to-female ratio 1.7:1 and adult to paediatric ratio 2.2:1. Fever (100%), change in mental status (100%), headache (80.02%), neck rigidity (52.01%), unconsciousness (48.99%), seizure (37.64%) and paralysis (11.06%) were the major clinical findings. The majority of cases (94%) were from rural areas. There was a significant association of JE cases with rainy season of the year i.e., June to August (P <0.001). Overall, 14.94 per cent deaths were reported in JE positive cases.
Interpretation & conclusions:
A higher occurrence of JE was observed in above 15 yr age group. Cases were mainly from rural areas, and there was clustering of cases in rainy season.
Keywords: Acute encephalitis syndrome, Assam, Japanese encephalitis, vaccination
Japanese encephalitis (JE) is a leading viral cause of acute encephalitis syndrome (AES) in Asia1. It is a mosquito-borne viral encephalitis that occurs in temperate and tropical regions of Asia and is maintained in a cycle of virus transmission between vertebrate-amplifying hosts (e.g., pigs, herons and egrets) and several Culex mosquito species2. Epidemiological data suggest that the disease primarily affects children under the age of 153.
JE virus transmission is widespread in India1. All the endemic States except Assam start reporting JE cases from July onwards, and attain a peak in September-October. In Assam, the cases start appearing from February and the peak is in the month of July4. The high case fatality rate (20-30%) and frequent residual neuropsychiatric damage in survivors (50-70%) make JE a major public health problem as about 50,000 cases and 10,000 deaths are reported each year, mostly amongst children2. There has been an increase in the disease burden and deaths due to AES including JE (8249 cases/1169 deaths, 8344 cases/1256 deaths, 7825 cases/1273 deaths and 9693 cases/1490 deaths, respectively in 2011, 2012, 2013 and 2014)5.
The present study was undertaken to investigate the JE positivity amongst AES cases in upper Assam districts during 2012 and 2014. Different parameters with their changing trend related to JE in terms of age, sex, geographical location, vaccination status, clinical presentation and seasonal variation were also studied.
Material & Methods
This hospital-based prospective cross-sectional study was conducted in the department of Microbiology, Assam Medical College and Hospital (AMCH), Dibrugarh, Assam, India, for a period of January 2012 to December 2014.
The study included all consecutive non-repetitive AES patients of different age groups and both sexes admitted to the Medicine and Pediatrics departments of AMCH as well as to the private hospitals of Dibrugarh and also the referred cases from civil hospitals of Tinsukia and Sivasagar districts. The inclusion criteria were the clinical case definition of AES as per the WHO guidelines6 according to which AES is defined as acute onset of fever and a change in mental status including symptoms such as confusion, disorientation or inability to talk and/or new onset of seizures excluding febrile convulsions in a person of any age at any time of year. Other early clinical findings may include an increase in irritability, somnolence or abnormal behaviour greater than that seen with usual febrile illness7.
Cases were reported using standard Case Investigation Form for documentation of clinical and demographic characteristics and Laboratory Request Form as per guidelines set by National Vector Borne Disease Control Programme (NVBDCP), Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India7. Patients were enrolled after obtaining informed/written consent from themselves/parents or guardians (in case of minors). Ethical clearance for the study was obtained from the Institutional Ethics Committee.
Both CSF (1-2 ml) and serum samples (2 ml) were collected under strict aseptic conditions. Only serum samples were collected in whom a lumbar puncture was not possible or was contraindicated. Blood samples were left at room temperature for 30 min for clot formation then serum was separated by centrifugation. Both serum and CSF samples were kept at 4-8°C if testing is done within 48 h, for short- and long-term storage kept in a deep freezer at −20° and at −80°C, respectively.
MAC ELISA technique was used for the detection of JE virus-specific IgM antibodies using kits acquired from ICMR-National Institute of Virology, Pune, India. Samples were reported as positive or negative or equivocal. In case of equivocal result, second serum sample from the patient was obtained 10-14 days after the first sample. The kits were supplied by NVBDCP, India.
Statistical analysis: The data were analyzed using Chi-square test to determine significance using Epi Info 7 software (Centers for Disease Control and Prevention, Atlanta, USA).
