Abstract
The quantitative estimation of amikacin (AMK) in AMK sulfate injection samples is reported using FTIR-derivative spectrometric method in a continuous flow system. Fourier transform of mid-IR spectra were recorded without any sample pretreatment. A good linear calibration (r>0.999, %RSD<2.0) in the range of 7.7–77.0 mg/mL was found. The results showed a good correlation with the manufacturer's and overall they all fell within acceptable limits of most pharmacopoeial monographs on AMK sulfate.
Keywords: Amikacin, FTIR derivative spectrometry, Continuous flow system, Pharmaceutical preparation, Injection, Sulfate
Graphical abstract
1. Introduction
Amikacin (AMK) is used to treat infections caused by Gram-negative bacteria. It is a semi-synthetic aminoglycoside derived from kanamycin, formulated as a disulfate salt (Fig. 1). The dosage form is normally supplied as a sterile solution for parenteral use [1].
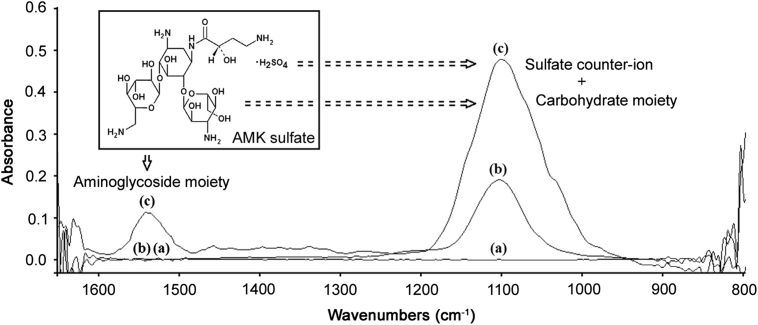
Fig. 1.
FTIR spectra of the AMK sulfate injections related substances. Excipients constituted by water, 1.3 mg/mL sodium bisulfite, and 5 mg/mL sodium citrate (a). Aqueous sulfuric acid solution, pH=4.5 (b). AMK sulfate standard (50 mg/mL) as AMK base (c). All spectra were obtained using water background.
Aminoglycoside antibiotics determination has been carried out by a wide variety of methods [2], [3], [4]. However, a direct UV–vis spectrophotometric estimation is not feasible [5]. The former Pharmacopoeia of United States (USP 24) [6] and later the European Pharmacopoeia (Ph. Eur. 6) [7] and British Pharmacopoeia (BP) [8] reported derivatization procedures of AMK prior to reversed-phase liquid chromatographic (LC) analysis. As it is too time consuming, LC methods based on non-derivatization procedures, such as universal aerosol-based detector [9], ligand displacement reaction [10], charged aerosol [11], evaporative light scattering [12] and resonance Rayleigh scattering [13] have been proposed. The pulsed electrochemical detection method [14], [15], [16] has been adopted in the recent USP monograph [1]. The major drawback of the detection approach coupled to LC is that it requires skills for implementation [17].
Pharmaceutical raw materials are also tested for sulfate content [18]. The actual pharmacopoeial monographs, Eur. Ph. [7] and USP [1] refer to sulfate counter-ion content as a molar ratio between AMK and H2SO4. The sulfate ion, outside the allowed range, could indicate that AMK is present as a free base or the sulfate ion, in excess, is present as an impurity [19]. Recently, our research group proposed a Fourier-transform infrared (FTIR) method for determining the sulfate counter-ion content in AMK sulfate injections [19].
FT-mid-IR in conjunction with a continuous flow system (CFS) was extensively used for quantitative estimation of active ingredients in pharmaceutical preparations [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. The spectrum is a marker for identity and purity of the active pharmaceutical ingredient (API) and also useful to detect impurities coming from the excipients.
