Abstract
Background
We conducted this study to examine life-course body size and physical activity, in relation to total and cause-specific mortality, which have not been studied in the low and middle-income countries in Asia.
Methods
The Golestan Cohort Study is a population-based cohort in northeastern Iran in which 50,045 people above the age of 40 have been followed since 2004. Participants were shown a validated pictogram to assess body size at ages 15, 30, and the time of recruitment. Information on occupational physical activity at these ages was also collected. Subjects were followed up annually, and cause of death was determined. Cox regression models were adjusted for age at cohort start, smoking, socioeconomic status, ethnicity, place of residence, education, and opium use. Models for body size were also adjusted for physical activity at the same age, and vice-versa.
Results
During a total of 252,740 person-years of follow-up (mean follow-up duration 5.1±1.3 years) through December 2011, 2,529 of the cohort participants died. Larger body sizes at ages 15 or 30 in both sexes were associated with increased overall mortality. Cancer mortality was more strongly associated with adolescent obesity, and cardiovascular mortality with early adulthood body size. Weight gain between these ages was associated with cardiovascular mortality. Obese adolescents who lost weight still had increased mortality from all medical causes in both sexes. Physical activity during adolescence and early adulthood had no association with mortality, but at cohort baseline higher levels of activity were associated with reduced mortality.
Conclusion
Mortality in this Middle-Eastern population was associated with obesity both during adolescence and early adult life.
Keywords: Obesity, Physical activity, Adolescence, Mortality, Cancer, Cardiovascular disease
Introduction
While the rising trend in the prevalence of obesity seems to have reached a plateau in the US [1] and some European countries [2], many low- and middle-income countries in Africa and Asia are still experiencing increasing rates of this condition, especially among children and adolescents [2]. In the year 2010, almost 43 million preschool children around the world were obese, and almost 80% of them lived in low- and middle-income countries [3]. Obesity and lack of physical activity are growing health problems in the Middle East, and in some countries around 30% of children and adolescents, especially girls, have been reported to be overweight or obese [4].
Childhood obesity can impact both childhood and adult health [5], and it is not clear whether its effect on adult morbidity and mortality is due to the higher rate of adult obesity among obese and inactive children or whether it has effects independent of adult body size [6]. Most of the evidence on the effect of obesity on mortality, cardiovascular disease and cancer come from studies in Western populations [7, 8]. Studies from Asian populations have shown a relationship between obesity and all-cause [9, 10] or cancer [11] mortality, along with increases in mortality among the underweight, which was more pronounced in India and Bangladesh [9]. These Asian studies have not included populations from the Middle East, and have not reported data on the health effects of body size in adolescence or early adult life. A recent systematic review showed that although there is considerable evidence for the long-term health effects of childhood and adolescent obesity, there is a paucity of such evidence from low- or middle-income countries [12]. There is a close relationship between the individual’s body size and level of activity [13]. The effect of physical activity on mortality has also been shown in several studies, yet there is not enough information about the effects of life-course activity on adult morbidity and mortality [14], especially in low- and middle-income countries. While some investigators have shown long-term effects of physical activity during early life on mortality and health outcomes [15], others believe that this effect fades after the age of 40 [14].
The Golestan Cohort Study has collected detailed information on lifetime exposures in a large group of the general population of Golestan Province, in northeastern Iran. In Iran, about 10% of adolescents have the metabolic syndrome [16], 23% are overweight or obese and 18% engage in low levels of physical activity [17]. In a previous publication from this study, we showed that adolescent and early adulthood obesity were associated with the risk of diabetes in adulthood [18]. In the current paper, we examine life-course body size and physical activity, in relation to total and cause-specific mortality in this large cohort.
Methods
The design of the Golestan Cohort Study (GCS) has been published before [19]. This study is a population-based cohort in northeastern Iran which has followed 50,045 people above the age of 40 since 2004. A total of 16,599 urban inhabitants older than 40 years were selected randomly from five areas of Gonbad City by systematic clustering based on household number, and 10,032 urban participants were enrolled, with participation rates of 70% for women and 50% for men. In rural areas, recruitment took advantage of the network of health houses, primary health care centers present in each group of villages. A total of 40,013 participants were enrolled from 326 villages, with participation rates of 84% for women and 70% for men. At cohort recruitment, between the years 2004 and 2008, all participants were interviewed by trained cohort staff and underwent blood pressure and anthropometric measurements. Individuals were weighed in light clothes using a standard scale. Height was measured in the full upright position, without shoes, and waist circumference was measured by a standard tape at the level of the umbilicus. BMI was calculated as weight in kilograms divided by squared height in meters. We asked about weight loss in the past year, its amount, and if it had been intentional (e.g. by diet or exercise).
The questionnaire contained detailed questions about lifetime exposures and habits. These included education, history of diseases, smoking, opium and alcohol use, and socioeconomic status. Education was defined based on the number of years spent in formal education (none, up to 8 years which is junior high school, high school, and university level). Questions about tobacco smoking, opiate use, and alcohol consumption included ever use (at least once a week for more than six months) and all dates of starting and quitting. Smoking pack-years were calculated using the data on the amount of smoking and durations, and smokers were classified based on quartiles of the calculated pack-years. To estimate the socioeconomic status, as described before [20], a composite wealth score was made using multiple correspondence analysis (MCA), based on house ownership, house size (tertiles), having a bath in the residence, and owning each of these: personal car, motorbike, television sets, refrigerator, freezer, personal computer, vacuum cleaner and washing machine. Quartiles of this wealth score were used to classify the socioeconomic status into low, middle-low, middle-high and high.
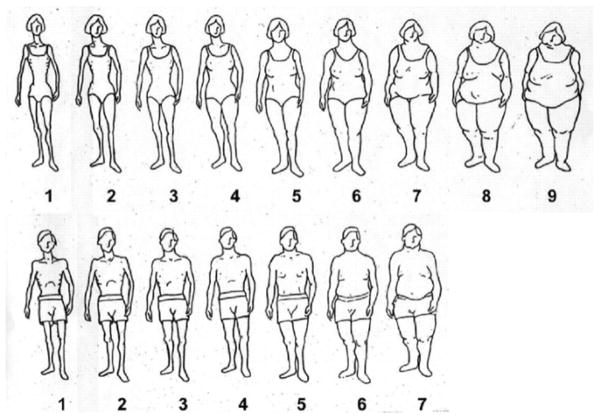
Because most people in our study population were illiterate, and lacked access to scales over most of their life, we showed each person a validated set of pictograms [21] (Figure 1) to report body size at ages 15, 30, and at the time of interview (cohort baseline). The pictures ranged from very thin (underweight) to very obese and had 7 images for men and 9 for women. We compared the pictograms at cohort baseline with the simultaneous BMI and waist circumference measurement (Table 1). The pictograms showed very good correlation with both BMI (Spearman correlation coefficient= 0.68 in women and 0.71 in men, p<0.0001), and waist circumference (Spearman correlation coefficient= 0.65 in women and 0.69 in men, p<0.0001). In both sexes, based on median BMI, pictogram 1 was defined as underweight, 2 and 3 as normal, 4 and 5 as overweight, and above 5 as obese. The last two pictograms in each sex were combined due to the relatively small number of individuals in each. Adolescent body size was defined as the picture chosen for age 15, early adulthood body size as the picture chosen for age 30, and body size at cohort baseline as the pictogram chosen by the participant at cohort recruitment. In total, 39 individuals could not identify their body size at age 15, and additionally 3 and 9 could not do so for ages 30 and at cohort baseline; these people were excluded from the corresponding analyses.