Results
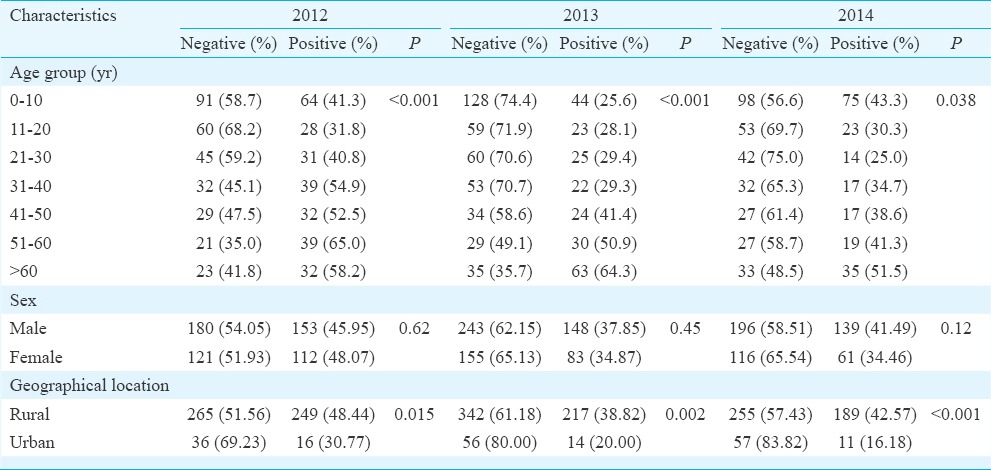
A total of 1707 clinically suspected AES cases were included in the study. Of these, 62.04 per cent were male and 37.96 per cent were female. Six hundred and ninety six (40.77 %) cases were diagnosed as JE positive. Of them, in 61.49 per cent (n=428) cases both CSF and serum, in 24.86 per cent (n=173) only CSF and in 13.65 per cent (n=95) only serum were positive. A significant year-wise difference in JE positivity was observed amongst AES cases (P <0.001). The highest number of positive cases were found in 2012 (46.82%) and lowest in 2013 (36.72%) (Table I).
Table I.
Distribution of Japanese encephalitis cases according to age group, sex and geographical location
Clinical presentation: Clinically, the JE cases were presented as viral encephalitis. Fever and change in mental status were the most common presentation and were present in all cases, followed by headache (80.02%), neck rigidity (52.01%), unconsciousness (48.99%), seizure (37.64%) and paralysis (11.06%) (Table II).
Table II.
Clinical presentation of Japanese encephalitis cases
Age, sex-wise and geographical distribution of JE-positive cases: Although the percentage of JE positivity was higher in males (63.22%) compared to females (36.78%), the difference was not significant (Table I). A significant variation was observed in the occurrence of JE with age (P <0.05) (Table I). Highest positivity was found amongst adults >51 yr in all the three years. JE cases were referred from different districts of upper Assam including Dibrugarh, Sivasagar, Tinsukia, etc. (Fig. 1), mainly from the rural parts (94%) compared to urban (Table I).
Fig. 1.
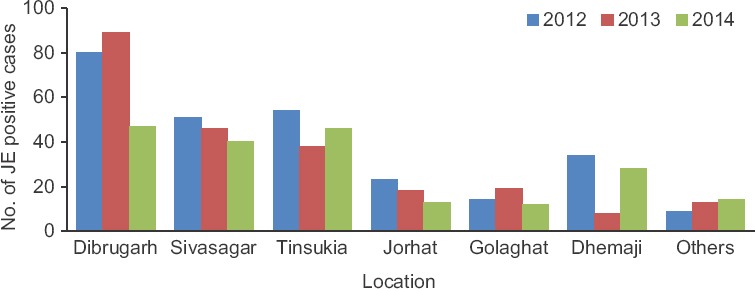
Geographical distribution of Japanese encephalitis-positive cases from 2012 to 2014.
Seasonal distribution and vaccination status: A significant association of JE cases with rainy season was observed i.e., June to August (P <0.001) (Fig. 2). Of the 1707 AES suspected cases, only 69 individuals were vaccinated with SA 14-14-2, and amongst them, only one was JE positive.
Fig. 2.
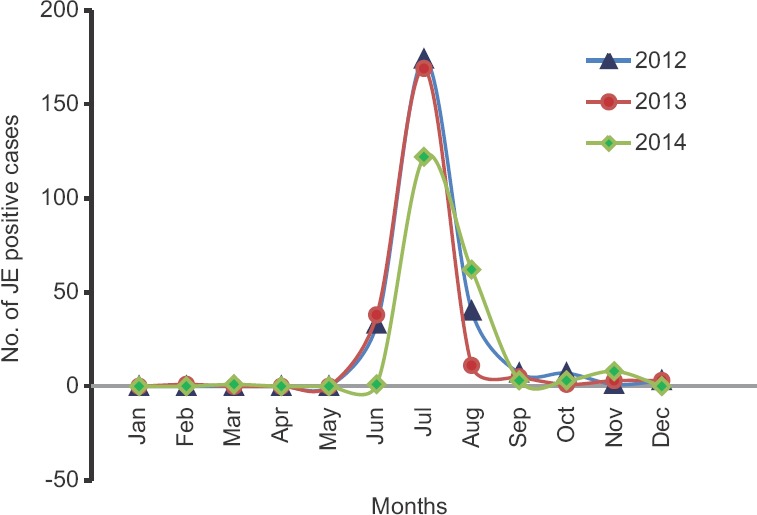
Monthly distribution of Japanese encephalitis-positive cases from 2012 to 2014.