A direct reagent-free determination of AMK content in parenteral formulations of AMK sulfate by CFS-FTIR-derivative spectrometry (DS) is proposed keeping in view principles of green analytical chemistry [19], [29], [30], [31], [32]. The advantage of the present method is to simultaneously quantify AMK base and sulfate counter-ion in AMK sulfate injections with a single spectrum.
2. Experimental
2.1. Reagents and samples
All chemicals used were of analytical-reagent grade. Water was obtained from a Milli-Q-TOC purification system (Millipore, Bedford, MA, USA). Reference standards: AMK sulfate stating 786 μg/mg as AMK base, purchased from Sigma-Aldrich (St Louis, MO, USA) and AMK sulfate with 99.95% purity, kindly provided by a pharmaceutical manufacturer of the region. Standard stock solution of AMK sulfate (100 mg/mL) was prepared in water. Pharmaceutical products were acquired from local drug stores and analyzed directly from the ampoules. Commercial samples containing a concentration higher than 50 mg/mL of AMK base were diluted with water to obtain required concentrations.
2.2. Apparatus
A Perkin–Elmer, model Spectrum 2000, FTIR spectrophotometer (Norwalk, CT, USA) was employed for acquisition of spectra. The FTIR equipment was connected to a monochannel flow system through a flow cell. A demountable liquid transmission cell (Wilmad Labglass, Buena, NJ, USA) with ZnSe windows (38 mm×19 mm size, 2 mm thick, and 0.05 mm optical pathlength) was used. An Ismatec IPC peristaltic pump (Glattbrugg, Switzerland) equipped with Tygon tubing was employed for sample and standard propulsion. A Rheodyne manual selecting valve (Alltech, Waukegan, USA) was used for carrying either sample solution or standard solution into the flow cell.
2.3. General procedure
A schematic diagram of the continuous flow system and its operation steps were presented earlier [27]. The system was optimized for leaks, air bubbles, and pump flow rate. Calibration or test sample solutions were continuously pumped in order to reach the flow cell. An injection valve was switched to the solution in turn. The continuous flowing stream of either samples or standards was monitored using the FTIR spectrometer. Each spectrum was automatically converted to its first-order derivative spectrum. The validation of the analytical method was carried out as it was described by us earlier [19].
3. Results and discussion
3.1. Identification, FTIR spectra of AMK sulfate and related excipients in aqueous phase
Infrared spectra of API were recorded under the optimum instrumental conditions. We can see that water showed the characteristic transparency zone localized close to the fingerprint region 1600–900 cm−1 (Fig. 1). AMK sulfate has two absorption bands, one broad and intense band observed in the range of 1220–938 cm−1 with a maximum at 1100 cm−1, and the other, with lesser intensity in the range of 1590–1480 cm−1 with a maximum at 1538 cm−1. The spectra also show that the presence of excipients, at the stated concentrations in the formulation, do not have any additional IR bands in the fingerprint region, except the sulfate from sulfuric acid.
3.2. Selection of the analytical spectral band and the analytical measurement criterion
The two mid-IR bands of AMK sulfate are due to contribution of carbohydrate moiety and hetero-oxy groups of the sulfate counter-ion. The less intense band observed in the range of 1590–1480 cm−1 arises from the aminoglycoside moiety [19]. In the present study, the latter spectral band was selected for further studies. This band was evaluated using DS to overcome spectral deviations and to choose the best analytical performance of the proposed FTIR method (Fig. 2). The first-order derivative locates the spectral region where minimal bandshape spectral variability was observed, both from inter sample variation and compared to the standard. Further, higher order derivatives of the AMK spectrum for different samples were evaluated. Considering the little advantage of using higher order derivative spectra, only first order derivative was selected for further studies.
