Abstract
A validated ultra-performance liquid chromatography mass spectrometric method (UPLC–MS/MS) was used for the simultaneous quantitation of candesartan (CN) and hydrochlorothiazide (HCT) in human plasma. The analysis was performed on UPLC–MS/MS system using turbo ion spray interface. Negative ions were measured in multiple reaction monitoring (MRM) mode. The analytes were extracted using a liquid–liquid extraction (LLE) method by using 0.1 mL of plasma volume. The lower limit of quantitation for CN and HCT was 1.00 ng/mL whereas the upper limit of quantitation was 499.15 ng/mL and 601.61 ng/mL for CN and HCT respectively. CN d4 and HCT-13Cd2 were used as the internal standards for CN and HCT respectively. The chromatography was achieved within 2.0 min run time using a C18 Phenomenex, Gemini NX (100 mm×4.6 mm, 5 µm) column with organic mixture:buffer solution (80:20, v/v) at a flow rate of 0.800 mL/min. The method has been successfully applied to establish the bioequivalence of candesartan cilexetil (CNC) and HCT immediate release tablets with reference product in human subjects.
Keywords: Candesartan cilexetil, Hydrochlorothiazide, UPLC–MS/MS, Bioequivalence, Candesartan cilexetil-hydrochlorothiazide (ATACAND HCT)
1. Introduction
Candesartan cilexetil (CNC) is an inactive prodrug of candesartan (CN) which is a new generation angiotensin II type 1 receptor blocker (ARB) mainly used for the treatment of hypertension. CNC is hydrolyzed completely by esterase in the intestinal wall during absorption to the active CN moiety during absorption from the gastrointestinal tract [1]. Chemically, it is described as (±)-1-Hydroxyethyl 2-ethoxy-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-7-benzimidazolecarboxylate, cyclohexyl carbonate (ester). The use of a prodrug form increases the bioavailability of CN. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure suggests that combination therapy should typically comprise of a thiazide-type diuretic as a first-line therapy for stage 2 hypertension [2]. The combination of an ARB and a low-dose diuretic exerts a synergistic effect in terms of both efficacy and minimization of the side effects owing to their complementary mechanisms of action [3]. Hydrochlorothiazide (HCT) is one of the most commonly used diuretic drugs along with ARBs. It reduces the amount of water in the body by increasing the flow of urine, which helps lower the blood pressure. Various studies conducted on the combination of HCT and CNC have shown enhanced blood pressure control as compared to the CNC monotherapy [4], [5], [6]. The formulation of CNC and HCT is available in combination with trade name Atacand HCT® in different strengths in the form of tablets. The available strengths for CNC+HCT are 16+12.5 mg, 32+12.5 mg and 32+25 mg. Although some literatures for the quantitation of CN and HCT alone or in combination of other drugs are available [7], [8], [9], [10], [11], [12], no simultaneous sensitive bioanalytical method has been published that could be applied for bioequivalence studies of human subject samples for all available dose strengths of CN+HCT combination. A recent report for the simultaneous quantification of CN and HCT by using liquid chromatography mass spectrometry (LC–MS/MS) could only be applied for the bioequivalence of 16+12.5 mg dose strength due to its very low validated calibration curve range [13]. So, the aim of this study was the development and validation of bioanalytical method for the simultaneous analysis of CN and HCT by using UPLC–MS/MS which could be applied easily on all available dose strengths of Atacand HCT®. This developed method is rapid, selective with good sensitivity, having a shorter analysis run time (2.0 min) and requires a very low plasma volume (0.1 mL) for each analysis. The method has been successfully applied to establish the bioequivalence of CNC and HCT 32+25 mg in-house developed immediate release tablets with reference product [Atacand HCT® (CNC−HCT) 32+25 mg immediate release tablets of Astra Zeneca LP, Wilmington] in healthy adult, human subjects, under fasting conditions.
2. Experimental
2.1. Chemicals and materials
Working standards namely CN (99.44%), HCT (100.00%) and CN d4 (99.21%) (IS1) were procured from Clearsynth Lab. Pvt. Ltd., India, while HCT-13Cd2 (97.00%) (IS2) was obtained from Splendid Lab., India. Control buffered di-potassium salt of ethylene diamine tetra acetic acid (K2EDTA) human plasma was procured from Mediplas Laboratories, Hyderabad, India. All other reagents/chemicals were of AR grade.
