Abstract
Creatinine, uric acid, hypoxanthine and xanthine are important diagnostic biomarkers in human urine for gouty arthritis or renal disease diacrisis. A simple method for simultaneous determination of these biomarkers in urine based on reversed-phase high-performance liquid chromatography (RP-HPLC) with ultraviolet (UV) detector was proposed. After pretreatment by dilution, centrifugation and filtration, the biomarkers in urine samples were separated by ODS-BP column by elution with methanol/50 mM NaH2PO4 buffer solution at pH 5.26 (5:95). Good linearity between peak areas and concentrations of standards was obtained for the biomarkers with correlation coefficients in the range of 0.9957–0.9993. The proposed analytical method has satisfactory repeatability (the recovery of data in a range of creatinine, uric acid, hypoxanthine and xanthine was 93.49–97.90%, 95.38–96.45%, 112.46–115.78% and 90.82–97.13% with standard deviation of <5%, respectively) and the limits of detection (LODs, S/N≥3) for creatinine, uric acid, hypoxanthine, and xanthine were 0.010, 0.025, 0.050 and 0.025 mg/L, respectively. The established method was proved to be simple, accurate, sensitive and reliable for the quantitation of gouty arthritis' biomarkers in human urine samples. The ratio of creatinine to uric acid was found to be a possible factor for assessment of gouty arthritis.
Keywords: Gouty arthritis, Creatinine, Uric acid, Hypoxanthine, Xanthine, High-performance liquid chromatography
Graphical abstract
1. Introduction
In clinical diagnosis, the uric acid concentration in gouty arthritis patients has been evaluated on (a) decreased destruction of uric acid, (b) overproduction of uric acid, and (c) an abnormality in the kidney excretion of uric acid [1]. Uric acid is the hepatic product of purine metabolism. After primary filtration by the kidney, the metabolic uric acid is reabsorbed into the blood circulation system or secreted into the urine [2]. The symptoms of gouty might be abnormal metabolism of kidney excretion of uric acid. In addition, creatinine is one of the most widely used biomarkers of kidney function [3]. Therefore, the study of simultaneous determination of uric acid and creatinine for diagnosis of gouty arthritis has been of importance.
The conventional method for the determination of these biomarkers in plasma and urine is based on the enzymatic conversion of urate to allantoin using uricase followed by colorimetric measurement [4], [5]. However, this method includes unstable reagents and suffers from interferences such as ascorbic acid and dopamine, which are present in biological fluids. In the past decades, different chromatographic methods were applied to determine uric acid or uric acid and creatinine, including ion-exchange liquid chromatography [6], paired-ion liquid chromatography [7], size-exclusion liquid chromatography [8], liquid chromatography/mass spectrometry [9], [10], [11], high-performance liquid chromatography (HPLC) [5], [12], [13], [14], [15], [16], [17], [18], [19], column-switching liquid chromatography [20], hydrophilic interaction chromatography [21] and capillary electrophoresis [22], [23], [24], [25], [26], [27].
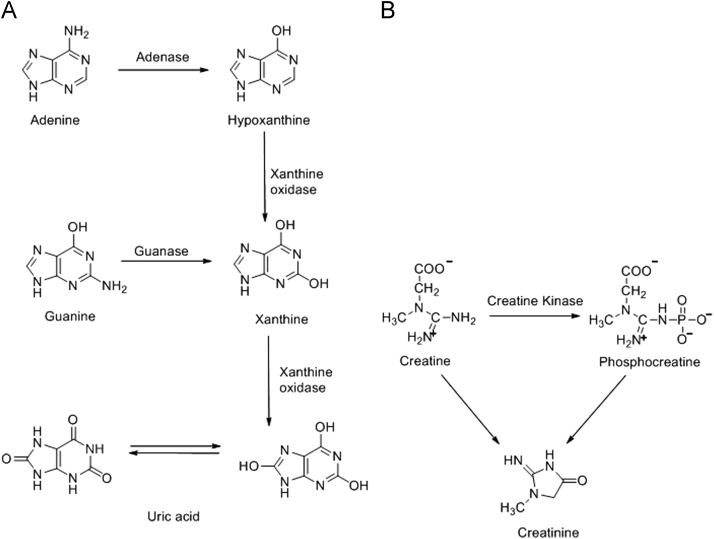
In human body, uric acid is the end-metabolic product of adenine and guanine as shown in Fig. 1A. Adenine is catalyzed by adenase to hypoxanthine, which is catalyzed by xanthine oxidase undergoing xanthine and finally to uric acid. Xanthine can also be produced from guanine by guanase catalysis [1]. Creatinine is excreted from creatine and phosphocreatine and this process occurs at fractional rates of 0.016 and 0.03 per day for creatine and phosphocreatine, respectively. It leads to the irreversible, non-enzymatic dehydration and loss of phosphate from phosphocreatine as shown in Fig. 1B. The amount of creatinine in the urine is proportional to the amount of creatine and creatine phosphate present in the human body [28]. Creatinine is one of the most widely used markers of renal function. Changes in urinary creatinine content can indicate the renal problems [3]. Herein, creatinine, uric acid, hypoxanthine and xanthine are important diagnostic biomarkers in human urine for gouty arthritis or renal disease.