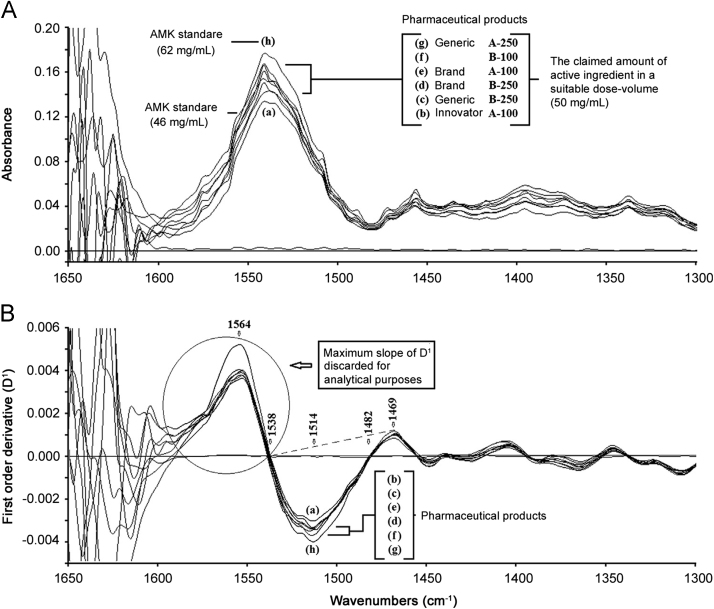
Fig. 2.
The zeroth (A) and first (B) derivative spectra of representative commercial AMK sulfate injections against two reference standard solutions. Each analyzed solution with a nominal concentration of 50 mg/mL AMK base. In Fig. 2A, 100 and 250 represent the declared concentration units in mg for 2 mL pharmaceutical products.
In order to choose the best analytical performance of the FTIR-DS determination of AMK in parenteral pharmaceutical formulations, different measurement criteria were evaluated (Table 1). As a compromise among sensitivity, precision, and band shape, the selected measurement criterion for determining AMK content was the peak area under the baseline in the range of 1538–1469 cm−1 (Fig. 2B). A derivative-window of 25 points was selected for analytical purposes.
Table 1.
Analytical results obtained for determining AMK base content in AMK sulfate injections by the proposed CFS-FTIR-DS method.
| Band peak parameters (units in cm−1) of the first derivative spectra |
Parameters derived from linear regressiona: Y=a+bX |
||||||
|---|---|---|---|---|---|---|---|
| Measurement mode | Location | Baseline | a±SD (×10−3) | b±SD (×10−3) | r | LOD | RSD |
| Total height | 1514 | Zero abs | 0.08±0.02 | 0.0648±0.0004 | 0.9996 | 2.1 | 0.004 |
| Corrected height | 1514 | 1538–1469 | 0.05±0.07 | 0.0728±0.0007 | 0.9995 | 2.2 | 0.005 |
| Total area | 1514 | Zero abs | 1±1 | 2.25±0.02 | 0.9987 | 3.5 | 0.30 |
| Corrected area | 1514 | 1538–1469 | 1.0±0.6 | 2.81±0.01 | 0.9997 | 1.6 | 0.15 |
Calibration curves obtained using eight standards of AMK sulfate, in triplicate, registered as its equivalent AMK concentration ranging from 7.7 to 77.0 (mg/mL); where X is the explanatory variable and Y is the dependent variable; the slope of the line is b, and a is the Y-intercept; SD=standard deviation; r=correlation coefficient; LOD=limit of detection in mg/mL AMK base calculated as 3σ(1/slope); RSD=relative standard deviation (%).