2.2. LC–MS/MS instrumentation and settings
A WATERS ultra-performance liquid chromatography (UPLC) system (Milford, MA, USA) with Phenomenex, Gemini NX C18 100 mm × 4.6 mm, 5 µm column was used in this study. The mobile phase consisted of organic mixture:buffer solution (80:20, v/v) (preparation is discussed in Section 2.4). The autosampler temperature was maintained at 5 °C and the flow rate was set at 0.800 mL/min. Ionization and detection of analyte and IS were performed on a triple quadrupole mass spectrometer, API-4000 Q-trap (MS/MS) equipped with turbo ion spray, from MDS Sciex (Toronto, Canada) operated in the negative ion mode. Quantitation was done using MRM mode to monitor protonated precursor→product ion transition of m/z 439.4→309.0, 295.9→204.8, 443.1→312.0 and 298.8→205.9 for CN, HCT, CN d4 and HCT 13Cd2, respectively. All the parameters of UPLC and MS/MS were controlled by analyst software version 1.5.1. The source dependent parameters maintained for analytes and internal standards were GS1 (nebulizer gas): 45 psi, GS2 (heater gas): 55 psi, ion spray voltage (ISV): −2000 V, turbo heater temperature (TEM): 475 °C, entrance potential (EP): −10 V, collision activation dissociation (CAD): 6 psi, and curtain gas (CUR): 25 psi. The compound dependent parameters like declustering potential (DP), collision energy (CE) and cell exit potential (CXP) were optimized at −50, −29 and −6 V for CN, −50, −31 and −18 V for IS1, −92, −33 and −15 V for HCT and −92, −33 and −9 V for IS2, respectively.
2.3. Preparation of standard stock and plasma samples
The standard stock solutions of CN (1 mg/mL), HCT (1 mg/mL), IS1 (1 mg/10 mL) and IS2 (1 mg/10 mL) were prepared by dissolving in requisite amount of methanol. The further dilutions from the stock solutions were prepared by using diluent solution (methanol:milli-Q water in the ratio of 50:50) for spiking in plasma to obtain the required calibration curve (CC) standards and quality control (QC) samples concentration. All the samples were protected from light during preparation, storage and handling. Calibration curve standards consisting of a set of eight non-zero concentrations of 1.00, 2.00, 62.93, 125.61, 225.12, 324.15, 425.57 and 499.15 ng/mL were prepared for CN while concentrations of 1.00, 2.00, 75.02, 149.44, 270.73, 390.05, 511.37 and 601.61 ng/mL were prepared for HCT. The quality control samples for CN consisting of concentrations for lower limit of quantitation quality control (LLOQ QC=1.00 ng/mL), low quality control (LQC=2.75 ng/mL), medium quality control (MQC=249.66 ng/mL) and high quality control (HQC=411.44 ng/mL) were prepared. While for HCT LLOQ QC 1.00 ng/mL, LQC 2.74 ng/mL, MQC 301.90 ng/mL and HQC 495.12 ng/mL were also prepared. After bulk spiking, 200 µL of spiked plasma samples was pipetted out in pre-labeled polypropylene tubes. The calibration curve standards and quality control samples were logged in ultra low temperature deep freezer (temp. range: −55 °C to −75 °C) except 30 samples each of LQC and HQC which were transferred for storage in cell frost deep freezer (temp. range: −17 °C to −27 °C) for the generation of long term stability at −22±5 °C. These samples were used for performing the method validation.
2.4. Preparation of mobile phase and liquid–liquid extraction method
Buffer solution A (5 mM ammonium acetate) was prepared by weighing approximately 385 mg of ammonium acetate in a 1000 mL reagent bottle followed by the addition of 1000 mL milli-Q water and filtered through a 0.22 µm membrane filter. Finally, the pH was adjusted with liquid ammonia to 7.5±0.1. Organic mixture was prepared as a mixture of methanol:acetonitrile in the ratio of 15:85 v/v (Solution B). Mobile phase was prepared by adding buffer solution A and solution B in the ratio of 80:20 (v/v). The solutions were used within 7 days from the date of preparation. A set of calibration curve standards and quality control samples were withdrawn from the deep freezer and allowed to thaw at room temperature in a water bath. 100 µL of plasma from the pre-labeled polypropylene tubes was aliquoted into labeled ria vials and 50 µL of internal standard dilution (IS1=400 ng/mL and IS2=500 ng/mL) was added and vortexed. 200 µL of formic acid:milli-Q water solution in the ratio of 99.500.5 (v/v) (solution C) was then added and samples were vortexed. 2 mL of extraction solution (tert-butyl-methyl-ether:dichloromethane:70:30, v/v was added and samples were vortexed for approximately 5 min. The samples were flash freezed for 1–2 min, supernatant was decanted off and evaporated to dryness at 40 °C at constant pressure in nitrogen evaporator. Finally, the dried samples were reconstituted with 200 µL of mobile phase and transferred into UPLC vials for analysis.
2.5. Method validation
The method was validated for selectivity, sensitivity, linearity, matrix effect, precision, accuracy, recovery and stability of analytes under different processing and storage conditions as per the USFDA guidelines [14]. The results of various stabilities (i.e., stock dilution stability at refrigerator temperature and room temperature, photo degradation test in light and dark and standard stock solution stability in refrigerator, auto sampler stability, reinjection reproducibility, freeze-thaw stability, long term stability at −65±10 °C and at −22±5 °C, reagent stability, bench top stability, dry ice stability, dry extract stability, extended bench top stability, wet extract stability in refrigerator, lipemic and haemolysed plasma stability), blood stability, effect of potentially interfering drugs, dilution integrity, recovery, ion suppression through infusion, ruggedness, robustness and extended batch verification met the acceptance criteria as per the USFDA guidelines [15].