Fig. 1.
Chemical structures of uric acid, creatinine, hypoxanthine and xanthine.
However, to the best of our knowledge, methods for simultaneous determination of uric acid, creatinine, xanthine and hypoxanthine in urine still have not been reported. Sometimes the spot concentration of uric acid does not represent the real level in the body because of the effect of biological clock. The 24 h monitoring uric acid is not applicable for clinical diagnosis. Therefore, the combination of uric acid, creatinine, hypoxanthine and xanthine is more instructional for correct and integrated diagnosis of gouty arthritis. In our study, a chromatographic method for simultaneous determination of these four biomarkers in urine was proposed. A simple pretreatment to remove the protein interferents was performed for real urine samples before chromatographic analysis. The samples were pretreated by dilution, centrifugation and filtration, and then were analyzed with HPLC. Urine samples from healthy and gouty volunteers were gathered. It was found in this study that the ratio of creatinine to uric acid may be considered as an additional assessment factor for diagnosis of gouty arthritis.
2. Experimental
2.1. Chemicals and reagents
Creatinine, uric acid, hypoxanthine and xanthine were purchased from Sigma-Aldrich (USA). Methanol supplied by J&K Scientific Ltd. (USA) was of HPLC grade. Sodium dihydrogen phosphate and sodium hydroxygen obtained from Beijing Chemical Reagent Company (China) were used for preparing HPLC buffer solutions. All chemicals were dissolved with de-ionized water.
Creatinine standard (200 mg/L) was freshly prepared in water. Uric acid standard (200 mg/L) was dissolved in basic aqueous solution at a pH value of 10.35 to increase the solubility. Hypoxanthine and xanthine standards (200 mg/L, respectively) were dissolved in 0.1 M sodium hydroxide solutions. The working solutions of standards were prepared from the stock standard solution. Calibration curves were prepared with the concentration ranging from 5 to 200 mg/L for the standards.
2.2. Urine samples preparation
Urine samples were collected from healthy volunteers and patients with gouty arthritis in plastic containers. Urine samples were diluted 5-fold with HPLC mobile phase and the proteins were precipitated by centrifugation at 6000 rpm for 10 min. The supernatants of urine were diluted 3-fold with HPLC mobile phase to facilitate. The pretreated samples were filtered through a 0.45 μm membrane for HPLC determination.
2.3. HPLC instrumentation and conditions
A Hitachi L-2000 high-performance liquid chromatography (Hitachi Corporation, Japan) equipped with L-2130 pump, L-2300 column oven, UV L-2400 spectrophotometer detector and D2000HSM software was used for analysis. The analytical column used in the experiments was a SinoChrom ODS-BP column (250 mm×4.6 mm, 5 μm). The mobile phase consisted of 5% methanol and 95% NaH2PO4 buffer solution (50 mM, pH 5.26) and the flow rate was 0.8 mL/min. The mobile phase solvents were filtered through a 0.45 μm membrane and degassed before use. The sample detection was carried out at the 210 nm wavelength with an injection volume of 20 μL.