Figure 1.
Body size pictograms used in the Golestan Cohort Study.
Table 1.
Mean and median baseline BMIs and waist circumferences of the pictogram scores in the Golestan Cohort Study
| Pictogram scores | Women BMI | Women WC (cm) | Men BMI | Men WC (cm) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | Mean (SD) | Median | |
| 1 | 21.3 (3.8) | 20.8 | 80.6 (11.2) | 80 | 19.8 (2.8) | 19.5 | 78.9 (8.4) | 78 |
| 2 | 22.9 (3.7) | 22.6 | 84.3 (10.7) | 84 | 21.0 (2.8) | 20.8 | 82.2 (8.5) | 81 |
| 3 | 25.0 (3.8) | 24.8 | 89.8 (10.6) | 90 | 22.9 (3.0) | 22.8 | 88.1 (9.4) | 88 |
| 4 | 27.2 (3.8) | 27.1 | 95.0 (10.2) | 95 | 25.4 (3.2) | 25.4 | 95.4 (9.7) | 96 |
| 5 | 29.7 (4.0) | 29.8 | 101.0 (10.1) | 101 | 28.4 (3.4) | 28.3 | 103.3 (9.5) | 104 |
| 6 | 31.9 (4.7) | 31.6 | 105.7 (10.8) | 106 | 30.5 (4.1) | 30.4 | 109.0 (10.5) | 109 |
| 7 | 33.7 (5.3) | 33.3 | 109.9 (11.6) | 110 | 32.6 (5.7) | 32.4 | 113.2 (13.5) | 114 |
| 8 | 34.7 (6.0) | 34.4 | 111.7 (12.2) | 111 | - | - | - | - |
| 9 | 36.3 (7.7) | 35.6 | 114.8 (12.6) | 116 | - | - | - | - |
BMI: body mass index; WC: waist circumference; SD: standard deviation.
Participants reported their level of physical activity at work during different periods of life on a scale from 1 to 4. Level 1 was defined as sedentary work, mostly done while sitting (e.g. driving). Level 2 involved standing or occasional walking (e.g. teaching). Level 3 activities were mainly indoor activities causing mild increase in heart rate and sweating (e.g. house keeping). Level 4 activities were those causing significant elevation in heart rate and sweating, and were usually performed outdoors (e.g. farming). All work activities from childhood to cohort baseline and their start and end dates were recorded. Adolescent work activity was defined as the level of activity with the longest duration before the age of 18. Work activity level in early adulthood and at cohort baseline were defined as the level of activity with the longest duration between the ages of 18 and 30 and after age 30, respectively. If two or more activity levels had the same duration in any of these periods, the one with higher intensity was considered as the level of activity for that period. At cohort baseline, physical activity during leisure time was also recorded in the form of number of minutes per day spent in light (e.g. walking), moderate (e.g. volleyball), and vigorous (e.g. running) activities. Only 121 and 28 people reported doing moderate and vigorous leisure-time activities, respectively, so these variables were not included in the analyses. Average daily duration of light activity for each person was categorized into 30-minute intervals.
The Golestan Cohort Study was approved by the Institutional Review Boards of the Digestive Disease Research Center (DDRC), the US National Cancer Institute (NCI), and the International Agency for Research on Cancer (IARC), and all participants gave written informed consent before enrollment.
Cause of death ascertainment
Details of the Golestan Cohort Study follow-up procedures have been published before [22]. Annual follow-up has had a 99% success rate so far. Any reported death is followed by a visit from a physician who completes a verbal autopsy questionnaire, validated for this population [22], by interviewing the closest relative of the deceased. At the same time, death certificates and all available medical documents are collected. Two internists independently review all documents, including the verbal autopsy information and medical records, and determine the cause of death. The cause of death is classified according to the international classification of diseases, 10th revision (ICD-10) codes. In case of disagreement between the two internists, all documents and the two initial diagnoses are reviewed by a third more experienced internist who makes the final diagnosis. If a final diagnosis cannot be made, the cause of death is classified as “unknown”. For this analysis, causes of death were categorized as medical or external (i.e. accidents, intoxication, suicide or other types of injury). The most common medical causes of death were cardiovascular disease (including ischemic heart disease (ICD-10 codes I20-I25), cerebrovascular disease (I60-I69), and other diseases of the circulatory system), and cancer (ICD-10 codes C00-C97). Follow-up continued until the subject was lost to follow-up, death occurred, or the first of January 2012, whichever came first.
Statistical analysis
We used Cox proportional hazards models, with age as the time variable, to estimate unadjusted and adjusted hazard ratios and 95% confidence intervals for total and cause-specific mortality in relation to body size and occupational physical activity at different periods of life. Since men and women reported their body size in different scales, all the analyses were stratified by sex. Participants were left-censored at the age of enrollment, and all models (crude or adjusted) were adjusted for age at cohort baseline. The adjusted models also included potential confounders (ethnicity, place of residence (urban or rural), education, quartiles of smoking in pack-years, opium use, and socioeconomic status. These variables were selected because they have been shown to affect mortality in general, or in this population [23]. Models for body size were also adjusted for physical activity at the same age, and vice-versa. We originally included number of pregnancies for women in the model, but since it made no change in the estimates, we decided to exclude it. We also tested an interaction term for body size and smoking in the models, but since this did not show a significant result in any of the models, we did not include it in the final models, and only used the main effects.
Because body size and physical activity at cohort enrollment could have been affected by diseases present at baseline, all models including these variables were adjusted for unintentional weight loss during the year before recruitment. Two kinds of sensitivity analyses were also done when analyzing these variables: excluding deaths during the first year after the study start, and excluding individuals having a history of chronic medical conditions (i.e. ischemic heart disease, heart failure, cerebrovascular disease, hypertension, diabetes mellitus, chronic pulmonary disease, and cancer) at cohort baseline. Because smoking may reduce body weight, we repeated all the analyses involving body size at cohort baseline for lifetime non-smokers, and all the analyses involving body size at age 30 for those who did not smoke at or before this age (i.e. non-smokers and smokers who started after 30).
Results
Out of 50,006 people who had valid recall of their adolescent body size, 10,428 (20.9%) were underweight, and 6,838 (13.7%) were obese (pictograms above 5) at the age of 15. Baseline characteristics of these groups are summarized in Table 2.
Table 2.