Selectivity of the method for endogenous substances was accessed at LLOQ level for CN and HCT in eight lots of normal plasma, four lots of lipemic plasma and four lots of haemolysed plasma containing K2EDTA as an anticoagulant. Sensitivity of the method was determined in six LLOQ samples for CN and HCT by quantitating against a calibration curve. The calibration curve data of three precision and accuracy batches were subjected for goodness of fit analysis. The back-calculated concentrations of calibration curve standards using 1/x and 1/x2 weighing were considered for finding the best fit for regression. Linearity was calculated using a regression equation with a weighting factor of 1/x2 for drug to IS concentration to produce the best fit for the concentration–detector response relationship for CN and HCT. Matrix effect was accessed at two concentration levels (LQC and HQC) in normal, lipemic and haemolysed plasma. For matrix effect in normal, lipemic and haemolysed plasma, 12 blank samples were processed from six normal plasma lots, six blank samples from three lipemic plasma lots and six blank samples from three haemolysed plasma lots. Two aliquots were used from each plasma lot and these post-extracted dried blank samples of matrix effect were prepared by reconstituting with the neat solutions containing CN and HCT at LQC and HQC concentrations representing the final extracted concentration for both the analytes as well as internal standards. The comparison samples were the same neat solutions prepared in mobile phase at LQC and HQC levels containing CN and HCT. Matrix effect was calculated as per the following formula:
The precision of the assay was calculated as percent coefficient of variation over the concentration range of LLOQ QC, LQC, MQC and HQC samples. The accuracy of the assay was calculated as the ratio of the mean values of the LLOQ QC, LQC, MQC and HQC samples to their respective nominal values. Intra-day precision and accuracy were calculated by analyzing the six replicates of quality control samples at four concentration levels within the single analytical run while the inter-day precision and accuracy were calculated by analyzing the 18 replicates of quality control samples at four concentration levels from three analytical runs on two consecutive days of validation.
To access the recovery of the extraction method followed for extraction of analytes and internal standards the aqueous comparison samples (LQC, MQC and HQC) were prepared by adding 40 µL aqueous dilution (20 µL aqueous dilution each of CN and HCT) from each respective quality control, 500 µL of IS dilution (approximately 400 ng/mL for IS1 and 500 ng/mL for IS2) and 3460 µL of mobile phase (representing 100% extraction). The aqueous samples (LQC, MQC and HQC) of CN and HCT were compared against six sets of extracted LQC, MQC and HQC samples. Recovery of internal standard was also compared at LQC, MQC and HQC levels.
The stability of CN and HCT in plasma was performed during their processing and storage. Bench top stability of CN and HCT was determined for 22 h using six sets each of LQC and HQC samples while extended bench top stability was determined for 7 h in spiked LQC and HQC samples at every step of sample processing. The freeze-thaw stability for the analytes was determined after completion of five freeze–thaw cycles by using six sets of LQC and HQC samples. Long term stability (at −65±10 °C and −22±5 °C) was carried out in plasma for 22 days. To access each kind of stability for CN and HCT in plasma six sets of LQC and HQC samples were processed and analyzed after required storage time against the freshly spiked calibration curve standards and freshly spiked quality control samples (freshly spiked comparison QC samples were prepared from the fresh stock solution of analytes at LQC and HQC levels) to calculate the % change. Stock dilution stability in refrigerator for CN, HCT, IS1 and IS2 was carried out for 22 days while stock dilution stability at room temperature and at 2–8 °C was carried out for 26 h. Photo degradation test samples of analytes and IS were kept for 26 h in light and dark. For the aqueous medium stability (except plasma) studies i.e., stock dilution stability at 2–8 °C and room temperature, photo degradation test in light and dark and standard stock solution stability 2–8 °C, two aqueous mixtures were prepared, one from the stability standard stock solution and the other from fresh standard stock solution (comparison stock). The response of stability dilution (already stored at room temperature, refrigerator temperature and in presence of light and dark) against the comparison dilution (prepared from freshly prepared stock solution) has been corrected using a correction factor. Six replicates of aqueous mixture from stability stock and comparison stock were injected.
The effect of potentially interfering drugs ( Ibuprofen, Caffeine, Acetaminophen and Acetyl salicylic acid) on CN and HCT analysis was performed by spiking PID's at their approximately Cmax in the LLOQ samples in triplicate. Robustness experiment was performed at low and high pH of buffer solutions, at different column oven temperatures (38 °C and 42 °C) and at different flow rates (0.840 µL/min and 0.760 µL/min). To evaluate ruggedness, precision and accuracy batch was processed and analyzed by different analysts using different columns and different sets of solutions.