3. Result and discussion
3.1. Optimization of HPLC analysis
Creatinine, uric acid, hypoxanthine and xanthine are very polar small molecules (see Fig. 1) showing very poor retention on ODS columns. Because of proper carbon content, the SinoChrom ODS-BP columns were suitable for the separation of the hydrophilic sample containing a high proportion of water in the mobile phase conditions with a strong retention. The concentrations of these biomarkers are very different in human urine. The separation of these biomarkers requires a highly aqueous mobile phase, which causes the retention loss of the analytes on the reversed-phase column, and equilibration of the column with an organic solvent after each run. To verify the applicability of the analytical protocol, the pH value and the composition of the HPLC mobile phase were thoroughly optimized. The reproducibility of qualitative and quantitative detection was evaluated.
For the better conditions of an analytical protocol, the mobile phase should be either free from or have a low level of organic solvents for the polar analytes. An eluent of modest elution force with a certain pH value to keep the analytes in neutral forms is required. Thus, organic/aqueous buffer solution was tested as the mobile phase. The methanol/NaH2PO4 buffer solution or methanol/NaAc buffer solution was tried as the mobile phases. NaH2PO4 buffer solution (50 mM, pH 5.26) and NaAc (20 mM, pH 7.40) were selected as the aqueous phase according to the relevant literature [6], [20]. Experimental results showed that uric acid and creatinine could be baseline separated by both mobile phases, but hypoxanthine and xanthine could not be separated by methanol/NaAc mobile phase. Thus, methanol/NaH2PO4 eluent was the final choice as the mobile phase.
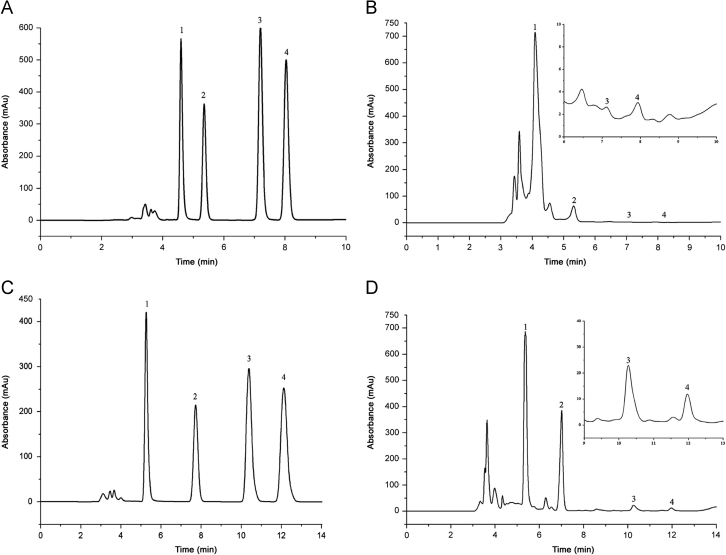
Organic phase ratio affected the retention time of biomarkers in the ODS-BP column, organic phase ratio was selected as 5% and 10%. As shown in Fig. 2, experimental results showed that the baseline separation of biomarkers could be achieved under the ratio of methanol 10% and 5% (Fig. 2A and C). At the 10% proportion of methanol, impurity peaks were close to targets as shown in Fig. 2B. To ensure the separation effect of the actual sample, the ultimate eluent was methanol/NaH2PO4 buffer solution (5:95).
Fig. 2.
Effect of the ratio of organic phase. (The flow rate was 0.8 mL/min, the column temperature was 25 °C, the UV detection wavelength was 210 nm, and the injection volume was 20 μL. 1, 2, 3 and 4 stand for creatinine, uric acid, hypoxanthine and xanthine, respectively. A and C are mixed standard while B and D are actual urine sample. The HPLC mobile phases were methanol/NaH2PO4 buffer solution (10:90) and methanol/NaH2PO4 buffer solution (5:95)).
Considering the effect of detect wavelength on the detected results, UV detection wavelength from 200 nm to 230 nm was estimated. Experimental results illustrated that the detection wavelength at 210 nm was good for the absorption of biomarkers. The UV absorbance of creatinine, uric acid, hypoxanthine and xanthine did not interfere with each other.