3.3. Effect of instrumental and experimental conditions
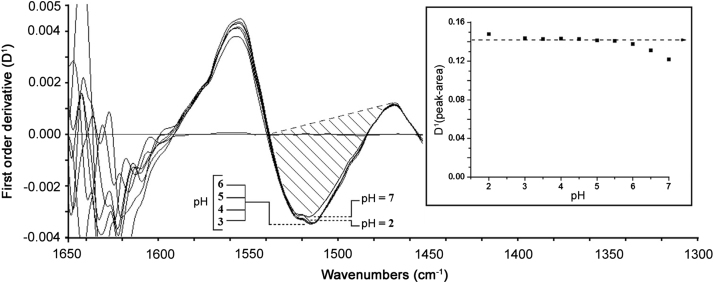
In this regard, the selected spectral analytical signal was evaluated by changing one variable at a time, each by triplicate, using solutions containing 50 mg/mL of AMK base. Accordingly, a nominal resolution of 4 cm−1, by accumulating 5 scans, was selected for analytical purposes as a compromise to the smoothness of bandshape, precision (<2% RSD) and spectral acquisition time (<30 s). The latter data and those obtained for the CFS parameters, such as flow rate (0.3 mL/min) and sample analysis frequency (13 h−1), were consistent with those obtained from our earlier work [19]. As depicted in Fig. 3, there was very minimal fluctuation on the analytical signal as a function of pH values between 3.0 and 5.5, where precision was better than the percentage unit.
Fig. 3.
Effect of the pH value of the sample solution on both signal intensity and spectral band shape according to the selected measurement criterion. The study was carried out using an AMK sulfate standard equivalent to 50 mg/mL AMK base and water as background.
3.4. Analytical figures of merit of the CFS-FTIR-DS method
Validation of the analytical method was performed to demonstrate its usefulness in the direct determination of AMK content in AMK sulfate injections. Corrected peak-area of the first order DS was used for obtaining the regression line (Table 1). Eight concentrations ranging from 7.7 mg/mL to 77.0 mg/mL (AMK base) were used to obtain the calibration curve. A straight line with an excellent correlation coefficient (>0.999) with negligible deviations from linearity at low and high concentrations was obtained. The limit of detection ((3σ(1/slope)) and the limit of quantification (10σ(1/slope)) were sufficient for analysis of parenteral formulations (Table 1).
The scatter in the results due to the instrumental variability was 1.4% (RSD, n=10). Intra-assay precision was less than 2% (RSD, n=6). The precision of the accuracy was assessed using a linear response function at three concentrations covering the range. The observed RSD ranged from 0.40% to 1.44%.
The robustness of the method was considered to be appropriate during normal usage because common parameters affecting other methods were not involved in the present proposal. The effect of pH variability of the sample solutions on the response signal was negligible in the range (3.5–5.5) stated by the USP (Fig. 3). Specificity research was conducted during the preliminary studies. As depicted in Fig. 1, the interferences study of coexisting foreign inorganic ions and organic compounds revealed no interference on the selected spectral region for quantization purposes. Background and baseline displacement that use to affect the intercept of a calibration function, but not its slope, was previously corrected by DS selecting the appropriate band wavenumber range. The IR spectroscopy studies revealed no apparent difference between the standard spectrum and the sample spectra (Fig. 2). Similarly, in spite of any disparity in the concentration, the latter studies revealed no apparent change in bandshape from sample to sample.
These parameters and the satisfactory results could somehow assure the accuracy of the method. Nevertheless, we carried out a recovery experiment. On the average, recoveries were within 97% and 103%, which is similar to or better than recoveries obtained by using other much more complicated methodologies (Table 2).
Table 2.
Representative recovery of AMK base added to AMK sulfate injections.
| Type of drug brand | AMK base (mg/mL±SD) (n=3) |
Recovery (%) | Standard addition calibration: Y=a+bX | r | ||
|---|---|---|---|---|---|---|
| Endogenous | Added | Found | ||||
| Innovator A | 11.8±0.6 | 23.08 | 35.4±0.4 | 102.4 | Y=0.034 1+0.002 79X | 0.999 |
| 38.46 | 49.3±0.2 | 97.7 | ||||
| 46.15 | 57.0±0.3 | 97.9 | ||||
| Brand A | 11.3±0.2 | 23.08 | 34.6±0.5 | 100.8 | Y=0.032 8+0.002 81X | 0.999 |
| 38.46 | 49.9±0.3 | 100.3 | ||||
| 46.15 | 57.6±0.4 | 100.1 | ||||
3.5. Application to pharmaceutical preparations
Finally, validation of the proposed methodology was carried out by its application to pharmaceutical preparations. The results obtained for determining AMK content in AMK sulfate injections by interpolation in the external calibration were in acceptable agreement with the nominal content claimed by the pharmaceutical manufacturers (Table 3). Moreover, all the obtained values for the commercial samples were within the limits of official pharmacopoeias.