Baseline characteristics of Golestan Cohort Study participants according to pictogram score at age 15
| Body size in adolescence | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Underweight (n= 10,428) | Normal (n= 21,533) | Overweight (n=11,207) | Obese (n=6838) | Total | ||
| sex | female | 8,272 (79.3) | 9,616 (44.7) | 5,224 (46.6) | 5,689 (83.2) | 28,801 (57.6) |
| male | 2,156 (20.7) | 11,917 (55.3) | 5,983 (53.4) | 1,149 (16.8) | 21,205 (42.4) | |
|
| ||||||
| Age a | 51.6 (8.5) | 51.3 (8.9) | 52.5 (9.2) | 54.9 (9.4) | 52.1 (9.0) | |
|
| ||||||
| Ethnicity | Turkmen | 7,295 (70.0) | 16,193 (75.2) | 8,584 (76.6) | 5,153 (75.4) | 37,225 (74.4) |
| non-turkmen | 3,133 (30.0) | 5,340 (24.8) | 2,623 (23.4) | 1,685 (24.6) | 12,781 (25.6) | |
|
| ||||||
| residence | urban | 2,540 (24.4) | 4,785 (22.2) | 1,855 (16.6) | 849 (12.4) | 10,029 (20.1) |
| rural | 7,888 (75.6) | 16,748 (77.8) | 9,352 (83.4) | 5,989 (87.6) | 39,977 (79.9) | |
|
| ||||||
| education | none | 8,240 (79.0) | 12,862 (59.7) | 7,607 (67.9) | 6,375 (93.2) | 35,084 (70.2) |
| Up to 8 years | 1,695 (16.3) | 5,911 (27.5) | 2,676 (23.9) | 421 (6.2) | 10,703 (21.4) | |
| High school | 396 (3.8) | 2,014 (9.4) | 712 (6.4) | 33 (0.5) | 3,155 (6.3) | |
| University | 97 (0.9) | 746 (3.5) | 212 (1.9) | 9 (0.1) | 1,064 (2.1) | |
|
| ||||||
| Smoking pack-year | Non-smoker | 9,298 (89.2) | 16,032 (74.5) | 7,954 (71.0) | 5,931 (86.7) | 39,215 (78.4) |
| Q1 (≤3) | 382 (3.7) | 1,344 (6.2) | 765 (6.8) | 353 (5.2) | 2,844 (5.7) | |
| Q2 (3–10) | 245 (2.3) | 1,386 (6.4) | 732 (6.5) | 213 (3.1) | 2,576 (5.2) | |
| Q3 (10–24) | 248 (2.4) | 1,462 (6.8) | 886 (7.9) | 167 (2.4) | 2,763 (5.5) | |
| Q4 (>24) | 255 (2.4) | 1,309 (6.1) | 870 (7.8) | 174 (2.5) | 2,608 (5.2) | |
|
| ||||||
| Opium use | 982 (9.4) | 3,802 (17.7) | 2,524 (22.5) | 1,180 (17.3) | 8,488 (17.0) | |
|
| ||||||
| Socioeconomic status | Low | 2,674 (25.6) | 5,196 (24.1) | 3,307 (29.5) | 2,741 (40.1) | 13,918 (27.8) |
| Low-middle | 2,208 (21.2) | 4,571 (21.2) | 2,619 (23.4) | 1,735 (25.4) | 11,133 (22.3) | |
| High-middle | 2,727 (26.2) | 5,583 (25.9) | 2,796 (24.9) | 1,476 (21.6) | 12,582 (25.2) | |
| High | 2,819 (27.0) | 6,183 (28.7) | 2,485 (22.2) | 886 (13.0) | 12,373 (24.7) | |
|
| ||||||
| Activity level | 1 (sedentary) | 872 (8.4) | 3,162 (14.7) | 1,343 (12.0) | 435 (6.4) | 5,812 (11.6) |
| 2 | 204 (2.0) | 563 (2.6) | 239 (2.1) | 102 (1.5) | 1,108 (2.2) | |
| 3 | 6,550 (62.8) | 8,194 (38.1) | 4,389 (39.2) | 4,478 (65.5) | 23,611 (47.2) | |
| 4 (active) | 2,802 (26.9) | 9,614 (44.6) | 5,236 (46.7) | 1,823 (26.7) | 19,475 (38.9) | |
|
| ||||||
| Body size at 30 | Underweight | 1,324 (12.7) | 440 (2.0) | 102 (0.9) | 109 (1.6) | 1,975 (3.9) |
| Normal | 6,925 (66.4) | 11,951 (55.5) | 2,248 (20.1) | 490 (7.2) | 21,614 (43.2) | |
| Overweight | 1,716 (16.5) | 8,392 (39) | 7,160 (63.9) | 2,521 (36.9) | 19,789 (39.6) | |
| Obese | 462 (4.4) | 749 (3.5) | 1,696 (15.1) | 3,718 (54.4) | 6,625 (13.2) | |
|
| ||||||
| Body size at baseline | Underweight | 775 (7.4) | 1,104 (5.1) | 651 (5.8) | 725 (10.6) | 3,255 (6.5) |
| Normal | 2,993 (28.7) | 7,200 (33.4) | 3,683 (32.9) | 2,099 (30.7) | 15,975 (32.0) | |
| Overweight | 4301 (41.2) | 9,903 (46.0) | 4,674 (41.7) | 2,683 (39.2) | 21,561 (43.1) | |
| Obese | 2,359 (22.6) | 3,319 (15.4) | 2,198 (19.6) | 1,330 (19.5) | 9,206 (18.4) | |
|
| ||||||
| Medical history at baseline | CVD | 720 (6.9) | 1,162 (5.4) | 699 (6.2) | 466 (6.8) | 3,047 (6.1) |
| Hypertension | 2,574 (24.7) | 3,607 (16.8) | 1,944 (17.3) | 1,740 (25.4) | 9,865 (19.7) | |
| DM | 922 (8.8) | 1,241 (5.8) | 712 (6.4) | 573 (8.4) | 3,448 (6.9) | |
| Cancer | 42 (0.4) | 68 (0.3) | 34 (0.3) | 15 (0.2) | 159 (0.3) | |
|
| ||||||
| Number of pregnancies* | 7.4 (3.3) | 7.1 (3.2) | 7.4 (3.3) | 8.1 (3.3) | 7.5 (3.3) | |
CVD: cardiovascular disease, DM: diabetes mellitus
Numbers show frequencies (percentage) except for age and number of pregnancies which are mean (SD)
During a total of 252,740 person-years of follow-up (mean follow-up duration 5.1±1.3 years) through December 2011, 2,529 of the cohort participants died. Mean age at death was 63.2±9.8 years. The most common causes of death was cardiovascular disease (n=1,318, 52.1%), followed by cancer (n=543, 21.5%). In 168 cases (6.7%) the cause of death was external, and in 53 (2.1%) it was unknown. The most common cardiovascular causes of death were ischemic heart disease (n=747, 56.7%) and cerebrovascular disease (n=405, 30.7%) which together accounted for 87.4% of the cardiovascular mortality.
In the fully-adjusted models, there were significant associations between each level of increase in adolescent or early adult body size and overall mortality in both sexes (p for trend<0.001) (Tables 3 and 4). The highest pictogram score during adolescence was associated with increased overall mortality in both women (HR= 1.58; 95% CI: 1.26–1.96) and men (HR=1.36; 95% CI: 1.11–1.66), compared with the normal pictogram. At age 30, similar associations were observed with overall mortality (HR= 1.44; 95% CI: 1.10–1.90 for women and HR=1.46; 95% CI: 1.16–1.82 for men). At cohort recruitment, being overweight (but not obese) was associated with reduced mortality in both sexes, which did not change after adjusting for unintentional weight loss in the year before cohort baseline. Unintentional weight loss itself was associated with increased mortality in women (HR= 1.40; 95% CI: 1.20–1.63), and to a lesser extent, in men (HR=1.15; 95% CI: 0.99–1.33).
Table 3.