Based on a series of tests, the optimum separation and detection conditions were achieved as isocratic elution program at 0.8 mL/min flow rate with an eluent of methanol/NaH2PO4 buffer solution (5:95). The chromatogram for standards of creatinine, uric acid, hypoxanthine and xanthine is shown in Fig. 2C. All biomarkers gave sharp and symmetric peaks and were well separated within 12 min.
3.2. Linearity, sensitivity and precision of the analysis method
The real urine sample of a volunteer was pretreated and determined. As shown in Fig. 2D, creatinine, uric acid, hypoxanthine and xanthine were found. Compared with uric acid and creatinine, hypoxanthine and xanthine presented a lower level in human urine. The relative standard deviation (RSD) values of the retention times were smaller than 1.3% (the real urine sample of a volunteer was replicated six times and gained RSD by calculating), indicating that the developed separation method was stable enough.
A series of standard solutions over different concentration ranges for each analyte was detected for calibration curves. The quantitative performance factors are listed in Table 1. The LODs (signal/noise ratio [S/N]≥3) were 0.010 mg/L for creatinine, 0.025 mg/L for uric acid, 0.050 mg/L for hypoxanthine and 0.025 mg/L for xanthine. The accuracy of the method was estimated by adding two levels of standards for each target to the urine sample. The recoveries of targets were in the range of 90.82–115.78% as listed in Table 2.
Table 1.
Linearity of analytical method.
| Constituents | Retention time (min) | Parameters of linear regression | Range of linearity (mg/L) | r2 |
|---|---|---|---|---|
| Creatinine | 5.14 | Y=94632X+94219 | 10–200 | 0.9993 |
| Uric acid | 7.41 | Y=89899X+274915 | 7.5–150 | 0.9957 |
| Hypoxanthine | 10.00 | Y=106554X+18426 | 5–100 | 0.9999 |
| Xanthine | 11.61 | Y=111030X−25303 | 5–100 | 0.9994 |
Table 2.
The recoveries and the detection limit of creatinine, uric acid, hypoxanthine and xanthine in the human urine sample.
| Analysts | Actual concentration of sample (mg/L) | Standard addeda (mg/L) | Mean recoveryb (%)±SD | LOD (mg/L) |
|---|---|---|---|---|
| Creatinine | 861.25 | 160 | 97.90±0.84 | 0.010 |
| 320 | 93.49±0.79 | |||
| Uric acid | 411.78 | 80 | 96.45±4.20 | 0.025 |
| 160 | 95.38±1.76 | |||
| Hypoxanthine | 51.96 | 50 | 112.46±1.08 | 0.050 |
| 100 | 115.78±0.94 | |||
| Xanthine | 54.44 | 20 | 97.13±3.16 | 0.025 |
| 50 | 90.82±0.96 | |||
Concentrations of standards are expressed as the equivalent concentrations added in the final injected solutions. Each level of standard added was replicated six times.
Calculated recovery (%)=(amount observed−original amount)/added amount×100%.
3.3. Application to assess of gouty arthritis
Urine samples from 16 volunteers were analyzed using the developed RP-HPLC method discussed above. Results are listed in Table 3. The concentrations of creatinine, uric acid, hypoxanthine and xanthine for the gouty group were in the range of 1086–2316 mg/L, 587–1163 mg/L, 79–150 mg/L and 25–90 mg/L, respectively. However, the concentrations of creatinine, uric acid, hypoxanthine and xanthine for the healthy group were in the range of 194–1621 mg/L, 52–553 mg/L, 15–156 mg/L and 25–171 mg/L, respectively. It could be seen that the concentration levels of creatinine and uric acid in the gouty group were much higher than those in the healthy group. However, compared with the healthy group, the concentration of xanthine and hypoxanthine for the gouty group changed slightly.
Table 3.