Table 3.
Analytical application of the proposed CFS-FTIR-DS method for determining AMK base content in AMK sulfate injections.
| Sample (Lot) | Type of branded drug a | Concentration per ampoule of AMK base |
Acceptance criterion |
|||||
|---|---|---|---|---|---|---|---|---|
| Claimed | Claimed (mg/mL) | Actual result±SDb (mg/mL) | (90.0–110.0) % BP | Pass/ Fail | (90.0–120.0) % USP and Ph. Int. | Pass/Fail | ||
| S1(A–D) | Brand A | 100 mg/2 mL | 50 | 50±2 | 45–55 | Pass | 45–60 | Pass |
| S2 | Innovator A | 100 mg/2 mL | 50 | 55±1 | 45–55 | Pass | 45–60 | Pass |
| S3 | Brand B | 100 mg/2 mL | 50 | 49.8±0.6 | 45–55 | Pass | 45–60 | Pass |
| S4 | Generic B | 100 mg/2 mL | 50 | 50±1 | 45–55 | Pass | 45–60 | Pass |
| S5 | Generic A | 250 mg/2 mL | 125 | 122±2 | 112.5–137.5 | Pass | 112.5–150.0 | Pass |
| S6 | Brand A | 500 mg/2 mL | 250 | 256±4 | 225–275 | Pass | 225–300 | Pass |
| S7(A-D) | Brand A | 500 mg/2 mL | 250 | 239±10 | 225–275 | Pass | 225–300 | Pass |
| S8 | Brand B | 500 mg/2 mL | 250 | 251.0±0.4 | 225–275 | Pass | 225–300 | Pass |
| S9 | Generic B | 500 mg/2 mL | 250 | 252±2 | 225–275 | Pass | 225–300 | Pass |
| S10 | Innovator B | 1000 mg/4 mL | 250 | 236±3 | 225–275 | Pass | 225–300 | Pass |
Brand, available from one or more manufacturers, distributors, and/or re-packagers by generic (nonproprietary) name; innovator “B” was manufactured by a foreign country; S1(A–D) and S7(A–D) mean parenteral products from different lots.
SD, standard deviation (n=3). The mean and the standard deviation of S1 and S7 belong to a set of lot data, each also analyzed by triplicate. BP, British Pharmacopoeia. USP, United States Pharmacopoeia. Ph. Int., The International Pharmacopoeia.