Overall mortality by body size and physical activity in different ages among women in Golestan Cohort Study
| n (person-year) | deaths (MR) | crude HR (95% CI) | adjusted HR (95% CI) | |
|---|---|---|---|---|
| Body size at 15 | ||||
| 1 | 8,271 (42252) | 253 (598) | 1.04 (0.86,1.27) | 1.05 (0.86,1.28) |
|
| ||||
| 2 | 5,776 (29753) | 162 (544) | 1 | 1 |
|
| ||||
| 3 | 3,838 (19562) | 118 (603) | 1.12 (0.89,1.43) | 1.09 (0.86,1.39) |
|
| ||||
| 4 | 2,682 (13485) | 96 (711) | 1.32 (1.02,1.70)* | 1.25 (0.97,1.61) |
|
| ||||
| 5 | 2,541 (12750) | 112 (878) | 1.45 (1.14,1.84)** | 1.34 (1.05,1.71)* |
|
| ||||
| 6 | 2,191 (11171) | 99 (886) | 1.35 (1.05,1.74)** | 1.21 (0.94,1.55) |
|
| ||||
| 7 | 1,213 (6078) | 77 (1266) | 1.70 (1.30,2.24)** | 1.50 (1.14,1.97)** |
|
| ||||
| >=8 | 2,285 (11243) | 166 (1476) | 1.77 (1.42,2.21)** | 1.58 (1.26,1.96)** |
|
| ||||
| per 1 level increase | 1.06 (1.04–1.09)** | |||
|
| ||||
| Body size at 30 | ||||
| 1 | 1,677 (8705) | 51 (585) | 0.93 (0.68,1.27) | 0.93 (0.68,1.27) |
|
| ||||
| 2 | 5,792 (29847) | 178 (596) | 1 | 1 |
|
| ||||
| 3 | 6,462 (32948) | 180 (546) | 0.93 (0.76,1.14) | 0.94 (0.76,1.15) |
|
| ||||
| 4 | 5,079 (25528) | 171 (669) | 1.14 (0.92,1.40) | 1.12 (0.91,1.39) |
|
| ||||
| 5 | 4,398 (22161) | 178 (803) | 1.28 (1.04,1.58)* | 1.21 (0.98,1.49) |
|
| ||||
| 6 | 2,997 (15176) | 159 (1047) | 1.50 (1.21,1.85)** | 1.36 (1.09,1.68)* |
|
| ||||
| 7 | 1,391 (7001) | 92 (1314) | 1.57 (1.22,2.02)** | 1.42 (1.10,1.83)** |
|
| ||||
| >=8 | 1,005 (4928) | 74 (1501) | 1.55 (1.18,2.04)** | 1.44 (1.10,1.90)** |
|
| ||||
| per 1 level increase | 1.08 (1.05–1.11)** | |||
|
| ||||
| Body size at cohort baseline | ||||
| 1 | 2,272 (11712) | 167 (1425) | 1.17 (0.94,1.44) | 1.12 (0.90,1.38) |
|
| ||||
| 2 | 3,282 (16864) | 176 (1043) | 1 | 1 |
|
| ||||
| 3 | 4,749 (24430) | 185 (757) | 0.84 (0.68,1.04) | 0.91 (0.74,1.12) |
|
| ||||
| 4 | 5,643 (28322) | 156 (550) | 0.67 (0.54,0.84)** | 0.75 (0.60,0.94)* |
|
| ||||
| 5 | 5,826 (29075) | 166 (570) | 0.70 (0.57,0.87)** | 0.81 (0.65,1.00)* |
|
| ||||
| 6 | 3,901 (19912) | 112 (562) | 0.70 (0.55,0.89)** | 0.82 (0.64,1.04) |
|
| ||||
| 7 | 1,933 (9874) | 66 (668) | 0.78 (0.58,1.03) | 0.92 (0.69,1.22) |
|
| ||||
| >=8 | 1,195 (6105) | 55 (900) | 0.97 (0.71,1.31) | 1.12 (0.83,1.52) |
|
| ||||
| per 1 level increase | 0.97 (0.94–1.00) | |||
|
| ||||
| Level of work activity at 15 | ||||
| 1 | 1,637 (8910) | 53 (594) | 0.84 (0.61,1.15) | 0.93 (0.67,1.28) |
|
| ||||
| 2 | 347 (2085) | 20 (959) | 1.12 (0.70,1.79) | 1.18 (0.73,1.89) |
|
| ||||
| 3 | 22,424 (115440) | 873 (756) | 1.00 (0.83,1.19) | 1.05 (0.87,1.26) |
|
| ||||
| 4 | 4,403 (19900) | 140 (703) | 1 | 1 |
|
| ||||
| per 1 level increase | 1.01 (0.92–1.12) | |||
|
| ||||
| Level of work activity at 30 | ||||
| 1 | 171 (901) | 3 (332) | 0.41 (0.13,1.28) | 0.46 (0.15,1.45) |
|
| ||||
| 2 | 198 (1278) | 7 (547) | 0.66 (0.31,1.40) | 0.73 (0.34,1.56) |
|
| ||||
| 3 | 21,200 (110822) | 795 (717) | 0.86 (0.75,0.98)* | 0.93 (0.80,1.08) |
|
| ||||
| 4 | 7,242 (33334) | 281 (842) | 1 | 1 |
|
| ||||
| per 1 level increase | 1.11 (0.97–1.27) | |||
|
| ||||
| Level of work activity at cohort baseline | ||||
| 1 | 732 (3890) | 68 (1748) | 1.44 (1.04,1.99)* | 1.47 (1.05,2.06)* |
|
| ||||
| 2 | 391 (2130) | 33 (1549) | 1.59 (1.06,2.38)* | 1.76 (1.16,2.68)** |
|
| ||||
| 3 | 24,593 (125986) | 904 (717) | 1.05 (0.83,1.32) | 1.17 (0.92,1.49) |
|
| ||||
| 4 | 3,095 (14329) | 81 (565) | 1 | 1 |
|
| ||||
| per 1 level increase | 0.86 (0.78–0.95)* | |||
HR: hazard ratio; MR: mortality rate; Cox regression models adjusted for age, smoking, socioeconomic status, ethnicity, residence, education, opium and number of pregnancies. Body size analyses also adjusted for physical activity at the same age, and vice versa.
p<0.01,
p<0.001
Table 4.