2.6. Bioequivalence study design
The bioequivalence study design comprised of a randomized, balanced, open label, two treatment, two period, two sequence, single dose, crossover, bioequivalence study by comparing in-house developed test product i.e., CNC and HCT (32+25 mg) immediate release tablets with reference product Atacand HCT® (CNC−HCT) (32+25 mg) immediate release tablets of Astra Zeneca LP, Wilmington, in healthy adult, human subjects, under fasting conditions. The protocol was approved by the relevant institutional ethics committee. All participants gave written consent and were informed of the aims and risks of the study. Inclusion criteria comprise age (18–45 years), body mass index (18.5–30.0 kg/height2) and absence of abnormalities on physical examination along with normal electrocardiogram and laboratory tests. Exclusion criteria comprise allergy to CNC, HCT, alcoholism, psychosis, smoking, diabetes or any disease which could compromise the haemopoietic, gastrointestinal, renal, hepatic, cardiovascular, and respiratory or central nervous systems. Moreover, all the procedures were based on the International Conference on Harmonization, E6 Good Clinical Practice guidelines [8]. As per the study protocol 1976 (38 subjects×26 time points×2 periods) clinical blood samples were to be collected from 38 subjects during two periods of the study. The clinical blood samples were collected in K2EDTA vacutainer from the subjects and intermediately stored in deep freezer at −20 °C on the days of plasma sample collection. Plasma was obtained by centrifugation at 4000 rpm at 4 °C for 15 min and stored at −20 °C until assay. All pharmacokinetic values were gained by the non-compartmental model and expressed as mean±SD.
3. Results and discussion
3.1. LC–MS/MS settings
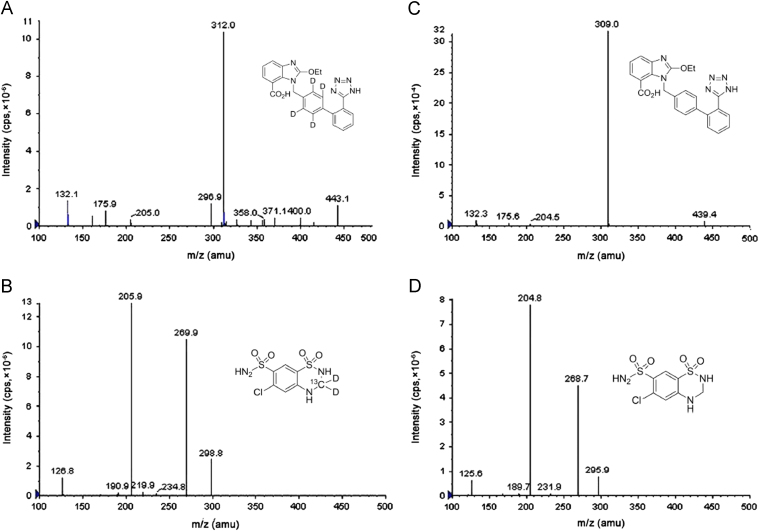
Internal standards CN d4 (IS1) and HCT13Cd2 (IS2) were expected showing nearly similar chromatographic behavior as of analytes because they were differing only in terms of possessing different isotopic atoms. The retention time (RT) of IS1 and CN was found to be 0.88 min and 0.89 min while RT for IS2 and HCT was found to be 1.14 min and 1.13 min. Furthermore, as expected the recovery of both the internal standards was similar to that of their parent compounds. Electron spray ionization (ESI) provided high ionization efficiencies for both analytes and IS in negative ion mode which resulted in higher sensitivity of the method. The product ion mass spectra of the [M−H]− ions for HCT and CN and their respective internal standards have been shown in Fig. 1.
Fig. 1.
The product ion mass spectra of the [M−H]− ions of (A) IS1, (B) CN, (C) IS2 and (D) HCT.
3.2. Sample preparation
Liquid–liquid extraction (LLE) method was used for sample preparation because of relatively low cost, good extraction efficiency as well as simple procedure. Various extraction solvent systems were tried but mixture of tert-butyl-methyl-ether and dichloromethane in the ratio of 70:30 (v/v) was found to be the most effective for extraction of both analytes and IS.
3.3. Method validation
3.3.1. Selectivity
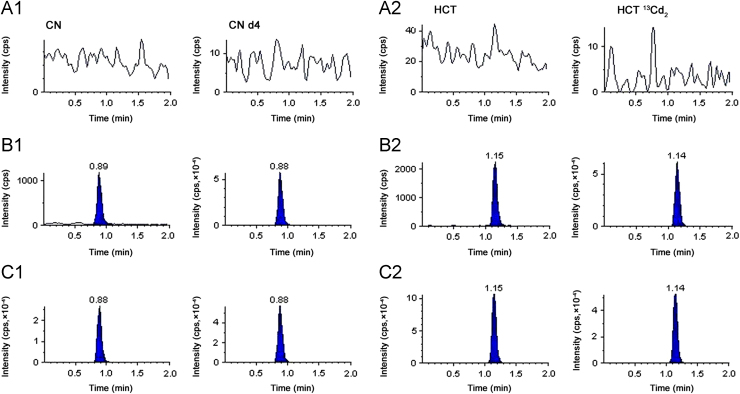
Fig. 2 consists of typical MRM chromatograms of a blank plasma sample, a plasma sample spiked with CN and HCT at the LLOQ level (1.00 ng/mL for both analytes) and a plasma sample from a healthy volunteer 1.5 h after the oral administration of the combination tablet. There was no significant interference from endogenous substances observed at the retention time of the analytes and internal standards in the extracted blank plasma sample.
Fig. 2.