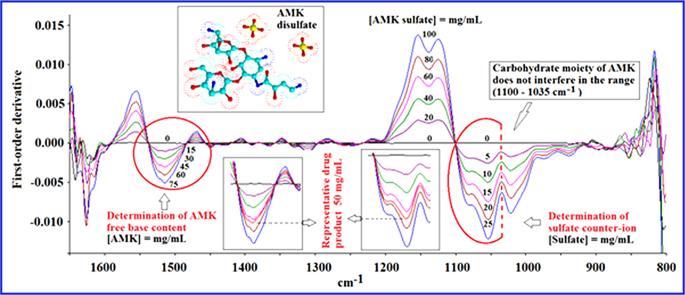
3.5.1. Applicability of the method in the simultaneous quantitation of AMK base and sulfate counter-ion in AMK sulfate injections
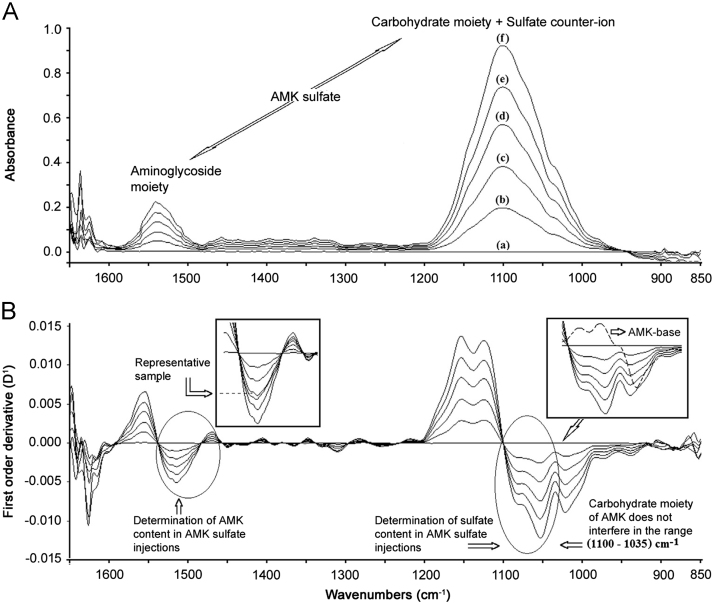
Fig. 4 shows the viability of simultaneously measuring AMK base and sulfate counter-ion in AMK sulfate injections with one spectrum. The two major spectral bands were monitored simultaneously. The possibility of measuring sulfate counter-ion in the presence of the AMK moiety in the spectral range, where carbohydrates present strong IR absorption, was discussed earlier by us [19].
Fig. 4.
Proposal for simultaneously determining the content of both AMK free base and sulfate counter-ion in AMK sulfate injections. Zeroth order spectra (A). First order spectra (B). The calibration curve was constructed with AMK sulfate standards at concentrations of 0, 20, 40, 60, 80 and 100 mg/mL (a)→(f). The left hand insert shows additionally a spectrum of a representative pharmaceutical sample with a nominal concentration of 50 mg/mL AMK base. The right hand insert shows how the AMK carbohydrate moiety does not interfere in the determination of sulfate counter-ion (discontinuous line).
4. Conclusion
The determination of active content of compounds in pharmaceutical preparations of AMK sulfate is often achieved by HPLC with electrochemical detection or pre-column derivatization. In this paper, we reported a simple, precise, and accurate methodology for the CFS-FTIR-DS determination of AMK content in aqueous AMK sulfate injections. The actual proposal is based on direct measurements of untreated samples. This green analytical method is an alternative to those that employ separating techniques coupled with either uncommon detection modes or those requiring derivatization procedures.
Since sulfate content is also subject to control as mandated in the pharmacopoeias, one of the strengths of the proposal is that the assay for AMK in AMK sulfate injections can be employed simultaneously to determine the sulfate counter-ion by monitoring two different spectral bands in just one spectrum. Therefore, the proposed CFS-FTIR-DS strategy represents a promising tool for characterizing the quality of both bulk drug materials and pharmaceutical formulations containing AMK sulfate. In addition, it can significantly reduce both expensive laboratory analysis and chemical waste.
Acknowledgments
The authors are grateful to the CDCHTA of the University of Los Andes for providing financial support through several approved projects. The authors are also thankful to the National Fund for Science, Technology and Innovation (FONACIT) of Venezuelan Ministry of Science and Technology for providing financial support, SPE 112–370 and Project G-2005000641.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2013.08.001.
Appendix A. Supplementary materials
Supplementary Material
References
- 1.The United States Pharmacopeia, USP, 34th ed., United States Pharmacopeial Convention, Rockville, MD, USA, 2011.