Overall mortality by body size and physical activity at different ages among men in the Golestan Cohort Study
| n (person-year) | deaths (MR) | crude HR (95% CI) | adjusted HR (95% CI) | |
|---|---|---|---|---|
| Body size at 15 | ||||
| 1 | 2,156 (10794) | 157 (1454) | 1.18 (0.98,1.05) | 1.15 (0.94,1.39) |
|
| ||||
| 2 | 5,482 (27686) | 306 (1105) | 1 | 1 |
|
| ||||
| 3 | 6,435 (32615) | 369 (1131) | 1.05 (0.90,1.22) | 1.03 (0.89,1.20) |
|
| ||||
| 4 | 4,086 (20250) | 289 (1427) | 1.23 (1.05,1.44)* | 1.16 (0.99,1.37) |
|
| ||||
| 5 | 1,897 (9344) | 170 (1819) | 1.36 (1.13,1.64)** | 1.22 (1.01,1.47)* |
|
| ||||
| >=6 | 1,149 (5509) | 147 (2668) | 1.51 (1.24,1.85)** | 1.36 (1.11,1.66)** |
|
| ||||
| per 1 level increase | 1.05 (1.01–1.09)* | |||
|
| ||||
| Body size at 30 | ||||
| 1 | 298 (1485) | 26 (1750) | 1.40 (0.93,2.12) | 1.32 (0.87,1.99) |
|
| ||||
| 2 | 2,651 (13350) | 161 (1205) | 1 | 1 |
|
| ||||
| 3 | 6,711 (33834) | 383 (1131) | 1.01 (0.84,1.22) | 1.08 (0.90,1.30) |
|
| ||||
| 4 | 6,841 (34336) | 427 (1243) | 1.09 (0.91,1.31) | 1.16 (0.96,1.39) |
|
| ||||
| 5 | 3,472 (17232) | 295 (1711) | 1.25 (1.03,1.51)* | 1.29 (1.07,1.57)* |
|
| ||||
| >=6 | 1,235 (5975) | 146 (2443) | 1.48 (1.18,1.85)** | 1.46 (1.16,1.82)** |
|
| ||||
| per 1 level increase | 1.08 (1.04–1.13)** | |||
|
| ||||
| Body size at cohort baseline | ||||
| 1 | 984 (4954) | 141 (2846) | 1.33 (1.09,1.63)* | 1.22 (0.99,1.49) |
|
| ||||
| 2 | 3,189 (16027) | 284 (1772) | 1 | 1 |
|
| ||||
| 3 | 4,759 (23833) | 293 (1229) | 0.78 (0.66,0.92)** | 0.85 (0.72,1.00)* |
|
| ||||
| 4 | 5,729 (28615) | 321 (1121) | 0.75 (0.64,0.88)** | 0.87 (0.75,1.03) |
|
| ||||
| 5 | 4,365 (21833) | 240 (1099) | 0.76 (0.64,0.90)** | 0.93 (0.78,1.12) |
|
| ||||
| >=6 | 2,179 (10940) | 158 (1444) | 0.91 (0.75,1.10) | 1.11 (0.91,1.35) |
|
| ||||
| per 1 level increase | 0.99 (0.95–1.02) | |||
|
| ||||
| Level of work activity at 15 | ||||
| 1 | 4,176 (21414) | 249 (1162) | 0.94 (0.82,1.08) | 1.03 (0.89,1.19) |
|
| ||||
| 2 | 764 (4201) | 64 (1523) | 1.13 (0.88,1.46) | 1.19 (0.92,1.54) |
|
| ||||
| 3 | 1,197 (6515) | 87 (1335) | 1.06 (0.85,1.32) | 1.12 (0.89,1.40) |
|
| ||||
| 4 | 15,097 (74206) | 1043 (1405) | 1 | 1 |
|
| ||||
| per 1 level increase | 0.98 (0.94–1.03) | |||
|
| ||||
| Level of work activity at 30 | ||||
| 1 | 229 (1186) | 20 (1686) | 1.10 (0.70,1.71) | 1.32 (0.84,2.06) |
|
| ||||
| 2 | 2,107 (11656) | 128 (1098) | 0.90 (0.75,1.08) | 1.12 (0.90,1.39) |
|
| ||||
| 3 | 2,343 (12544) | 142 (1132) | 0.99 (0.83,1.18) | 1.05 (0.87,1.26) |
|
| ||||
| 4 | 16,555 (80950) | 1153 (1424) | 1 | 1 |
|
| ||||
| per 1 level increase | 0.94 (0.86–1.02) | |||
|
| ||||
| Level of work activity at cohort baseline | ||||
| 1 | 636 (3203) | 94 (2934) | 1.60 (1.29,1.98)** | 1.51 (1.21,1.88)** |
|
| ||||
| 2 | 2,320 (12795) | 152 (1187) | 0.93 (0.78,1.10) | 1.19 (0.96,1.46) |
|
| ||||
| 3 | 3,072 (16357) | 182 (1112) | 0.91 (0.77,1.06) | 0.95 (0.81,1.13) |
|
| ||||
| 4 | 15,206 (73981) | 1015 (1371) | 1 | 1 |
|
| ||||
| per 1 level increase | 0.89 (0.84–0.95)** | |||
HR: hazard ratio; MR: mortality rate; Cox regression models adjusted for age, smoking, socioeconomic status, ethnicity, residence, education, opium and number of pregnancies. Body size analyses also adjusted for physical activity at the same age, and vice versa.
p<0.01,
p<0.001
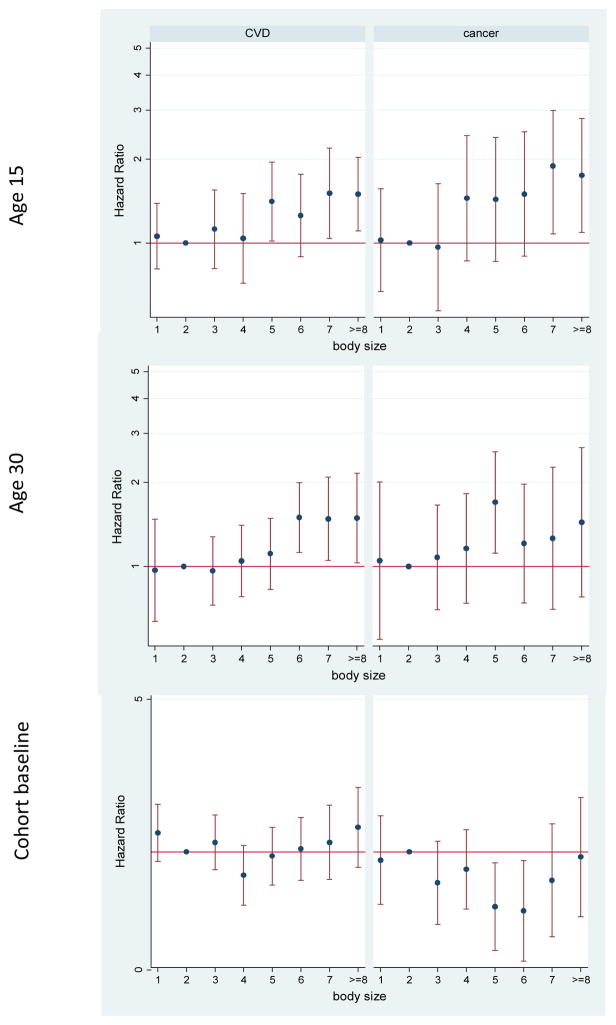
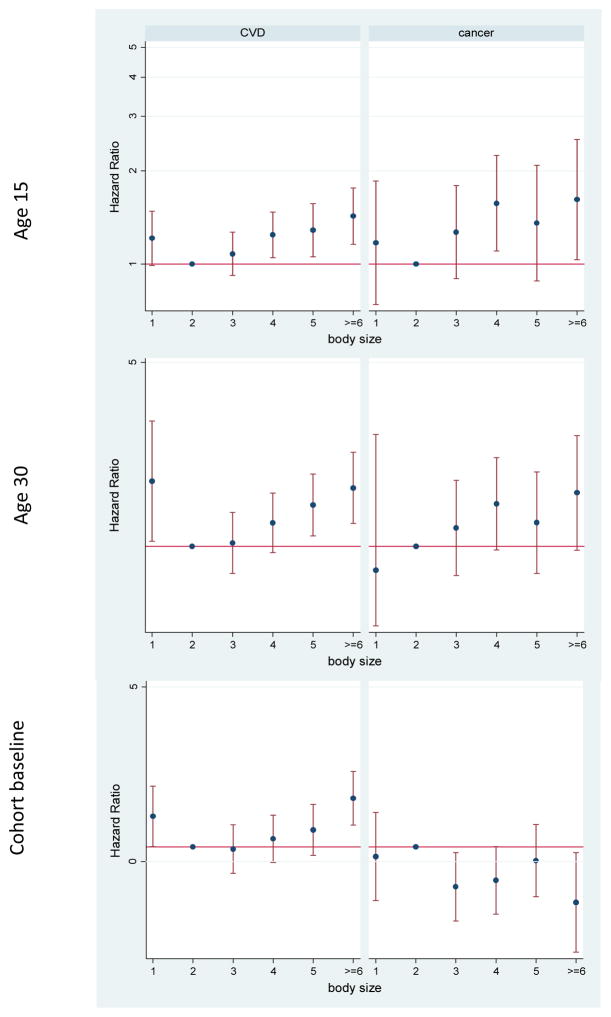
Excluding external causes of death only caused slight increases in some of the associations described above (Supplementary Table 1). The patterns of the association between body size and cause-specific mortality were different for cardiovascular disease and cancer (Figures 2 and 3). Cancer mortality was more strongly associated with adolescent obesity, and cardiovascular mortality with early adulthood body size (Supplementary Table 1). In both men and women, being overweight at cohort baseline was associated with reduced risk of cancer mortality. In addition, in men, being obese at cohort baseline was associated with a higher risk of cardiovascular mortality and a reduced risk of cancer mortality (Supplementary Table 1). In men, underweight at all ages was also associated with increased cardiovascular mortality. Since some of the associations at age 30 and cohort baseline may be confounded by smoking, we repeated the analyses in people who did not smoke at or before those ages (Supplementary Table 2). Most of the changes were seen only in men; underweight was no longer associated with cardiovascular mortality, and the reduced cancer mortality among obese and overweight men was attenuated and was not significant anymore.