Representative MRM chromatograms of blank plasma sample (A1 and A2), a plasma sample spiked with CN (B1) and HCT (B2) at the LLOQ level (1.00 ng/mL for both analytes) and a plasma sample from a healthy volunteer 1.5 h after the oral administration of the combination tablet. (C1 for CN and C2 for HCT). CN (left panels A1, B1 and C1) and its ISTD-CN d4 (right panels A1, B1and C1) and HCT (left panels A2, B2and C2) and its ISTD-HCT 13Cd2 (right panels A2, B2 and C2).
3.3.2. Sensitivity and linearity
In sensitivity the observed accuracies were 110.17% and 103.50% with a precision of 4.60% and 3.29% for CN and HCT respectively. The assay was linear over the concentration range 1.00–499.15 ng/mL for CN and 1.00–601.61 ng/mL for HCT. The observed slope, intercept and r2 for CN were respectively 0.0083, 0.0163 and 0.9980 while for HCT were 0.0160, 0.0059 and 0.9990. Therefore, the typical equations of calibration curves are as follows:
where y represents the analyte/IS peak area ratio and x represents the plasma concentration of the analyte. LLOQ was 1.00 ng/mL for both CN and HCT and was adequate for clinical PK studies following oral administration of therapeutic doses for all available formulations of Atacand HCT®.
3.3.3. Matrix effect
The percentage matrix effects of low and high QC samples were 6.16 and 2.31 for CN while 6.44 and 2.49 for HCT respectively. It was found to be 6.79 and 1.57 for IS1 and 7.27 and 3.72 for IS2 respectively. The results were within the acceptance criteria and indicate that ion suppression or enhancement due to the plasma matrix was consistent and would not interfere with the quantitation of analytes.
3.3.4. Precision and accuracy
Table 1 summarizes back calculated concentrations of calibration curve standards for CN and HCT whereas Table 2 represents the intra-day and inter-day precision and accuracy data. The intra-day precision for CN was ≤4.95% and accuracy was ≥94.01%. An intra-day precision for HCT was ≤7.04% and accuracy was ≥94.55%.
Table 1.
Back calculated concentration of calibration curve standards for candesartan and hydrochlorothiazide (n=3).
| Analyte | Standard concentration (ng/mL) | Mean (ng/mL) | SD | CV (%) | Nominal (%) | Slope | Intercept | r2 |
|---|---|---|---|---|---|---|---|---|
| Candesartan | 1.00 | 1.06 | 0.04 | 3.82 | 105.67 | 0.0083 | 0.0163 | 0.9980 |
| 2.00 | 2.08 | 0.04 | 1.69 | 104.17 | ||||
| 62.93 | 67.55 | 1.58 | 2.34 | 107.34 | ||||
| 125.61 | 128.52 | 1.98 | 1.54 | 102.32 | ||||
| 225.12 | 224.26 | 2.24 | 1.00 | 99.62 | ||||
| 324.15 | 310.19 | 5.26 | 1.70 | 95.69 | ||||
| 425.57 | 404.11 | 4.10 | 1.01 | 94.96 | ||||
| 499.15 | 488.92 | 4.96 | 1.01 | 97.95 | ||||
| Hydrochlorothiazide | 1.00 | 1.05 | 0.07 | 6.22 | 104.67 | 0.0160 | 0.0059 | 0.9990 |
| 2.00 | 2.10 | 0.04 | 2.08 | 105.00 | ||||
| 75.02 | 79.34 | 1.14 | 1.44 | 105.76 | ||||
| 149.44 | 148.36 | 2.61 | 1.76 | 99.28 | ||||
| 270.73 | 274.59 | 1.63 | 0.59 | 101.42 | ||||
| 390.05 | 380.98 | 4.40 | 1.16 | 97.67 | ||||
| 511.37 | 491.30 | 9.66 | 1.97 | 96.07 | ||||
| 601.61 | 600.15 | 6.54 | 1.09 | 99.76 | ||||
Table 2.
Inter-day and intra-day precision and accuracy of the method for candesartan and hydrochlorothiazide.
| Analyte | Level | Concentration added (ng/mL) | Inter-day (n=6) |
Intra-day (n=18) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean conc. found (ng/mL) | Nominal (%) | CV (%) | Mean conc. found (ng/mL) | Nominal (%) | CV (%) | |||
| Candesartan | LLOQQC | 1.00 | 1.03 | 102.67 | 4.95 | 1.03 | 102.67 | 4.65 |
| LQC | 2.75 | 2.72 | 98.91 | 3.26 | 2.68 | 97.41 | 4.13 | |
| MQC | 249.66 | 235.09 | 94.16 | 2.56 | 245.21 | 98.22 | 3.88 | |
| HQC | 411.44 | 386.78 | 94.01 | 2.87 | 402.50 | 97.83 | 3.67 | |
| Hydrochloro-thiazide | LLOQQC | 1.00 | 1.00 | 100.17 | 7.04 | 1.00 | 100.17 | 6.62 |
| LQC | 2.74 | 2.66 | 97.02 | 4.67 | 2.70 | 98.52 | 3.71 | |
| MQC | 301.90 | 292.56 | 96.91 | 2.39 | 305.45 | 101.17 | 4.12 | |
| HQC | 495.12 | 468.15 | 94.55 | 3.20 | 483.36 | 97.62 | 3.16 | |
Whereas the inter-day precision for CN was ≤4.65% and accuracy was ≥97.41%. An inter-day precision for HCT was ≤6.62% and accuracy was ≥97.62%.