- 2.Isoherranen N., Soback S. Chromatographic methods for analysis of aminoglycoside antibiotics. J. AOAC Int. 1999;82(5):1017–1045. [PubMed] [Google Scholar]
- 3.Soltés L. Aminoglycoside antibiotics—two decades of their HPLC bioanalysis. Biomed. Chromatogr. 1999;13(1):3–10. doi: 10.1002/(SICI)1099-0801(199902)13:1<3::AID-BMC811>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Stead D.A. Current methodologies for the analysis of aminoglycosides. J. Chromatogr. B. 2000;747(1-2):69–93. doi: 10.1016/s0378-4347(00)00133-x. [DOI] [PubMed] [Google Scholar]
- 5.Ovalles J.F., Brunetto M.R., Gallignani M. A new method for the analysis of amikacin using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) derivatization and high-performance liquid chromatography with UV-detection. J. Pharm. Biomed. Anal. 2005;39(1–2):294–298. doi: 10.1016/j.jpba.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 6.The United States Pharmacopeia, USP, 24th ed., United States Pharmacopeial Convention, Rockville, MD, USA, 2000.
- 7.European Pharmacopoeia, Ph. Eur., sixth ed., European Pharmacopoeia Commission, Strasbourg, Council of Europe, Strasbourg, France, 2008. Available from: http://www.uspbpep.com/ep60/amikacin%20sulphate%201290e.pdf
- 8.British Pharmacopoeia Commission, British Pharmacopoeia, BP, vol. III, The Stationery Office, London, UK, 2012. Available from: http://bp2012.infostar.com.cn/Bp2012.aspx?a=query&title=%22Amikacin+Injection%22&tab=a-z+index&l=A&xh=1
- 9.Olšovská J., Kameník Z., Cajthaml T. Hyphenated ultra high-performance liquid chromatography–nano quantity analyte detector technique for determination of compounds with low UV absorption. J. Chromatogr. A. 2009;1216(30):5774–5778. doi: 10.1016/j.chroma.2009.05.088. [DOI] [PubMed] [Google Scholar]
- 10.Yang M., Tomellini S.A. Non-derivatization approach to high-performance liquid chromatography-fluorescence detection for aminoglycoside antibiotics based on a ligand displacement reaction. J. Chromatogr. A. 2001;939(1-2):59–67. doi: 10.1016/s0021-9673(01)01337-1. [DOI] [PubMed] [Google Scholar]
- 11.C. Crafts, M. Plante, I. Acworth, et al., Rapid and Sensitive Analysis of Aminoglycoside Antibiotics using RRLC with Corona Ultra Detection, LPN 2545-01, ESA—A Dionex Company, Chelmsford, MA, USA, 2010. Available from: 〈http://www.dionex.com/en-us/webdocs/83431-PO-HPLC-Corona-ultra-08July2010-LPN2545-01.pdf〉 (accessed 23.01.13).
- 12.Galanakis E.G., Megoulas N.C., Solich P. Development and validation of a novel LC non-derivatization method for the determination of amikacin in pharmaceuticals based on evaporative light scattering detection. J. Pharm. Biomed. Anal. 2006;40(5):1114–1120. doi: 10.1016/j.jpba.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Peng J., Tang J. Description and validation of coupling high performance liquid chromatography with resonance Rayleigh scattering in aminoglycosides determination. Anal. Chim. Acta. 2011;706(2):199–204. doi: 10.1016/j.aca.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Adams E., Van Vaerenbergh G., Roets E. Analysis of amikacin by liquid chromatography with pulsed electrochemical detection. J. Chromatogr. A. 1988;819(1–2):93–97. doi: 10.1016/s0021-9673(98)00394-x. [DOI] [PubMed] [Google Scholar]
- 15.Dionex Corporation, Analysis of the Aminoglycoside Antibiotics Kanamycin and Amikacin Matches USP Requirements, Application Note 267, LPN 2663, Sunnyvale, CA, USA, 2011. Available from: 〈http://www.dionex.com/en-us/webdocs/109787-AN267-IC-KanamycinAmikacin-HPAEPAD-18Jan2011-LPN2663-R2.pdf〉 (accessed 23.01.13).