Analytical results of creatinine, uric acid, hypoxanthine and xanthine in the human urine sample.
| Urine sample | Creatinine (mg/L) | Uric acid (mg/L) | Hypoxanthine (mg/L) | Xanthine (mg/L) | Ratio of creatinine to uric acid |
|---|---|---|---|---|---|
| Gouty group | |||||
| 1 | 2316.31 | 1163.22 | 150.24 | 85.54 | 1.99 |
| 2 | 1731.57 | 1146.86 | 125.89 | 72.21 | 1.51 |
| 3 | 1622.91 | 1069.85 | 91.94 | 25.17 | 1.52 |
| 4 | 1057.32 | 719.82 | 100.78 | 90.55 | 1.47 |
| 5 | 1314.07 | 874.40 | 79.59 | 59.64 | 1.50 |
| 6 | 1086.87 | 587.47 | 80.28 | 90.66 | 1.85 |
| Healthy group | |||||
| 7 | 861.25 | 411.78 | 51.96 | 62.88 | 2.09 |
| 8 | 1521.93 | 388.97 | 55.83 | 54.44 | 3.91 |
| 9 | 463.63 | 177.85 | 15.95 | 39.10 | 2.61 |
| 10 | 1621.70 | 390.32 | 30.99 | 32.60 | 4.15 |
| 11 | 659.74 | 321.80 | 61.43 | 29.24 | 2.05 |
| 12 | 1248.28 | 553.37 | 145.52 | 64.06 | 2.26 |
| 13 | 194.90 | 52.84 | 57.04 | 25.15 | 3.69 |
| 14 | 665.17 | 223.70 | 156.60 | 135.55 | 2.97 |
| 15 | 1291.88 | 454.14 | 66.16 | 171.03 | 2.84 |
| 16 | 568.61 | 173.48 | 133.50 | 121.81 | 3.28 |
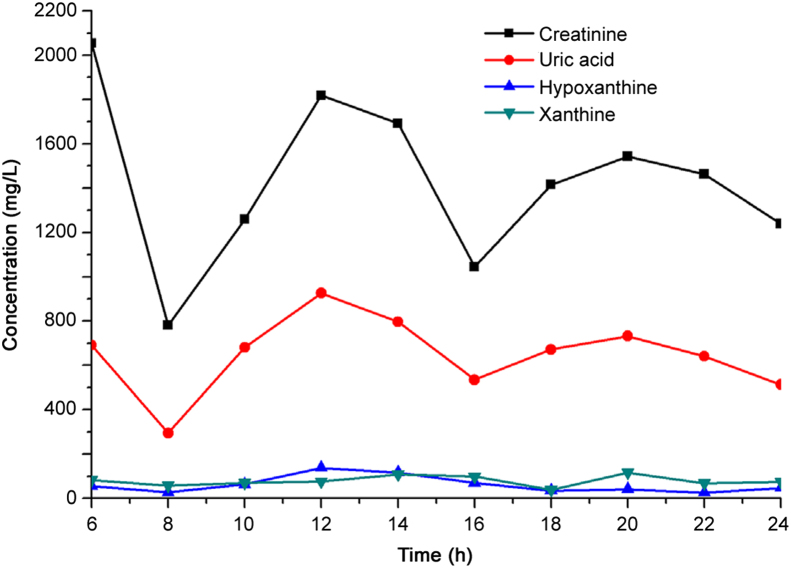
There was an 18 h monitoring period to test the changes of targets' concentration at different biological clocks. Urine samples of healthy individuals were collected at the interval of 2 h from 6:00 to 24:00 and last for three days. As shown in Fig. 3, the concentrations of biomarkers changed with time. But the concentrations of creatinine and uric acid significantly changed. In the morning, the concentrations of creatinine and uric acid were high because the two biomarkers were accumulated during sleep. When the volunteer stayed hungry (near 12:00 and 18:00), the concentrations of creatinine and uric acid increased. And the concentrations of biomarkers were different under various conditions. For examples, the daily food, high levels of purine foods especially, affects the concentration of biomarkers in human urine. The consumption of cranberry fruit juice has an effect on the concentration of urinary uric acid [21]. The ingestion of Tofu (bean curd) would increase the plasma concentration of uric acid, together with increasing uric acid clearance and urinary excretion of uric acid. The increase of uric acid in plasma concentration was very small [29]. Therefore, the point concentration of biomarkers was not fit to be a reliable index for screening test of gouty arthritis assessment.
Fig. 3.
A time course of the concentration of creatinine, uric acid, hypoxanthine and xanthine in human urine.