3.3.5. Recovery and stability
The mean recoveries of CN in low, medium and high QC samples were 89.02%, 96.90% and 112.19%, respectively (mean recovery=99.37±11.86%) and those of HCT were 100.65%, 94.16% and 102.49% (mean recovery=99.10±4.41%). Mean recoveries of IS1 and IS2 were 99.91±10.74% and 98.45±4.40%, respectively. The data show that the simple LLE procedure efficiently extracts all four compounds from human plasma. Table 3 summarizes stability data and shows there were no stability-related issues that might cause problems in application of the assay to pharmacokinetic study.
Table 3.
Stability data of CN and HCT in processed QC samples for different stability activities at different conditions (n=6).
| Stability experiment | Analyte | Concentration added (ng/mL) | Mean concentration found in stability samples (ng/mL) | Nominal (%) | CV (%) | Mean concentration found in comparison samples (ng/mL) | Nominal (%) | CV (%) | Change (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bench top stability (22 h) | CN | 2.75 | 2.71 | 98.67 | 2.97 | 2.58 | 93.82 | 4.71 | 6.10 |
| 411.44 | 402.84 | 97.91 | 2.98 | 399.52 | 97.12 | 1.78 | −0.81 | ||
| HCT | 2.74 | 2.69 | 98.18 | 0.94 | 2.48 | 90.63 | 3.37 | −0.17 | |
| 495.12 | 476.11 | 96.16 | 2.32 | 473.41 | 95.69 | 3.63 | −0.49 | ||
| Auto sampler stability (50 h) | CN | 2.75 | 2.69 | 97.76 | 2.04 | 2.57 | 93.33 | 5.10 | 4.19 |
| 411.44 | 390.53 | 94.92 | 2.63 | 405.07 | 98.47 | 3.44 | 3.60 | ||
| HCT | 2.74 | 2.71 | 99.03 | 2.97 | 2.65 | 96.87 | 3.45 | 2.67 | |
| 495.12 | 473.16 | 95.56 | 2.30 | 474.55 | 95.92 | 2.41 | 0.37 | ||
| Freeze–thaw stability (5-cycles) | CN | 2.75 | 2.78 | 101.15 | 1.60 | 2.58 | 93.82 | 4.71 | −0.42 |
| 411.44 | 401.52 | 97.59 | 2.67 | 399.52 | 97.12 | 1.78 | −0.48 | ||
| HCT | 2.74 | 2.70 | 98.24 | 2.26 | 2.48 | 90.63 | 3.37 | −0.37 | |
| 495.12 | 480.11 | 96.97 | 0.32 | 473.41 | 95.69 | 3.63 | −1.34 | ||
| Dry extract stability (69 h) | CN | 2.75 | 2.74 | 99.58 | 3.54 | 2.57 | 93.33 | 5.10 | 1.33 |
| 411.44 | 404.22 | 98.25 | 2.27 | 405.07 | 98.47 | 3.44 | 0.23 | ||
| HCT | 2.74 | 2.69 | 98.11 | 2.04 | 2.65 | 96.87 | 3.45 | 2.51 | |
| 495.12 | 470.54 | 95.03 | 1.58 | 474.55 | 95.92 | 2.41 | 0.92 | ||
| Wet extract stability (69 h) | CN | 2.75 | 2.75 | 99.82 | 3.33 | 2.57 | 93.33 | 5.10 | 2.56 |
| 411.44 | 411.24 | 99.95 | 0.54 | 405.07 | 98.47 | 3.44 | −1.51 | ||
| HCT | 2.74 | 2.68 | 97.93 | 1.17 | 2.65 | 96.87 | 3.45 | −0.06 | |
| 495.12 | 472.49 | 95.43 | 2.28 | 474.55 | 95.92 | 2.41 | 0.51 | ||
| Lipemic plasma stability (4 days) | CN | 2.75 | 2.77 | 100.61 | 4.34 | 2.58 | 93.82 | 4.71 | 0.87 |
| 411.44 | 406.56 | 98.81 | 1.58 | 399.52 | 97.12 | 1.78 | −1.74 | ||
| HCT | 2.74 | 2.74 | 99.94 | 3.54 | 2.48 | 90.63 | 3.37 | −1.58 | |
| 495.12 | 481.39 | 97.23 | 1.90 | 473.41 | 95.69 | 3.63 | −1.61 | ||
| Haemolysed plasma stability (4 days) | CN | 2.75 | 2.71 | 98.61 | 3.94 | 2.58 | 93.82 | 4.71 | 4.75 |
| 411.44 | 409.87 | 99.62 | 1.53 | 399.52 | 97.12 | 1.78 | −2.57 | ||
| HCT | 2.74 | 2.69 | 98.11 | 2.04 | 2.48 | 90.63 | 3.37 | 2.95 | |
| 495.12 | 486.11 | 98.18 | 1.30 | 473.41 | 95.69 | 3.63 | −2.60 | ||
| Long term stability at −65±10 °C (22 days) | CN | 2.75 | 2.69 | 97.88 | 2.26 | 2.50 | 90.97 | 1.28 | 0.70 |
| 411.44 | 384.37 | 93.42 | 1.07 | 383.30 | 93.21 | 0.64 | −0.22 | ||
| HCT | 2.74 | 2.77 | 100.97 | 4.34 | 2.58 | 94.16 | 2.88 | −2.33 | |
| 495.12 | 465.62 | 94.04 | 0.85 | 462.62 | 93.44 | 0.92 | −0.65 | ||
| Long term stability at −22±5 °C (22 days) | CN | 2.75 | 2.69 | 97.82 | 0.94 | 2.50 | 90.97 | 1.28 | 2.20 |
| 411.44 | 381.22 | 92.66 | 1.20 | 383.30 | 93.21 | 0.64 | 0.60 | ||
| HCT | 2.74 | 2.71 | 98.97 | 3.94 | 2.58 | 94.16 | 2.88 | −3.55 | |
| 495.12 | 465.19 | 93.95 | 1.39 | 462.62 | 93.44 | 0.92 | −0.55 | ||
3.3.6. Other parameters
The outcomes of other parameters like ruggedness, reinjection reproducibility, effect of potential interfering drugs (PID), dilution integrity, extended batch verification and robustness were found to be within the acceptance criteria as per the USFDA guidelines [15].