- 16.Zawilla N.H., Li B., Hoogmartens J. Improved reversed-phase liquid chromatographic method combined with pulsed electrochemical detection for the analysis of amikacin. J. Pharm. Biomed. Anal. 2007;43(1):168–173. doi: 10.1016/j.jpba.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Nicolas A. Control of the quality of antibiotics in the European Pharmacopoeia: recent development in the case of aminoglycosides. Ann. Pharm. Fr. 2007;65(3):174–182. doi: 10.1016/s0003-4509(07)90033-4. [DOI] [PubMed] [Google Scholar]
- 18.Rocheleau M.J. Analytical methods for determination of counter-ions in pharmaceutical salts. Curr. Pharm. Anal. 2008;4(1):25–32. [Google Scholar]
- 19.Ovalles J.F., Gallignani M., Rondón R. Proposal for determining sulfate counter ion in amikacin sulfate formulations by Fourier-transform infrared derivative spectroscopy. Curr. Pharm. Anal. 2013;9(1):20–30. [Google Scholar]
- 20.Cadet F., Garrigues S., de la Guardia M. Quantitative Analysis, Infrared. In: Meyers R.A., editor. Encyclopedia of Analytical Chemistry. Wiley & Sons; Chichester: 2012. [Google Scholar]
- 21.Schindler R., Lendl B. FTIR spectroscopy as detection principle in aqueous flow analysis. Anal. Commun. 1999;36(4):123–126. [Google Scholar]
- 22.Gallignani M., Brunetto M.R. Infrared detection in flow analysis—developments and trends (review) Talanta. 2004;64(5):1127–1146. doi: 10.1016/j.talanta.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Armenta S., Garrigues S., de la Guardia M. Recent developments in flow-analysis vibrational spectroscopy. Trends Anal. Chem. 2007;26(8):775–787. [Google Scholar]
- 24.Konoz E., Mohsen A.H., Samadizadeh M. Quantitative analysis of lorazepam in pharmaceutical formulation through FTIR spectroscopy. E-Journal of Chemistry. 2012;9(4):2232–2238. [Google Scholar]
- 25.Ventura-Gayete J.F., de la Guardia M., Garrigues S. On-line sample treatment and FT-IR determination of doxylamine succinate in pharmaceuticals. Talanta. 2006;70(5):1100–1106. doi: 10.1016/j.talanta.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Moros J., Garrigues S., de la Guardia M. Quality control Fourier transform infrared determination of diazepam in pharmaceuticals. J. Pharm. Biomed. Anal. 2007;43(4):1277–1282. doi: 10.1016/j.jpba.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Ovalles F., Gallignani M., Rondón R. Determination of sulphate for measuring magnesium sulphate in pharmaceuticals by flow analysis-Fourier transforms infrared spectroscopy. Lat. Am. J. Pharm. 2009;28(2):173–182. [Google Scholar]
- 28.Ayala C., Brunetto M.R., Ovalles F. Determinación de atenolol en productos farmacéuticos por espectrometría infrarroja con transformada de Fourier (FTIR) Rev. Tec. Fac. Ing. Univ. Zulia. 2009;32(3):238–248. [Google Scholar]
- 29.Silva F.M., Lacerda P.S.B., Jones-Junior J. Desenvolvimento sustentável e química verde. Quim. Nova. 2005;28(1):103–110. [Google Scholar]
- 30.Armenta S., Garrigues S., de la Guardia M. Green analytical chemistry. Trends Anal. Chem. 2008;27(6):497–511. [Google Scholar]
- 31.Moros J., Garrigues S., de la Guardia M. Vibrational spectroscopy provides a green tool for multi-component analysis. Trends Anal. Chem. 2010;29(7):578–591. [Google Scholar]
- 32.Cascant M., Kuligowski J., Garrigues S. Determination of sugars in depilatory formulations: a green analytical method employing infrared detection and partial least squares regression. Talanta. 2011;85(4):1721–1729. doi: 10.1016/j.talanta.2011.06.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material