In our study, the ratio of creatinine to uric acid was found to be a possible factor for assessment of gouty arthritis. As presented in Table 3, the value of uric acid No. 12 in the healthy group was 553.37 mg/L, nearly 600 mg/L. However, the ratio of creatinine to uric acid was 2.26, beyond 2.0. In Table 3, the ratio of creatinine to uric acid of No. 1 in the gouty group was 1.99, nearly 2.0. However, the value of uric acid was 1163.22 mg/L. Hence, only the values of uric acid at 600 mg/L might not be an accurate value for diagnosis. It appears that the ratio of creatinine to uric acid together with considering the values of uric acid could be used as a clinical assessment of gouty arthritis.
4. Conclusions
A simple and reliable RP-HPLC method has been developed for simultaneous determination of creatinine, uric acid, hypoxanthine and xanthine in human urine. It has a simple pretreated procedure and a relatively short detection time. The developed method has been successfully applied to determine urine samples from healthy and gouty volunteers. The ratio of creatinine to uric acid was found to be a possible factor for assessment of gouty arthritis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21275088).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Joan K. The biological significance of uric acid and guanine excretion. Biol. Rev. 1959;34(3):265–294. [Google Scholar]
- 2.Choi H.K., Mount D.B., Reginato A.M. Pathogenesis of Gout. Ann. Intern. Med. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 3.Levey A.S., Perrone R.D., Madias N.E. Serum creatinine and renal function. Ann. Rev. Med. 1988;39:465–490. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 4.Samanidou V.F., Metaxa A.S., Papadoyannis I.N. Direct simultaneous determination of uremic toxins: creatine, creatinine, uric acid and xanthine in human biofluids by HPLC. J. Liq. Chromatogr. Relat. Technol. 2002;25:43–57. [Google Scholar]
- 5.Jen J.F., Hsiao S.L., Liu K.H. Simultaneous determination of uric acid and creatinine in urine by an eco-friendly solvent-free high performance liquid chromatographic method. Talanta. 2002;58:711–717. doi: 10.1016/s0039-9140(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama Y., Yamasaki K., Sato H. Simultaneous determination of urinary creatinine and UV-absorbing amino acids using a novel low-capacity cation-exchange chromatography for the screening of inborn errors of metabolism. J. Chromatogr. B. 2005;816:333–338. doi: 10.1016/j.jchromb.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Moral P.G., Diez M.T., Resines J.A. Simultaneous measurements of creatinine and purine derivatives in ruminant's urine using ion-pair HPLC. J. Liq. Chromatogr. Relat. Technol. 2003;26:2961–2968. [Google Scholar]
- 8.Kochansky C.J., Strein T.G. Determination of uremic toxins in biofluids: creatinine, creatine, uric acid and xanthenes. J. Chromatogr. B. 2000;747:217–227. doi: 10.1016/s0378-4347(00)00119-5. [DOI] [PubMed] [Google Scholar]
- 9.Perello J., Sanchis P., Grases F. Determination of uric acid in urine, saliva and calcium oxalate renal calculi by high-performance liquid chromatography/mass spectrometry. J. Chromatogr. B. 2005;824:175–180. doi: 10.1016/j.jchromb.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Dai X.H., Fang X., Zhang C.M. Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method. J. Chromatogr. B. 2007;857:287–295. doi: 10.1016/j.jchromb.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Kwon W., Kim J.Y., Suh S. Simultaneous determination of creatinine and uric acid in urine by liquid chromatography–tandem mass spectrometry with polarity switching electrospray ionization. Forensic Sci. Int. 2012;221:57–64. doi: 10.1016/j.forsciint.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Cooper N., Ksohravan R., Erdmann C. Quantification of uric acid, xanthine and hypoxanthine in human serum by HPLC for pharmacodynamic studies. J. Chromatogr. B. 2006;837:1–10. doi: 10.1016/j.jchromb.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y.M., Pietrzyk R.A., Whitson P.A. Quantification of urinary uric acid in the presence of thymol and thimerosal by high-performance liquid chromatography. J. Chromatogr. A. 1997;763:187–192. doi: 10.1016/s0021-9673(96)00740-6. [DOI] [PubMed] [Google Scholar]