3.4. Bioequivalence study
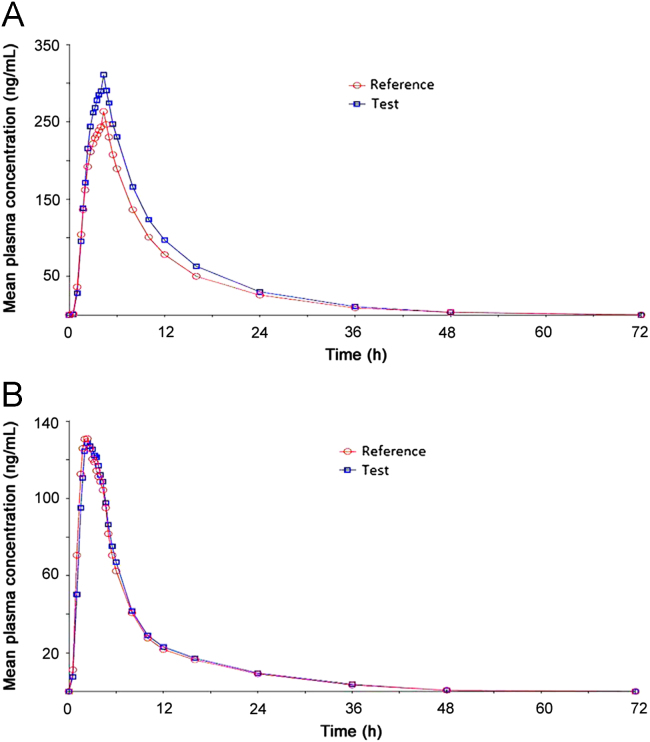
The method was applied for the analysis of plasma samples obtained from the pharmacokinetic study. The study was conducted as a randomized, balanced, open label, two treatment, two period, two sequence, single dose, crossover, bioequivalence study of comparing test product [CNC and HCT 32+25 mg immediate release tablet] with reference product [Atacand HCT® (CNC and HCT) 32+25 mg immediate release tablets] in 59 healthy adult, human subjects, under fasting conditions. Each subject received a tablet of CNC (32 mg) and HCT (25 mg) of test or reference product and a wash out period of 7 days was maintained between two consecutive administrations of the investigational products. Blood samples were collected using K2EDTA vaccutainers at the following time points: Pre-dose and at 0.50, 1.00, 1.50, 1.75, 2.00, 2.33, 2.67, 3.00, 3.25, 3.50, 3.75, 4.00, 4.33, 4.67, 5.00, 5.50, 6.00, 8.00, 10.00, 12.00, 16.00, 24.00, 36.00, 48.00 and 72.00 h postdose. Pharmacokinetic parameters were calculated from the subjects who had successfully completed periods I and II of the study. Some of the main pharmacokinetic parameters are given in Table 4. The mean plasma concentration versus time profile is shown in Fig. 3.
Table 4.
Pharmacokinetic parameters after administration of immediate release CN+HCT (32+25 mg) tablets in human subjects (n=59).
| Pharmacokinetic parameters | Candesartan |
Hydrochlorothiazide |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test |
Reference |
90% CI for T/R a | Test |
Reference |
90% CI for T/Ra | |||||
| Mean | RSD (%) | Mean | RSD (%) | Mean | RSD (%) | Mean | RSD (%) | |||
| Cmax(ng/mL) | 353.04 | 118.73 | 296.82 | 84.85 | 112.31–124.51 | 164.79 | 41.71 | 167.84 | 38.46 | 92.35–102.85 |
| AUC0−t (ng h/mL) | 3079.21 | 824.71 | 2581.70 | 588.34 | 112.99–123.60 | 1039.96 | 213.62 | 1023.37 | 223.78 | 99.62–103.96 |
| AUC0−∞ (ng h/mL) | 3128.76 | 835.57 | 2631.63 | 593.35 | 112.67–123.09 | 1083.58 | 217.33 | 1066.47 | 221.92 | 99.65–103.70 |
90% Confidence interval for the ratio of the mean test/reference.