Figure 2.
Adjusted hazard ratios for mortality due to cardiovascular disease (CVD) and cancer by body size in different ages among women in the Golestan Cohort Study
Figure 3.
Adjusted hazard ratios for mortality due to cardiovascular disease (CVD) and cancer by body size in different ages among men in the Golestan Cohort Study
Table 5 shows the effect of weight change on mortality due to medical causes. As the table shows, people who had been obese at either adolescence or early adulthood, including obese adolescents who lost weight, had increased mortality due to all medical causes. Cardiovascular mortality was particularly associated with weight gain between ages 15 and 30 in both sexes. Cancer mortality was still higher in female obese adolescents who lost weight. Men who were underweight at 15 and remained underweight at 30 had increased cardiovascular mortality. When we restricted the weight change analyses to people who did not smoke at or before age 30 (data not shown), this association disappeared. The other change in non-smoking men was a significantly higher cardiovascular mortality among those who had been obese at both ages (HR=1.66; 95% CI: 1.11–2.49). Cancer mortality and mortality among women were unaffected by restriction to non-smokers.
Table 5.
The effects of change in body size between the ages of 15 and 30 on cancer and cardiovascular mortality in the Golestan Cohort Study
| Body size at ages 15 and 30 | Women | Men | ||||
|---|---|---|---|---|---|---|
|
| ||||||
|
All medical causes
a HR (95% CI) |
Cardiovascular HR (95% CI) |
Cancer HR (95% CI) |
All medical causesa HR (95% CI) |
Cardiovascular HR (95% CI) |
Cancer HR (95% CI) |
|
| Underweight at both | 0.82 (0.57–1.19) | 0.89 (0.55–1.44) | 0.96 (0.49–1.91) | 1.43 (0.91–2.25) | 1.80 (1.04–3.12)* | 0.35 (0.05–2.50) |
| Underweight only at 15 b | 0.95 (0.79–1.13) | 0.98 (0.77–1.24) | 0.83 (0.58–1.20) | 1.07 (0.89–1.30) | 1.15 (0.90–1.47) | 1.03 (0.69–1.54) |
| Normal at both | 1 | 1 | 1 | 1 | 1 | 1 |
| Obese only at 15 | 1.37 (1.12–1.68)** | 1.33 (1.00–1.75) | 1.56 (1.05–2.30)* | 1.37 (1.10–1.72)* | 1.29 (0.94–1.78) | 1.23 (0.76–2.01) |
| Obese only at 30 | 1.48 (1.20–1.83)** | 1.71 (1.30–2.25)** | 0.71 (0.40–1.27) | 1.46 (1.17–1.83)** | 1.57 (1.17–2.10)** | 1.27 (0.77–2.08) |
| Obese at both | 1.36 (1.14–1.62)** | 1.41 (1.12–1.78)* | 1.30 (0.90–1.86) | 1.23 (0.93–1.61) | 1.32 (0.93–1.88) | 1.34 (0.78–2.32) |
HR: hazard ratio; Cox regression models adjusted for age, smoking, socioeconomic status, ethnicity, residence, education, and opium. Body size analyses also adjusted for physical activity at the same age, and vice versa.
Excluding accidents, suicide and all other external causes of death.
There were not enough people in the group “underweight only at 30”
p<0.01,
p<0.001
During adolescence and early adulthood, physical activity had no significant association with adult mortality rates (Tables 3 and 4). Overall mortality was higher in individuals who were less active at cohort base in both women (HR=0.86 for each level of increase in activity level; p=0.005), and men (HR=0.89 for each level of increase in activity level; p<0.001). In men, the duration of light leisure activity at cohort baseline was significantly associated with reduced mortality (HR=0.93; 95%CI: 0.91–0.96 for each 30-minute increase in daily leisure activity), while in women there was no association between leisure activity and mortality.
Supplementary Table 3 shows the results of sensitivity analyses for models involving body size and activity at cohort baseline. Since these associations can potentially be due to reverse causation, we reexamined these excluding deaths during the first year of follow-up, and excluding deaths among those with a diagnosis of chronic diseases at baseline. These analyses showed similar mortality reduction in overweight individuals and mortality increase in less active people.
Discussion
In this study we showed an increased mortality rate among people who were obese as adolescents and young adults. Cancer mortality was especially affected by adolescent obesity, while weight gain during early adult life seemed more important for cardiovascular mortality. We observed little effect for physical activity at work in this study, except for less favorable outcomes for people who were sedentary after the age of 40 (cohort baseline).
While some investigators believe that the effect of adolescent obesity on mortality is mediated through this continued trend during adult life, others have shown an independent effect [12]. There are only a few studies on mortality reporting body size at more than one time point since an early age, and most of them, similar to what we found, show some independent effect of adolescent obesity, particularly on non-cardiovascular mortality [24]. In the Norwegian health survey, Engeland et al. [25] observed increased mortality associated with adolescent obesity, which persisted in women after adjusting for adult BMI.
Obesity has been shown to increase the risk of cancer in several organs, including the adenocarcinoma of esophagus, thyroid, colon, kidney, and post-menopausal breast [26]. Our group has previously shown an independent association between adolescent body size and the increased risk of esophageal squamous cell carcinoma, the most common cause of cancer mortality in this population, which was present even in those who lost weight between age 15 and 30 [27]. Most previous studies on the effect of childhood obesity on mortality have either not reported cancer mortality or have had too few cancer deaths for accurate estimates [12]. However, those which did have large enough sample sizes showed increased risk of adult cancer death in obese children [24, 28]. The relationship between obesity and cancer may be mediated through insulin resistance, increased insulin-like growth factor-1 (IGF-1) secretion and/or growth hormone resistance in the liver [29]. Both IGF-1 and insulin promote cell proliferation and inhibit apoptosis. This mechanism might be relevant for childhood and adolescent obesity, as body fatness in adolescents has been shown to increase the risk of diabetes in adulthood in our population [18] and in the Nurses’ Health Study [30]. The accumulation of white adipose tissue (WAT) may be another way in which obesity can affect cancer. In a new line of research, animal studies have shown that WAT-derived adipose stromal cells (ASC) can be recruited by tumors and serve as adipocyte progenitors which promote tumor growth [31]. As a result of the expansion of the adipose tissue in obesity, the number of WAT-derived ASCs increases in obese individuals [32]. This process seems to be independent of the concurrent fat content of the animal’s diet [31], and we hypothesize that this mechanism may be relevant to the effects of adolescence obesity on mortality, since WAT expansion can occur over a more extended period of time.