- 14.Marklund N., Ostman B., Nalmo L. Hypoxanthine, uric acid and allantoin as indicators of in vivo free radical reactions description of a HPLC method and human brain microdialysis data. Acta Neurochir. (Wien) 2000;142:1135–1142. doi: 10.1007/s007010070042. [DOI] [PubMed] [Google Scholar]
- 15.George S.K., Dipu M.T., Mehra U.R. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J. Chromatogr. B. 2006;832:134–137. doi: 10.1016/j.jchromb.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 16.Kandar R., Drabkova P., Hampl R. The determination of ascorbic acid and uric acid in human seminal plasma using an HPLC with UV detection. J. Chromatogr. B. 2011;879:2834–2839. doi: 10.1016/j.jchromb.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Zuo Y.G., Wang C.J., Zhou J.P. Simultaneous determination of creatinine and uric acid in human urine by high-performance liquid chromatography. Anal. Sci. 2008;24:1589–1592. doi: 10.2116/analsci.24.1589. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y.D. Simultaneous determination of creatine, uric acid, creatinine and hippuric acid in urine by high performance liquid chromatography. Biomed. Chromatogr. 1998;12:47–49. doi: 10.1002/(SICI)1099-0801(199803/04)12:2<47::AID-BMC717>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Li X.N., Franke A.A. Fast HPLC–ECD analysis of ascorbic acid, dehydroascorbic acid and uric acid. J. Chromatogr. B. 2009;877:853–856. doi: 10.1016/j.jchromb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Seki T., Yamaji K., Orita Y. Simultaneous determination of uric acid and creatinine in biological fluids by column-switching liquid chromatography with ultraviolet detection. J. Chromatogr. A. 1996;730:139–145. doi: 10.1016/0021-9673(95)01218-4. [DOI] [PubMed] [Google Scholar]
- 21.Zuo Y.G., Yang Y., Zhu Z. Determination of uric acid and creatinine in human urine using hydrophilic interaction chromatography. Talanta. 2011;83:1707–1710. doi: 10.1016/j.talanta.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 22.Pormsila W., Krahenbuhl S., Hauser P.C. Capillary electrophoresis with contactless conductivity detection for uric acid determination in biological fluids. Anal. Chim. Acta. 2009;636:224–228. doi: 10.1016/j.aca.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhao S.L., Wang J.S., Ye F.G. Determination of uric acid in human urine and serum by capillary electrophoresis with chemiluminescence detection. Anal. Biochem. 2008;378:127–131. doi: 10.1016/j.ab.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Xing X.P., Shi X., Zhang M.J. CE determination of creatinine and uric acid in saliva and urine during exercise. Chromatographia. 2008;67:985–988. [Google Scholar]
- 25.Jia L., Chen X., Wang X.R. Simultaneous determination of creatinine and uric acid in human urine by capillary zone electrophoresis. J. Liq. Chromatogr. Relat. Technol. 1999;22:2433–2442. [Google Scholar]
- 26.Lee H.L., Chen S.C. Microchip capillary electrophoresis with electrochemical detector for precolumn enzymatic analysis of glucose, creatinine, uric acid and ascorbic acid in urine and serum. Talanta. 2004;64:750–757. doi: 10.1016/j.talanta.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Munoz J.A., Lopez-Mesas M., Valiente M. Development and validation of a simple determination of urine metabolites (oxalate, citrate, uric acid and creatinine) by capillary zone electrophoresis. Talanta. 2010;81:392–397. doi: 10.1016/j.talanta.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Truis S.P. Separation methods applicable to urinary creatine and creatinine. J. Chromatogr. B. 2002;781:93–106. doi: 10.1016/s1570-0232(02)00617-7. [DOI] [PubMed] [Google Scholar]
- 29.Yamakita J., Yamamoto T., Moriwaki Y. Effect of tofu (bean curd) ingestion and on uric acid metabolism in healthy and gouty subjects. Adv. Exp. Med. Biol. 1998;431:839–842. doi: 10.1007/978-1-4615-5381-6_161. [DOI] [PubMed] [Google Scholar]