Fig. 3.
Mean plasma concentration–time curve for CN (A) and HCT (B) compared with standard (Atacand HCT®).
4. Conclusion
As shown above, the CN and HCT compounds are highly stable in biological matrix when experiments were performed under different stability conditions like bench top, freeze–thaw, long term (−65 °C and −22 °C) and auto sampler etc. The recovery of the method is very good with no matrix effect. Due to the extended calibration curve range (at both LLOQ and ULOQ levels) the method could be utilized to quantify CN and HCT for all available dose strengths. Due to short analysis run time (2 min) and low plasma sample volume consumption (0.1 mL) the method is highly economical in comparison to all the published literature. The method has been successfully used in a pharmacokinetic study, where 59 healthy male volunteers were given a fixed-dose of CNC+HCT (32+25 mg) immediate release tablet. Furthermore, another added advantage, a single analytical column was used to chromatograph about 3500 extracts and there was no requirement to clean this column during the entire study sample analysis.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Gao F., Zhang Z., Bu H. Nanoemulsion improves the oral absorption of candesartan cilexetil in rats: performance and mechanism. J. Controlled Release. 2011;149:168–174. doi: 10.1016/j.jconrel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Flack J.M. Maximising antihypertensive effects of angiotensin II receptor blockers with thiazide diuretic combination therapy: focus on irbesartan/hydrochlorothiazide. Int. J. Clin. Pract. 2007;61:2093–2102. doi: 10.1111/j.1742-1241.2007.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H.Y., Hong B.K., Chung W.J. Phase IV, 8-week, multicenter, randomized, active treatment–controlled, parallel group, efficacy, and tolerability study of high-dose candesartan cilexetil combined with hydrochlorothiazide in Korean adults with stage II hypertension. Clin. Ther. 2011;33:1043–1056. doi: 10.1016/j.clinthera.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Campbell M., Sonkodi S., Soucek M. A candesartan cilexetil/hydrochlorothiazide combination tablet provides effective blood pressure control in hypertensive patients inadequately controlled on monotherapy. Clin. Exp. Hypertens. 2001;23:345–355. doi: 10.1081/ceh-100102672. [DOI] [PubMed] [Google Scholar]
- 5.Bönner G., Fuchs W. Fixed combination of candesartan with hydrochlorothiazide in patients with severe primary hypertension. Curr. Med. Res. Opin. 2004;20:597–602. doi: 10.1185/030079904125003395. [DOI] [PubMed] [Google Scholar]
- 6.Wing L., Arnolda L., Upton J. Candesartan and hydrochlorothiazide in isolated systolic hypertension. Blood Press. 2003;12:246–254. doi: 10.1080/08037050310014954. [DOI] [PubMed] [Google Scholar]
- 7.Karra V.K., Pilli N.R., Inamadugu J.K. Simultaneous determination of pioglitazone and candesartan in human plasma by LC–MS/MS and its application to a human pharmacokinetic study. J. Pharm. Anal. 2012;2:167–173. doi: 10.1016/j.jpha.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belal T.S., Daabees H.G., Abdel-Khalek M.M. New simple spectrophotometric method for determination of the binary mixtures (atorvastatin calcium and ezetimibe; candesartan cilexetil and hydrochlorothiazide) in tablets. J. Pharm. Anal. 2013;3 (2) doi: 10.1016/j.jpha.2012.10.004. 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao F., Zhang M., Cui X. Simultaneous quantitation of hydrochlorothiazide and metoprolol in human plasma by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010;52:149–154. doi: 10.1016/j.jpba.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Li H., He J., Liu Q. Simultaneous determination of hydrochlorothiazide and reserpine in human urine by LC with a simple pre-treatment. Chromatographia. 2011;73:171–175. doi: 10.1007/s10337-010-1821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pradhan K.K., Mishra U.S., Pattnaik S. Development and validation of a stability-indicating UV spectroscopic method for candesartan in bulk and formulations. Indian J. Pharm. Sci. 2011;73:693–696. doi: 10.4103/0250-474X.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wankhede S.B., Raka K.C., Wadkar S.B. Spectrophotometric and HPLC methods for simultaneous estimation of amlodipine besilate, losartan potassium and hydrochlorothiazide in tablets. Indian J. Pharm. Sci. 2010;72:136–140. doi: 10.4103/0250-474X.62239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharathi D.V., Hotha K.K., Chatki P.K. LC–MS/MS method for simultaneous estimation of candesartan and hydrochlorothiazide in human plasma and its use in clinical pharmacokinetics. Bioanalysis. 2012;4:1195–1204. doi: 10.4155/bio.12.83. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research (CBER), Guidance for Industry: ICHE6 Good Clinical Practice, 1996, 〈http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073122.pdf〉.
- 15.Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), May 2001.