Although several studies have shown increased risk of cardiovascular mortality and morbidity in obese children [12], a systematic review found little evidence that the effect of childhood obesity was independent of the effect of adult obesity [33]. Insulin resistance is again one of the main players, and the accompanying dyslipidemia, hypertension and vascular changes, which may not be noticed clinically during childhood, can lead to later morbidity and mortality [34]. Childhood obesity can increase carotid artery intimal thickness (a marker for atherosclerosis), and the cumulative effect of childhood obesity continued into adulthood can further contribute to this effect [35]. Our results, showing a prominent effect for early adulthood obesity on later cardiovascular mortality, and the significant effect of weight gain after adolescence on this risk, seem to support these findings in the setting of Iran, as a middle-income country in the Middle East. We also observed a higher rate of hypertension and diabetes in the adults who reported being obese as adolescents. Adolescent obesity has been projected to increase the rate of obesity among adults by 30–37% in men and 34–44% in women, this has been estimated to increase both the incidence and mortality of coronary heart disease among young adults [36].
In this population about 14% of the participants reported being obese as adolescents, but the effect of adolescent body size was not limited to those in the extreme categories; rather there was a significant increasing trend for mortality for each level of increase in body size. Previous studies have also shown a linear association between adolescenct obesity and mortality [6] and cancer incidence [37], even in BMI ranges not usually classified as overweight or obese [38]. These results may have important implications for prevention, i.e. preventive strategies must not only focus on overweight and obese children, but rather should encourage weight management strategies such as a healthy diet and physical activity for all children and adolescents.
The effect of physical activity on mortality has been studied before, and many studies seem to show a protective effect, which may be independent [39] or may be mediated through weight loss [13]. Physical activity during childhood and adolescence seems to play a modest role in adult disease, and this effect seems to be short lasting and disappear after some time [40]. In the Finnish Twin Cohort Study [15], co-twins with less physical activity had increased adult mortality, but the effect was only seen in dizygotic (and not monozygotic) twins, and so the authors could not exclude residual genetic confounding. Longitudinal studies such as the Oslo Youth Study [14], and The Amsterdam Growth and Health Longitudinal Study [41] have found no evidence for a beneficial effect of physical activity during adolescence beyond the age of 40. We also did not find a consistent relationship between earlier occupational activity and adult mortality in this population, although we did show that people who were active after the age of 40 had lower mortality. The effects of baseline leisure activity on mortality were not different from those of occupational physical activity. These findings support a modest independent protective effect of physical activity during late adult life on mortality.
Previous studies have shown a possible protective effect of being overweight in higher ages [42]. This may be due to confounding by preexisting diseases or smoking, which can both reduce body weight and increase mortality [43]. Our analyses showed that these findings persisted after excluding death during the first year, and after excluding people with any chronic condition that might lead to such confounding. Yet among non-smokers, the protective effect of being overweight at cohort baseline attenuated, and the increased cardiovascular mortality seen among underweight men was not significant. In their study of a large group of young Swedish men, Neovius et al. observed that the effect of smoking and obesity during late adolescence on subsequent mortality was not synergistic, although each was associated with an independent risk [44]. They also reported that in non-smokers, unlike smokers, even being extremely underweight was not associated with increased mortality. In contrast, the Asian Cohort Consortium reported significantly increased mortality among the underweight, even in non-smokers [9]. It should be noted, however, that we had a different pattern of cause-specific mortality than that in the Asian Cohort Consortium, where non-cancer mortality was the second cause of death (after cardiovascular disease), and the causes of such mortality, including infections, may be directly associated with malnutrition and decreased immunocompetence.
There is considerable controversy about the definition of childhood and adolescent obesity in different populations [5]. And there are only a few studies with data available on lifetime anthropometric measurements. Instead, most studies rely on the recall of body sizes, which may be biased, especially towards underreporting [45]. The pictogram is a useful method to assess body fatness during early life, and has been previously used in several large studies [46]. We showed that this method is consistent with the simultaneous measurements of BMI and waist circumference, and thus may prove valuable, particularly in similar settings where there are no recorded anthropometric measurements. Our experience shows that this method may actually be more useful than some methods recalling prior weight, particularly when the study population’s average level of education is limited, as it relies on visual rather than numeric memory of body size. The results of our pictogram analyses were consistent with both our previous understanding of adolescence obesity [18, 27], and the previous findings using the same visual method [30].
One of the advantages of this study was the availability of detailed information on many confounders. Both mortality and life-course body change have been shown to correlate with socioeconomic status [47], although there have been few similar studies which could adjust for such factors. We were also able to adjust for the cumulative amount of smoking throughout life, and tailor analyses according to the age when smoking started, because of the detailed information available on this important confounder. Also, to the best of our knowledge, this was the first study on the effect of life-course obesity on mortality which has included physical activity and its changes throughout life.
Like many similar studies, one of the limitations of our evaluation was that we had to rely on the recall of earlier body size and physical activity. While this may lead to some misclassification, it has been shown that this misclassification is usually non-differential, and lean and obese people probably show the same accuracy in remembering their childhood body size [48]. We had data on occupational activity level during earlier ages, and leisure activity was only assessed at cohort baseline. This limitation is unlikely to affect the results to a great extent, since in the lifestyle of this mainly rural population, most of the physical activity happens at work. This is reflected by the very small number of individuals engaging in moderate or vigorous leisure-time activity at cohort baseline. Left censoring of the mortality data is another limitation of our study, which may have weakened some of the associations because of their association with early mortality [42]. This may be particularly true for physical activity, whose effect has been shown to be time-dependent. On the other hand, by excluding death during younger ages, the impact of mortality due to genetic causes and structural abnormalities was reduced. In any case, our results are most relevant for people who have survived to reach 40 (our cohort’s minimum enrollment age).
This study showed that adolescent and early adult obesity is associated with increased overall mortality, and mortality due to cardiovascular disease and cancer in this Middle Eastern population. A great part of the increased mortality due to adolescent obesity may not be reversible in later years. These findings are relevant to public health professionals, educators, and policy makers, since it underscores the importance of life-course weight management. It seems that in this population there is not so much an association between underweight and mortality, unlike that observed in other Asian populations.
Supplementary Material
Acknowledgments
FUNDING
This work was supported in part by the intramural research program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute; the Digestive Disease Research Center of Tehran University of Medical Sciences [grant No 82-603]; and by the International Agency for Research on Cancer.