Disease outcome: Overall 13.58 per cent mortality was recorded amongst JE cases (36 deaths in 265 cases) in 2012, 19.05 per cent (44 in 231 cases) in 2013 and 12 per cent (24 in 200 cases) in 2014. Association between patient outcome and JE positivity was significant in 2012 and 2013 (P <0.05). Mortality was found to be higher in children.
Discussion
In the present study, the most common presentation was fever similar to the observations of other authors8,9,10,11. Change in mental status was also a common feature of all cases which was comparable with earlier studies8,9.
The aetiology of AES may be multifactorial. Viruses, bacteria, Mycobacteria, Rickettsia, Toxoplasma and malaria (due to Plasmodium falciparum) may cause acute encephalitis. Viruses are the most common and important aetiological causes of acute encephalitis. JE and dengue are prevalent in South East Asia12. In our study, 40.77 per cent of AES cases were JE positive which was lower than the report of 55.36 per cent JE positivity amongst children in Karnataka13. A decline was observed in the percentage of JE cases from 46.82 in 2012 to 36.72 per cent in 2013 and 39.06 per cent in 2014.
Similar to other studies, we also observed rural predilection of JE cases10,14,15. The JE virus is particularly common in rural areas where irrigated rice fields attract the natural avian hosts and provide abundant breeding site for the vector. In urban settings, the potential for an outbreak of JE is low although transmission can occur.
The study showed clustering of cases in monsoon i.e., starting from June, the peak was in July-August similar to other studies from Assam9,16. With the onset of winter, number of JE cases declined substantially, except for a few scattered cases. Studies from different states of India14,17,18 also showed higher JE positivity during rainy season because the paddy fields covered with stagnant water serves as good breeding environment for the vector.
Although JE has been known as a paediatric disease and the same has been reported by others10,11,13, the present study showed a higher positivity in adults. This observation was similar to other studies from the same institution9,16. This may be the influence of the vaccination programme targeting children aged 1-15 yr in this area or more exposure of adults to vector mosquitoes during their outdoor activities such as cultivation. The vaccination programme was started in a phased manner in Assam in 2006 from Dibrugarh and Sivasagar districts of upper Assam. In 2007, Jorhat and Golaghat and in 2008, Tinsukia and Dhemaji districts were involved. Considering the high case load of JE in adults, vaccination campaign was also started in adult population in 201119. In this study, the average case fatality of JE cases was found to be 14.94 per cent, which was lower than reported by others9,20.
In conclusion, the study showed a high JE positivity amongst AES cases but with a declining trend over three years. It also highlighted on the association of JE with different parameters such as age, season and geographical location (rural/urban). Our study had certain limitations. Tests to detect JE in vaccinated individuals, to investigate other co-circulating Flaviviruses such as dengue, West Nile in AES cases to exclude antigenic cross-reactivity and the cross-reactive results with the JE IgM ELISA kit could not be incorporated in the present study. The JE surveillance system and the vaccination programme need to be strengthened in the state. The case management and referral system should be improved to avoid any complication and mortality.
Acknowledgment
The authors thank Dr A.K. Adhikari, Principal Cum Chief Superintendent, Assam Medical College & Hospital, Dibrugarh, Assam, for allowing the study to be done at the Department of Microbiology, AMCH, Dibrugarh, Assam. The authors acknowledge National Vector Borne Disease Control Programme (NVBDCP), Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India and National Institute of Virology (NIV), Pune, India, for supply of ELISA kits and Department of Biotechnology, Government of India for providing the infrastructure. The authors also acknowledge Drs A.K. Borthakur, Aparna Sonowal, Shrimati Sunjan Gogoi, Sarvshree Charitra Saikia, Mondeep Thapa and Subhash Das, for assistance during the course of the study.
Footnotes
Conflicts of Interest: None.
References
- 1.Joshi PL. Research priorities in Acute Encephalitis Syndrome (AES) J Commun Dis. 2014;46:86–92. [Google Scholar]
- 2.Geneva: WHO; 2003. [accessed on June 30, 2017]. World Health Organization. WHO-Recommended standards for surveillance of selected vaccine-preventable diseases. Available from: http://apps.who.int/iris/bitstream/10665/68334/1/WHO_V-B_03.01_eng.pdf . [Google Scholar]
- 3.New Delhi: WHO; 2006. [accessed on June 15, 2017]. National Institute of Communicable Diseases - World Health Organization. Guidelines for prevention and control of Japanese Encephalitis. Available from: http://nicd.nic.in/writereaddata/linkimages/je3038660088.pdf . [Google Scholar]
- 4.National Vector Borne Disease Control Programme, Government of India. Operational Guidelines National Programme for Prevention & Control of Japanese Encephalitis/Acute Encephalitis Syndrome. 2014. [accessed on June 20, 2017]. Available from: http://www.nvbdcp.gov.in/Doc/JE-AES-Prevention-Control(NPPCJA).pdf .