Footnotes
Conflicts of interest: None
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. jama.2012.39 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Rokholm B, Baker JL, Sorensen TI. The levelling off of the obesity epidemic since the year 1999--a review of evidence and perspectives. Obes Rev. 2010;11(12):835–46. doi: 10.1111/j.1467-789X.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 3.de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257–64. doi: 10.3945/ajcn.2010.29786. ajcn.2010.29786 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Mirmiran P, Sherafat-Kazemzadeh R, Jalali-Farahani S, Azizi F. Childhood obesity in the Middle East: a review. East Mediterr Health J. 2010;16(9):1009–17. [PubMed] [Google Scholar]
- 5.Lakshman R, Elks CE, Ong KK. Childhood obesity. Circulation. 2012;126(14):1770–9. doi: 10.1161/CIRCULATIONAHA.111.047738. 126/14/1770 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–37. doi: 10.1056/NEJMoa072515. 357/23/2329 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–37. doi: 10.1001/jama.298.17.2028. 298/17/2028 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364(8):719–29. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jee SH, Sull JW, Park J, Lee S, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. New Engl J Med. 2006;355(8):779–87. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 11.Parr CL, Batty GD, Lam TH, Barzi F, Fang X, Ho SC, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11(8):741–52. doi: 10.1016/S1470-2045(10)70141-8. S1470-2045(10)70141-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 2011;35(7):891–8. doi: 10.1038/ijo.2010.222. ijo2010222 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156(9):832–41. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 14.Kvaavik E, Klepp KI, Tell GS, Meyer HE, Batty GD. Physical fitness and physical activity at age 13 years as predictors of cardiovascular disease risk factors at ages 15, 25, 33, and 40 years: extended follow-up of the Oslo Youth Study. Pediatrics. 2009;123(1):e80–6. doi: 10.1542/peds.2008-1118. 123/1/e80 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Waller K, Kujala UM, Rantanen T, Kauppinen M, Silventoinen K, Koskenvuo M, et al. Physical activity, morbidity and mortality in twins: a 24-year prospective follow-up. Eur J Epidemiol. 2010;25(10):731–9. doi: 10.1007/s10654-010-9493-x. [DOI] [PubMed] [Google Scholar]
- 16.Esmaillzadeh A, Mirmiran P, Azadbakht L, Etemadi A, Azizi F. High prevalence of the metabolic syndrome in Iranian adolescents. Obesity (Silver Spring) 2006;14(3):377–82. doi: 10.1038/oby.2006.50. 14/3/377 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Hajian-Tilaki K, Heidari B. Prevalences of overweight and obesity and their association with physical activity pattern among Iranian adolescents aged 12–17 years. Public Health Nutr. 2012:1–7. doi: 10.1017/S1368980012001048. S1368980012001048 [pii] [DOI] [PMC free article] [PubMed]
- 18.Golozar A, Khademi H, Kamangar F, Poutschi H, Islami F, Abnet CC, et al. Diabetes mellitus and its correlates in an Iranian adult population. PLoS One. 2011;6(10):e26725. doi: 10.1371/journal.pone.0026725. PONE-D-11-11782 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourshams A, Khademi H, Malekshah AF, Islami F, Nouraei M, Sadjadi AR, et al. Cohort Profile: The Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol. 2010;39(1):52–9. doi: 10.1093/ije/dyp161. dyp161 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Abedi-Ardekani B, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38(4):978–88. doi: 10.1093/Ije/Dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshtkar AA, Semnani S, Pourshams A, Khademi H, Roshandel G, Boffetta P, et al. Pictogram use was validated for estimating individual’s body mass index. J Clin Epidemiol. 2010;63(6):655–9. doi: 10.1016/j.jclinepi.2009.08.014. S0895-4356(09)00266-2 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Khademi H, Etemadi A, Kamangar F, Nouraie M, Shakeri R, Abaie B, et al. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLoS One. 2010;5(6):e11183. doi: 10.1371/journal.pone.0011183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khademi H, Malekzadeh R, Pourshams A, Jafari E, Salahi R, Semnani S, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50,000 adults in Iran. BMJ. 2012;344:e2502. doi: 10.1136/bmj.e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350–5. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 25.Engeland A, Bjorge T, Tverdal A, Sogaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology. 2004;15(1):79–85. doi: 10.1097/01.ede.0000100148.40711.59. [DOI] [PubMed] [Google Scholar]
- 26.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. S0140-6736(08)60269-X [pii] [DOI] [PubMed] [Google Scholar]
- 27.Etemadi A, Golozar A, Kamangar F, Freedman ND, Shakeri R, Matthews C, et al. Large body size and sedentary lifestyle during childhood and early adulthood and esophageal squamous cell carcinoma in a high-risk population. Ann Oncol. 2012;23(6):1593–600. doi: 10.1093/annonc/mdr494. mdr494 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168(1):30–7. doi: 10.1093/aje/kwn096. kwn096 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408. nrc1408 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Yeung EH, Zhang C, Louis GM, Willett WC, Hu FB. Childhood size and life course weight characteristics in association with the risk of incident type 2 diabetes. Diabetes Care. 2010;33(6):1364–9. doi: 10.2337/dc10-0100. dc10-0100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72(20):5198–208. doi: 10.1158/0008-5472.CAN-12-0294. 72/20/5198 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Maumus M, Sengenes C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93(10):4098–106. doi: 10.1210/jc.2008-0044. jc.2008-0044 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and adult cardiovascular disease risk: a systematic review. Int J Obes (Lond) 2010;34(1):18–28. doi: 10.1038/ijo.2009.61. ijo200961 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Raghuveer G. Lifetime cardiovascular risk of childhood obesity. Am J Clin Nutr. 2010;91(5):1514S–9S. doi: 10.3945/ajcn.2010.28701D. ajcn.2010.28701D [pii] [DOI] [PubMed] [Google Scholar]
- 35.Freedman DS, Patel DA, Srinivasan SR, Chen W, Tang R, Bond MG, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008;32(5):749–56. doi: 10.1038/sj.ijo.0803798. 0803798 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357(23):2371–9. doi: 10.1056/NEJMsa073166. 357/23/2371 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Jeffreys M, Smith GD, Martin RM, Frankel S, Gunnell D. Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. Int J Cancer. 2004;112(2):348–51. doi: 10.1002/ijc.20423. [DOI] [PubMed] [Google Scholar]
- 38.van Dam RM, Willett WC, Manson JE, Hu FB. The relationship between overweight in adolescence and premature death in women. Ann Intern Med. 2006;145(2):91–7. doi: 10.7326/0003-4819-145-2-200607180-00006. 145/2/91 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–703. doi: 10.1056/NEJMoa042135. 351/26/2694 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Twisk JW, Kemper HC, van Mechelen W. Prediction of cardiovascular disease risk factors later in life by physical activity and physical fitness in youth: introduction. Int J Sports Med. 2002;23(Suppl 1):S5–7. doi: 10.1055/s-2002-28454. [DOI] [PubMed] [Google Scholar]
- 41.Twisk JW, Kemper HC, van Mechelen W. The relationship between physical fitness and physical activity during adolescence and cardiovascular disease risk factors at adult age. The Amsterdam Growth and Health Longitudinal Study. Int J Sports Med. 2002;23(Suppl 1):S8–14. doi: 10.1055/s-2002-28455. [DOI] [PubMed] [Google Scholar]
- 42.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163(10):938–49. doi: 10.1093/aje/kwj114. kwj114 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–78. doi: 10.1056/NEJMoa055643. NEJMoa055643 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Neovius M, Sundstrom J, Rasmussen F. Combined effects of overweight and smoking in late adolescence on subsequent mortality: nationwide cohort study. BMJ. 2009;338:b496. doi: 10.1136/bmj.b496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132(6):1156–63. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- 46.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171(11):1183–94. doi: 10.1093/aje/kwq045. kwq045 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardy R, Wadsworth M, Kuh D. The influence of childhood weight and socioeconomic status on change in adult body mass index in a British national birth cohort. Int J Obes Relat Metab Disord. 2000;24(6):725–34. doi: 10.1038/sj.ijo.0801238. [DOI] [PubMed] [Google Scholar]
- 48.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.