- 5.National Vector Borne Disease Control Programme, Government of India. Annual Report 2014-2015. [accessed on June 20, 2017]. Available from: http://www.nvbdcp.gov.in/Doc/Annual-report-NVBDCP-2014-15.pdf .
- 6.Solomon T, Thao TT, Lewthwaite P, Ooi MH, Kneen R, Dung NM, et al. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bull World Health Organ. 2008;86:178–86. doi: 10.2471/BLT.07.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Directorate of National Vector Borne Diseases Control Programme. Guidelines for Surveillance of Acute Encephalitis Syndrome (with Special Reference to Japanese Encephalitis) 2006. [accessed on May 15, 2014]. Available from: http://www.nvbdcp.gov.in/Doc/AES%20guidelines.pdf .
- 8.Sen Gupta SN, Sen MK, Das PK, Bhattacharya DP, Rath BB. Clinical profile of the epidemic of Japanese encephalitis at Bankura. Indian J Med Res. 1976;64:1393–402. [PubMed] [Google Scholar]
- 9.Patgiri SJ, Borthakur AK, Borkakoty B, Saikia L, Dutta R, Phukan SK. An appraisal of clinicopathological parameters in Japanese encephalitis and changing epidemiological trends in upper Assam, India. Indian J Pathol Microbiol. 2014;57:400–6. doi: 10.4103/0377-4929.138732. [DOI] [PubMed] [Google Scholar]
- 10.Phukan AC, Borah PK, Mahanta J. Japanese encephalitis in Assam, Northeast India. Southeast Asian J Trop Med Public Health. 2004;35:618–22. [PubMed] [Google Scholar]
- 11.Parida M, Dash PK, Tripathi NK, Ambuj, Sannarangaiah S, Saxena P, et al. Japanese encephalitis outbreak, India, 2005. Emerg Infect Dis. 2006;12:1427–30. doi: 10.3201/eid1209.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra UK, Tan CT, Kalita J. Seizures in encephalitis. Neurol Asia. 2008;13:1–13. [Google Scholar]
- 13.Avabratha SK, Sulochana P, Nirmala G, Vishwanath B, Veerashankar M, Bhagyalakshmi K. Japanese encephalitis in children in Bellary Karnataka: Clinical profile and sequelae. Int J Biomed Res. 2012;3:100–5. [Google Scholar]
- 14.Bandyopadhyay B, Bhattacharyya I, Adhikary S, Mondal S, Konar J, Dawar N, et al. Incidence of Japanese encephalitis among acute encephalitis syndrome cases in West Bengal, India. Biomed Res Int. 2013;2013:896749. doi: 10.1155/2013/896749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar A, Taraphdar D, Mukhopadhyay B, Kumar M, Mukhopadhyay S, Chatterjee S. Influence of socio-economic status and environmental factors on serologically diagnosed Japanese encephalitis cases in the state of West Bengal, India during 2005-2010. Health. 2012;4:6–12. [Google Scholar]
- 16.Borthakur AK, Das N, Bora BJ. Data from the World Health Organization (WHO) National Network laboratory for Japanese encephalitis. J Glob Infect Dis. 2013;5:76–9. doi: 10.4103/0974-777X.112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anuradha SK, Surekha YA, Sathyanarayan MS, Suresh S, Satish P, Mariraj J, et al. Epidemiological aspects of Japanese encephalitis in Bellary, Karnataka, India. Int J Biol Med Res. 2011;2:691–5. [Google Scholar]
- 18.Kumari R, Joshi PL. A review of Japanese encephalitis in Uttar Pradesh, India. WHO South East Asia J Public Health. 2012;1:374–95. doi: 10.4103/2224-3151.207040. [DOI] [PubMed] [Google Scholar]
- 19.Directorate of Health Services (Family Welfare), Government of Assam, India. Immunization. [accessed on June 29, 2017]. Available from: http://www.dhsfw.assam.gov.in/portlets/immunization .
- 20.Baruah HC, Biswas D, Patgiri D, Mahanta J. Clinical outcome and neurological sequelae in serologically confirmed cases of Japanese encephalitis patients in Assam, India. Indian Pediatr. 2002;39:1143–8. [PubMed] [Google Scholar]


